25 Years of Research in Human Lactation: From Discovery to Translation
Abstract
:1. Introduction
2. Breast Anatomy
| Breast Anatomy Number of ductal openings on the nipple is 4–18 (previously 15–20) Milk ducts branch close to the nipple The conventionally described lactiferous sinuses do not exist Milk ducts can reside close to the skin surface and are easily compressible Most of the glandular tissue is found within 30 mm of the nipple |
3. Milk Ejection
| Milk ejection Milk ejection is critical for milk removal Milk ejection patterns are unique to the individual Milk ejection patterns do not change with stimulus (breastfeeding or pumping or different pumping patterns) Milk ejection patterns do not change over lactation Milk ejection patterns do not change between lactations |
4. Secretory Activation
Secretory activation is marked by
|
5. Milk Production
6. Breast Anatomy and Milk Production
7. Breast Physiology
8. Medications
9. Factors Impacting Milk Removal
Effectiveness of milk removal from the breast is enhanced by:
|
10. Sucking Swallowing and Breathing
11. Dynamics of Breastfeeding
Suck Swallow Breath Co-Ordination
Milk removal by breastfeeding infants
|
12. Nipple Pain
13. Nipple Shields
14. Ankyloglossia
15. Preterm Infants
16. Influences of Maternal Body Composition on Milk Composition
17. Gastric Emptying and Infant Body Composition
18. Preterm Infants
Gastric emptying of preterm infants
|
19. Term Infants
20. Proteins
21. Immune Factors
22. Appetite Hormones
23. Glucocorticoids
24. Carbohydrates
25. Lipids
Gastric emptying in term infants
|
26. Infant Health
27. Human Milk Microbiome
28. Donor Human Milk
29. Human Milk Cellular Content
30. Breastfeeding during COVID
31. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rollins, N.C.; Bhandari, N.; Hajeebhoy, N.; Horton, S.; Lutter, C.K.; Martines, J.C.; Piwoz, E.G.; Richter, L.M.; Victora, C.G.; Lancet Breastfeeding Series Group. Why invest, and what it will take to improve breastfeeding practices? Lancet 2016, 387, 491–504. [Google Scholar] [CrossRef]
- WHO Global Breastfeeding Scorecard, 2018: Enabling Women to Breastfeed through Better Policies and Programmes; WHO: Geneva, Switzerland, 2018.
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef]
- Azad, M.B.; Nickel, N.C.; Bode, L.; Brockway, M.; Brown, A.; Chambers, C.; Goldhammer, C.; Hinde, K.; McGuire, M.; Munblit, D.; et al. Breastfeeding and the origins of health: Interdisciplinary perspectives and priorities. Matern. Child Nutr. 2021, 17, e13109. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A. On the Anatomy of the Breast. Available online: http://jdc.jefferson.edu/cgi/viewcontent.cgi?article=1059&context=cooper (accessed on 23 November 2016).
- Cooper, A.P. The Anatomy of the Breast; Longman, Orme, Green, Brown, and Longman: London, UK, 1840. [Google Scholar]
- Ramsay, D.T.; Kent, J.C.; Hartmann, A.; Hartmann, P.E. Anatomy of the lactating human breast redefined with ultrasound imaging. J. Anat. 2005, 206, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, D.T.; Kent, J.C.; Owens, R.A.; Hartmann, P.E. Ultrasound imaging of milk ejection in the breast of lactating women. Pediatrics 2004, 113, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fetherston, C. Risk factors for lactation mastitis. J. Hum. Lact. 1998, 14, 101–109. [Google Scholar] [CrossRef]
- Love, S.M.; Barsky, S.H. Anatomy of the nipple and breast ducts revisited. Cancer 2004, 101, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.B.; Kent, J.C.; Casey, T.M.; Owens, R.A.; Hartmann, P.E. Breast growth and the urinary excretion of lactose during human pregnancy and early lactation: Endocrine relationships. Exp. Physiol. 1999, 84, 421–434. [Google Scholar] [CrossRef]
- Wilson-Clay, B.; Hoover, C. Response to review of the breastfeeding atlas. J. Hum. Lact. Off. J. Int. Lact. Consult. Assoc. 2006, 22, 389. [Google Scholar]
- Gooding, M.J.; Finlay, J.; Shipley, J.A.; Halliwell, M.; Duck, F.A. Three-dimensional ultrasound imaging of mammary ducts in lactating women: A feasibility study. J. Ultrasound Med. 2010, 29, 95–103. [Google Scholar] [CrossRef] [Green Version]
- McClellan, H.L.; Miller, S.J.; Hartmann, P.E. Evolution of lactation: Nutrition v. protection with special reference to five mammalian species. Nutr. Res. Rev. 2008, 21, 97–116. [Google Scholar] [CrossRef]
- Truchet, S.; Honvo-Houeto, E. Physiology of milk secretion. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 367–384. [Google Scholar] [CrossRef]
- Cobo, E.; De Bernal, M.M.; Gaitan, E.; Quintero, C.A. Neurohypophyseal hormone release in the human. II. Experimental study during lactation. Am. J. Obstet. Gynecol. 1967, 97, 519–529. [Google Scholar] [CrossRef]
- Ramsay, D.T.; Mitoulas, L.R.; Kent, J.C.; Cregan, M.D.; Doherty, D.A.; Larsson, M.; Hartmann, P.E. Milk flow rates can be used to identify and investigate milk ejection in women expressing breast milk using an electric breast pump. Breastfeed. Med. 2006, 1, 14–23. [Google Scholar] [CrossRef]
- Sakalidis, V.S.; Ivarsson, L.; Haynes, A.G.; Jager, L.; Scharer-Hernandez, N.G.; Mitoulas, L.R.; Prime, D.K. Breast shield design impacts milk removal dynamics during pumping: A randomized controlled non-inferiority trial. Acta Obstet. Gynecol. Scand. 2020, 99, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.C.; Ramsay, D.T.; Doherty, D.; Larsson, M.; Hartmann, P.E. Response of breasts to different stimulation patterns of an electric breast pump. J. Hum. Lact. 2003, 19, 179–186; quiz 187–178, 218. [Google Scholar] [CrossRef] [PubMed]
- Prime, D.K.; Geddes, D.T.; Spatz, D.L.; Robert, M.; Trengove, N.J.; Hartmann, P.E. Using milk flow rate to investigate milk ejection in the left and right breasts during simultaneous breast expression in women. Int. Breastfeed. J. 2009, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, H.; Kent, J.C.; Hartmann, P.E.; Geddes, D.T. Asynchronous milk ejection in human lactating breast: Case series. J. Hum. Lact. 2015, 31, 254–259. [Google Scholar] [CrossRef]
- Gardner, H.; Lai, C.T.; Ward, L.; Geddes, D. Detection of Milk Ejection Using Bioimpedance Spectroscopy in Lactating Women during Milk Expression Using an Electric Breast Pump. J. Mammary Gland Biol. Neoplasia 2019, 24, 177–184. [Google Scholar] [CrossRef]
- McNeilly, A.S.; Robinson, I.C.; Houston, M.J.; Howie, P.W. Release of oxytocin and prolactin in response to suckling. Br. Med. J. (Clin. Res. Ed.) 1983, 286, 257–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prime, D.K.; Geddes, D.T.; Hepworth, A.R.; Trengove, N.J.; Hartmann, P.E. Comparison of the patterns of milk ejection during repeated breast expression sessions in women. Breastfeed. Med. 2011, 6, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.; Kent, J.C.; Lai, C.T.; Mitoulas, L.R.; Cregan, M.D.; Hartmann, P.E.; Geddes, D.T. Milk ejection patterns: An intra-individual comparison of breastfeeding and pumping. BMC Pregnancy Childbirth 2015, 15, 156. [Google Scholar] [CrossRef] [Green Version]
- Gardner, H.; Kent, J.C.; Prime, D.K.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Milk ejection patterns remain consistent during the first and second lactations. Am. J. Hum. Biol. 2017, 29, e22960. [Google Scholar] [CrossRef]
- Barlow, S.M.; Lund, J.P.; Estep, M.; Kolta, A. Central pattern generators for orofacial movements and speech. In Handbook of Mammalian Vocalization; Brudzynski, S.M., Ed.; Academic Press: San Diego, CA, USA, 2009; Volume 19, pp. 351–370. [Google Scholar]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Geddes, D.T.; Larsson, M.; Doherty, D.A.; Hartmann, P.E. Importance of vacuum for breastmilk expression. Breastfeed. Med. 2008, 3, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Ramsay, D.T.; Mitoulas, L.R.; Kent, J.C.; Larsson, M.; Hartmann, P.E. The use of ultrasound to characterize milk ejection in women using an electric breast pump. J. Hum. Lact. 2005, 21, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Luther, E.; Arballo, J.; Sala, N.; Cordero Funes, J. Suckling pressure in humans: Relationship to oxytocin-reproducing reflex milk ejection. J. Appl. Physiol. 1974, 36, 350–353. [Google Scholar] [CrossRef]
- Boss, M.; Gardner, H.; Hartmann, P. Normal Human Lactation: Closing the gap. F1000Research 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Gardner, H.; Lai, C.T.; Ward, L.C.; Geddes, D. Thermal physiology of the lactating nipple influences the removal of human milk. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.W.; Hartmann, P.E. Initiation of human lactation: Secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 2007, 12, 211–221. [Google Scholar] [CrossRef]
- Lepe, M.; Bacardi Gascon, M.; Castaneda-Gonzalez, L.M.; Perez Morales, M.E.; Jimenez Cruz, A. Effect of maternal obesity on lactation: Systematic review. Nutr. Hosp. 2011, 26, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D.J.; Perez-Escamilla, R. Identification of risk factors for delayed onset of lactation. J. Am. Diet Assoc. 1999, 99, 450–454; quiz 455–456. [Google Scholar] [CrossRef]
- Tie, W.J.; Gardner, H.; Lai, C.T.; Hepworth, A.R.; Al-Tamimi, Y.; Paech, M.J.; Hartmann, P.E.; Geddes, D.T. Changes in milk composition associated with pethidine-PCEA usage after Caesarean section. Matern. Child Nutr. 2017, 13, e12275. [Google Scholar] [CrossRef] [PubMed]
- Cregan, M.D.; De Mello, T.R.; Kershaw, D.; McDougall, K.; Hartmann, P.E. Initiation of lactation in women after preterm delivery. Acta Obstet. Gynecol. Scand. 2002, 81, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Neville, M.C.; Morton, J. Physiology and endocrine changes underlying human lactogenesis II. J. Nutr. 2001, 131, 3005S–3008S. [Google Scholar] [CrossRef] [PubMed]
- Hoban, R.; Patel, A.L.; Medina Poeliniz, C.; Lai, C.T.; Janes, J.; Geddes, D.; Meier, P.P. Human Milk Biomarkers of Secretory Activation in Breast Pump-Dependent Mothers of Premature Infants. Breastfeed. Med. 2018, 13, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Hoban, R.; Medina Poeliniz, C.; Somerset, E.; Tat Lai, C.; Janes, J.; Patel, A.L.; Geddes, D.; Meier, P.P. Mother’s Own Milk Biomarkers Predict Coming to Volume in Pump-Dependent Mothers of Preterm Infants. J. Pediatr. 2021, 228, 44–52.e43. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.T.; Gardner, H.; Geddes, D. Comparison of Inductively Coupled Plasma Optical Emission Spectrometry with an Ion Selective Electrode to Determine Sodium and Potassium Levels in Human Milk. Nutrients 2018, 10, 1218. [Google Scholar] [CrossRef] [Green Version]
- Fok, D.; Aris, I.M.; Ho, J.H.; Chan, Y.H.; Rauff, M.; Lui, J.K.; Cregan, M.D.; Hartmann, P.; Chong, Y.S.; Mattar, C.N. Early initiation and regular breast milk expression reduces risk of lactogenesis II delay in at-risk Singaporean mothers in a randomised trial. Singapore Med. J. 2019, 60, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Meier, P.P.; Engstrom, J.L.; Janes, J.E.; Jegier, B.J.; Loera, F. Breast pump suction patterns that mimic the human infant during breastfeeding: Greater milk output in less time spent pumping for breast pump-dependent mothers with premature infants. J. Perinatol. 2012, 32, 103–110. [Google Scholar] [CrossRef]
- Post, E.D.; Stam, G.; Tromp, E. Milk production after preterm, late preterm and term delivery; effects of different breast pump suction patterns. J. Perinatol. 2016, 36, 47–51. [Google Scholar] [CrossRef]
- Perrella, S.L.; Nancarrow, K.; Rea, A.; Murray, K.; Geddes, D.T.; Simmer, K.N. Estimates of Preterm Infants’ Breastfeeding Transfer Volumes Are Not Reliably Accurate. Adv. Neonatal Care 2020, 20, E93–E99. [Google Scholar] [CrossRef]
- Perrella, S.L.; Williams, J.; Nathan, E.A.; Fenwick, J.; Hartmann, P.E.; Geddes, D.T. Influences on breastfeeding outcomes for healthy term and preterm/sick infants. Breastfeed. Med. 2012, 7, 255–261. [Google Scholar] [CrossRef]
- Coward, W.A.; Sawyer, M.B.; Whitehead, R.G.; Prentice, A.M.; Evans, J. New method for measuring milk intakes in breast-fed babies. Lancet 1979, 2, 13–14. [Google Scholar] [CrossRef]
- Kent, J.C.; Ashton, E.; Hardwick, C.M.; Rea, A.; Murray, K.; Geddes, D.T. Causes of perception of insufficient milk supply in Western Australian mothers. Matern. Child Nutr. 2021, 17, e13080. [Google Scholar] [CrossRef]
- Coward, W.A.; Cole, T.J.; Sawyer, M.B.; Prentice, A.M. Breast-milk intake measurement in mixed-fed infants by administration of deuterium oxide to their mothers. Hum. Nutr. Clin. Nutr. 1982, 36, 141–148. [Google Scholar] [PubMed]
- Arthur, P.G.; Hartmann, P.E.; Smith, M. Measurement of the milk intake of breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.T.; Hale, T.W.; Simmer, K.; Hartmann, P.E. Measuring milk synthesis in breastfeeding mothers. Breastfeed. Med. 2010, 5, 103–107. [Google Scholar] [CrossRef]
- Roznowski, D.M.; Wagner, E.A.; Riddle, S.W.; Nommsen-Rivers, L.A. Validity of a 3-Hour Breast Milk Expression Protocol in Estimating Current Maternal Milk Production Capacity and Infant Breast Milk Intake in Exclusively Breastfeeding Dyads. Breastfeed. Med. 2020, 15, 630–638. [Google Scholar] [CrossRef]
- Kent, J.C.; Gardner, H.; Lai, C.T.; Hartmann, P.E.; Murray, K.; Rea, A.; Geddes, D.T. Hourly Breast Expression to Estimate the Rate of Synthesis of Milk and Fat. Nutrients 2018, 10, 1144. [Google Scholar] [CrossRef] [Green Version]
- Kent, J.C.; Prime, D.K.; Garbin, C.P. Principles for maintaining or increasing breast milk production. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Yamanouchi, I. Breast-feeding frequency during the first 24 h after birth in full-term neonates. Pediatrics 1990, 86, 171–175. [Google Scholar] [PubMed]
- Kent, J.C.; Gardner, H.; Geddes, D.T. Breastmilk Production in the First 4 Weeks after Birth of Term Infants. Nutrients 2016, 8, 756. [Google Scholar] [CrossRef] [Green Version]
- Hill, P.D.; Aldag, J.C.; Chatterton, R.T.; Zinaman, M. Comparison of milk output between mothers of preterm and term infants: The first 6 weeks after birth. J. Hum. Lact. 2005, 21, 22–30. [Google Scholar] [CrossRef]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, J.C.; Hepworth, A.R.; Sherriff, J.L.; Cox, D.B.; Mitoulas, L.R.; Hartmann, P.E. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed. Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrom, J.L.; Meier, P.P.; Jegier, B.; Motykowski, J.E.; Zuleger, J.L. Comparison of milk output from the right and left breasts during simultaneous pumping in mothers of very low birthweight infants. Breastfeed. Med. 2007, 2, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef]
- Cox, D.B.; Owens, R.A.; Hartmann, P.E. Blood and milk prolactin and the rate of milk synthesis in women. Exp. Physiol. 1996, 81, 1007–1020. [Google Scholar] [CrossRef]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 h: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef]
- Kent, J.C.; Hepworth, A.R.; Langton, D.B.; Hartmann, P.E. Impact of Measuring Milk Production by Test Weighing on Breastfeeding Confidence in Mothers of Term Infants. Breastfeed. Med. 2015, 10, 318–325. [Google Scholar] [CrossRef]
- Coentro, V.S.; Geddes, D.T.; Perrella, S.L. Altered sucking dynamics in a breastfed infant with Down syndrome: A case report. Int. Breastfeed. J. 2020, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G.; Finley, D.A.; Strode, M.A.; Lonnerda, L.B. Relationship of maternal age to breast milk volume and composition. In Human Lactation 2: Maternal and Environmental Factors; Hamosh, M., Goldman, A.S., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 263–273. [Google Scholar]
- Dos Santos, C.O.; Dolzhenko, E.; Hodges, E.; Smith, A.D.; Hannon, G.J. An epigenetic memory of pregnancy in the mouse mammary gland. Cell Rep. 2015, 11, 1102–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winocour, S.; Lemaine, V. Hypoplastic breast anomalies in the female adolescent breast. Semin. Plast. Surg. 2013, 27, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Kam, R.L.; Amir, L.H.; Cullinane, M. Is There an Association Between Breast Hypoplasia and Breastfeeding Outcomes? A Systematic Review. Breastfeed. Med. 2021, 16, 594–602. [Google Scholar] [CrossRef]
- Rivera, O.C.; Geddes, D.T.; Barber-Zucker, S.; Zarivach, R.; Gagnon, A.; Soybel, D.I.; Kelleher, S.L. A common genetic variant in zinc transporter ZnT2 (Thr288Ser) is present in women with low milk volume and alters lysosome function and cell energetics. Am. J. Physiol. Cell Physiol. 2020, 318, C1166–C1177. [Google Scholar] [CrossRef] [PubMed]
- Garbin, C.P.; Deacon, J.P.; Rowan, M.K.; Hartmann, P.E.; Geddes, D.T. Association of nipple piercing with abnormal milk production and breastfeeding. JAMA 2009, 301, 2550–2551. [Google Scholar] [CrossRef]
- Geddes, D.T. Inside the lactating breast: The latest anatomy research. J. Midwifery Womens Health 2007, 52, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Dai, S.; Wang, C.; Zeng, S.; Chen, J.; Cen, Y. Do Breast Implants Influence Breastfeeding? A Meta-Analysis of Comparative Studies. J. Hum. Lact. 2018, 34, 424–432. [Google Scholar] [CrossRef]
- Kraut, R.Y.; Brown, E.; Korownyk, C.; Katz, L.S.; Vandermeer, B.; Babenko, O.; Gross, M.S.; Campbell, S.; Allan, G.M. The impact of breast reduction surgery on breastfeeding: Systematic review of observational studies. PLoS ONE 2017, 12, e0186591. [Google Scholar] [CrossRef] [Green Version]
- Cregan, M.D.; Hartmann, P.E. Computerized breast measurement from conception to weaning: Clinical implications. J. Hum. Lact. 1999, 15, 89–96. [Google Scholar] [CrossRef]
- Pang, W.W.; Geddes, D.T.; Lai, C.T.; Chan, S.Y.; Chan, Y.H.; Cheong, C.Y.; Fok, D.; Chua, M.C.; Lim, S.B.; Huang, J.; et al. The association of maternal gestational hyperglycemia with breastfeeding duration and markers of milk production. Am. J. Clin. Nutr. 2021. [Google Scholar] [CrossRef]
- Verd, S.; Barriuso, L.; Gich, I.; Gutierrez, A.; Nadal-Amat, J.; Carreras, E. Risk of early breastfeeding cessation among symmetrical, small for gestational age infants. Ann. Hum. Biol. 2013, 40, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Prior, E.; Santhakumaran, S.; Gale, C.; Philipps, L.H.; Modi, N.; Hyde, M.J. Breastfeeding after cesarean delivery: A systematic review and meta-analysis of world literature. Am. J. Clin. Nutr. 2012, 95, 1113–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drayton, B.A.; Patterson, J.A.; Nippita, T.A.; Ford, J.B. Red blood cell transfusion after postpartum haemorrhage and breastmilk feeding at discharge: A population-based study. Aust. N. Z. J. Obstet. Gynaecol. 2016, 56, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Chessman, J.; Patterson, J.; Nippita, T.; Drayton, B.; Ford, J. Haemoglobin concentration following postpartum haemorrhage and the association between blood transfusion and breastfeeding: A retrospective cohort study. BMC Res. Notes 2018, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Fetherston, C.M.; Lai, C.T.; Hartmann, P.E. Relationships between symptoms and changes in breast physiology during lactation mastitis. Breastfeed. Med. 2006, 1, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Fetherston, C.M.; Lai, C.T.; Mitoulas, L.R.; Hartmann, P.E. Excretion of lactose in urine as a measure of increased permeability of the lactating breast during inflammation. Acta Obstet. Gynecol. Scand. 2006, 85, 20–25. [Google Scholar] [CrossRef]
- Fetherston, C.M.; Wells, J.I.; Hartmann, P.E. Severity of mastitis symptoms as a predictor of C-reactive protein in milk and blood during lactation. Breastfeed. Med. 2006, 1, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Twigger, A.J.; Kuffer, G.K.; Geddes, D.T.; Filgueria, L. Expression of Granulisyn, Perforin and Granzymes in Human Milk over Lactation and in the Case of Maternal Infection. Nutrients 2018, 10, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, E.; Woodd, S.L.; Benova, L. Incidence of and Risk Factors for Lactational Mastitis: A Systematic Review. J. Hum. Lact. 2020, 36, 673–686. [Google Scholar] [CrossRef]
- Fetherston, C.M.; Lai, C.T.; Hartmann, P.E. Recurrent blocked duct(s) in a mother with immunoglobulin A deficiency. Breastfeed. Med. 2008, 3, 261–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geddes, D.T. Ultrasound imaging of the lactating breast: Methodology and application. Int. Breastfeed. J. 2009, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljazaf, K.; Hale, T.W.; Ilett, K.F.; Hartmann, P.E.; Mitoulas, L.R.; Kristensen, J.H.; Hackett, L.P. Pseudoephedrine: Effects on milk production in women and estimation of infant exposure via breastmilk. Br. J. Clin. Pharmacol. 2003, 56, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, E.W.; Davey, K.; Page-Sharp, M.; Hartmann, P.E.; Simmer, K.; Ilett, K.F. Dose-effect study of domperidone as a galactagogue in preterm mothers with insufficient milk supply, and its transfer into milk. Br. J. Clin. Pharmacol. 2008, 66, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Grzeskowiak, L.E.; Wlodek, M.E.; Geddes, D.T. What Evidence Do We Have for Pharmaceutical Galactagogues in the Treatment of Lactation Insufficiency?—A Narrative Review. Nutrients 2019, 11, 974. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, M.; Robertson, S.; Friedman, A.; Klaus, M. Effect of frequent breast-feeding on early milk production and infant weight gain. Pediatrics 1983, 72, 307–311. [Google Scholar]
- Mitoulas, L.R.; Lai, C.T.; Gurrin, L.C.; Larsson, M.; Hartmann, P.E. Efficacy of breast milk expression using an electric breast pump. J. Hum. Lact. 2002, 18, 344–352. [Google Scholar] [CrossRef]
- Kent, J.C.; Geddes, D.T.; Hepworth, A.R.; Hartmann, P.E. Effect of warm breastshields on breast milk pumping. J. Hum. Lact. 2011, 27, 331–338. [Google Scholar] [CrossRef]
- Prime, D.K.; Garbin, C.P.; Hartmann, P.E.; Kent, J.C. Simultaneous breast expression in breastfeeding women is more efficacious than sequential breast expression. Breastfeed. Med. 2012, 7, 442–447. [Google Scholar] [CrossRef] [Green Version]
- Prime, D.K.; Kent, J.C.; Hepworth, A.R.; Trengove, N.J.; Hartmann, P.E. Dynamics of milk removal during simultaneous breast expression in women. Breastfeed. Med. 2012, 7, 100–106. [Google Scholar] [CrossRef]
- Patel, A.L.; Johnson, T.J.; Robin, B.; Bigger, H.R.; Buchanan, A.; Christian, E.; Nandhan, V.; Shroff, A.; Schoeny, M.; Engstrom, J.L.; et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F256–F261. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Yamanouchi, I. The relationship between rooming-in/not rooming-in and breast-feeding variables. Acta Paediatr. Scand. 1990, 79, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Spatz, D.L.; Davanzo, R.; Muller, J.A.; Powell, R.; Rigourd, V.; Yates, A.; Geddes, D.T.; van Goudoever, J.B.; Bode, L. Promoting and Protecting Human Milk and Breastfeeding in a COVID-19 World. Front. Pediatr. 2020, 8, 633700. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.T.; Rea, A.; Mitoulas, L.R.; Kent, J.C.; Simmer, K.; Hartmann, P.E.; Geddes, D. Short-term rate of milk synthesis and expression interval of preterm mothers. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 105, 266–269. [Google Scholar] [CrossRef] [Green Version]
- Geddes, D.T.; Kent, J.C.; McClellan, H.L.; Garbin, C.P.; Chadwick, L.M.; Hartmann, P.E. Sucking characteristics of successfully breastfeeding infants with ankyloglossia: A case series. Acta Paediatr. 2010, 99, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.T.; Langton, D.B.; Gollow, I.; Jacobs, L.A.; Hartmann, P.E.; Simmer, K. Frenulotomy for breastfeeding infants with ankyloglossia: Effect on milk removal and sucking mechanism as imaged by ultrasound. Pediatrics 2008, 122, e188–e194. [Google Scholar] [CrossRef] [PubMed]
- Garbin, C.P.; Sakalidis, V.S.; Chadwick, L.M.; Whan, E.; Hartmann, P.E.; Geddes, D.T. Evidence of improved milk intake after frenotomy: A case report. Pediatrics 2013, 132, e1413–e1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, V.; Douglas, P.S.; Hill, P.S.; Walsh, L.J.; Tennant, M. Frenotomy for tongue-tie in Australian children, 2006–2016: An increasing problem. Med. J. Aust. 2018, 208, 88–89. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.S.; Kinniburgh, B.; Metcalfe, A.; Razaz, N.; Sabr, Y.; Lisonkova, S. Temporal trends in ankyloglossia and frenotomy in British Columbia, Canada, 2004–2013: A population-based study. CMAJ Open 2016, 4, E33–E40. [Google Scholar] [CrossRef] [Green Version]
- Hale, M.; Mills, N.; Edmonds, L.; Dawes, P.; Dickson, N.; Barker, D.; Wheeler, B.J. Complications following frenotomy for ankyloglossia: A 24-month prospective New Zealand Paediatric Surveillance Unit study. J. Paediatr. Child Health 2020, 56, 557–562. [Google Scholar] [CrossRef]
- Geddes, D.T.; Cannon, A.; Perrella, S.; Whan, E.; Rae, A.; Murray, K. Effectiveness of Milk Transfer Does Not Improve in Breastfed Tongue-Tied Infants Following Frenotomy. Breastfeed. Med. 2018, 13, 517. [Google Scholar]
- Mills, N.; Geddes, D.T.; Amirapu, S.; Mirjalili, S.A. Understanding the Lingual Frenulum: Histological Structure, Tissue Composition, and Implications for Tongue Tie Surgery. Int. J. Otolaryngol. 2020, 2020, 1820978. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.; Keough, N.; Geddes, D.T.; Pransky, S.M.; Mirjalili, S.A. Defining the anatomy of the neonatal lingual frenulum. Clin. Anat. 2019, 32, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.; Pransky, S.M.; Geddes, D.T.; Mirjalili, S.A. What is a tongue tie? Defining the anatomy of the in-situ lingual frenulum. Clin. Anat. 2019, 32, 749–761. [Google Scholar] [CrossRef] [Green Version]
- LeFort, Y.; Evans, A.; Livingstone, V.; Douglas, P.; Dahlquist, N.; Donnelly, B.; Leeper, K.; Harley, E.; Lappin, S. Academy of Breastfeeding Medicine Position Statement on Ankyloglossia in Breastfeeding Dyads. Breastfeed. Med. 2021, 16, 278–281. [Google Scholar] [CrossRef]
- Douglas, P.; Geddes, D. Practice-based interpretation of ultrasound studies leads the way to more effective clinical support and less pharmaceutical and surgical intervention for breastfeeding infants. Midwifery 2018, 58, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, A.M.; Sakalidis, V.S.; Lai, C.T.; Perrella, S.L.; Geddes, D.T. Vacuum characteristics of the sucking cycle and relationships with milk removal from the breast in term infants. Early Hum. Dev. 2016, 96, 1–6. [Google Scholar] [CrossRef]
- Geddes, D.T.; Chooi, K.; Nancarrow, K.; Hepworth, A.R.; Gardner, H.; Simmer, K. Characterisation of sucking dynamics of breastfeeding preterm infants: A cross sectional study. BMC Pregnancy Childbirth 2017, 17, 386. [Google Scholar] [CrossRef] [Green Version]
- Boyce, J.O.; Reilly, S.; Skeat, J.; Cahir, P.; Academy of Breastfeeding Medicine. ABM Clinical Protocol #17: Guidelines for Breastfeeding Infants with Cleft Lip, Cleft Palate, or Cleft Lip and Palate-Revised 2019. Breastfeed. Med. 2019, 14, 437–444. [Google Scholar] [CrossRef]
- Paul, V.K.; Singh, M.; Deorari, A.K.; Pacheco, J.; Taneja, U. Manual and pump methods of expression of breast milk. Indian J. Pediatr. 1996, 63, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Prime, D.K.; Geddes, D.; Spatz, S.L.; Trengove, N.J.; Hartmann, P.E. The effect of breastshield size and anatomy on milk removal in women. J. Hum. Lact. 2010, 26, 433. [Google Scholar]
- McClellan, H.L.; Kent, J.C.; Hepworth, A.R.; Hartmann, P.E.; Geddes, D.T. Persistent Nipple Pain in Breastfeeding Mothers Associated with Abnormal Infant Tongue Movement. Int. J. Environ. Res. Public Health 2015, 12, 10833–10845. [Google Scholar] [CrossRef]
- Ueda, T.; Yokoyama, Y.; Irahara, M.; Aono, T. Influence of psychological stress on suckling-induced pulsatile oxytocin release. Obstet. Gynecol. 1994, 84, 259–262. [Google Scholar]
- Mizuno, K.; Ueda, A. Changes in sucking performance from nonnutritive sucking to nutritive sucking during breast- and bottle-feeding. Pediatr. Res. 2006, 59, 728–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.E.; Short, R.V. Changes in breast sensitivity at puberty, during the menstrual cycle, and at parturition. Br. Med. J. 1977, 1, 1188–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitoulas, L.R.; Ramsay, D.T.; Kent, J.C.; Larsson, M.; Hartmann, P.E. Identification of factors affecting breast pump efficacy. Adv. Exp. Med. Biol. 2004, 554, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Lundeberg, T.; Uvnas-Moberg, K. Studies on cutaneous blood flow in the mammary gland of lactating rats. Acta Physiol. Scand. 1996, 158, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolridge, M.W. The ‘anatomy’ of infant sucking. Midwifery 1986, 2, 164–171. [Google Scholar] [CrossRef]
- Weber, F.; Woolridge, M.W.; Baum, J.D. An ultrasonographic study of the organisation of sucking and swallowing by newborn infants. Dev. Med. Child Neurol. 1986, 28, 19–24. [Google Scholar] [CrossRef]
- Uvnas-Moberg, K.; Eriksson, M. Breastfeeding: Physiological, endocrine and behavioural adaptations caused by oxytocin and local neurogenic activity in the nipple and mammary gland. Acta Paediatr. 1996, 85, 525–530. [Google Scholar] [CrossRef]
- Wolf, L.S.; Glass, R.P. Functional Anatomy and Physiology of the Suck/Swallow/Breathe Triad. In Feeding and Swallowing Disorders in Infancy; Disabilities, H.I.O., Ed.; Hammill Institute on Disabilities: Austin, TX, USA, 1992; p. 475. [Google Scholar]
- Page, D.C. “Real” early orthodontic treatment. From birth to age 8. Funct. Orthod. 2003, 20, 48–54. [Google Scholar]
- Page, D.C. Breastfeeding is early functional jaw orthopedics (an introduction). Funct. Orthod. 2001, 18, 24–27. [Google Scholar]
- Narbutyte, I.; Narbutyte, A.; Linkeviciene, L. Relationship between breastfeeding, bottle-feeding and development of malocclusion. Stomatologija 2013, 15, 67–72. [Google Scholar]
- Tamura, Y.; Horikawa, Y.; Yoshida, S. Co-ordination of tongue movements and peri-oral muscle activities during nutritive sucking. Dev. Med. Child Neurol. 1996, 38, 503–510. [Google Scholar] [CrossRef]
- Geddes, D.T.; Kent, J.C.; Mitoulas, L.R.; Hartmann, P.E. Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum. Dev. 2008, 84, 471–477. [Google Scholar] [CrossRef]
- McClellan, H.L.; Sakalidis, V.S.; Hepworth, A.R.; Hartmann, P.E.; Geddes, D.T. Validation of nipple diameter and tongue movement measurements with B-mode ultrasound during breastfeeding. Ultrasound Med. Biol. 2010, 36, 1797–1807. [Google Scholar] [CrossRef]
- Elad, D.; Kozlovsky, P.; Blum, O.; Laine, A.F.; Po, M.J.; Botzer, E.; Dollberg, S.; Zelicovich, M.; Ben Sira, L. Biomechanics of milk extraction during breast-feeding. Proc. Natl. Acad. Sci. USA 2014, 111, 5230–5235. [Google Scholar] [CrossRef] [Green Version]
- Geddes, D.T.; Sakalidis, V.S.; Hepworth, A.R.; McClellan, H.L.; Kent, J.C.; Lai, C.T.; Hartmann, P.E. Tongue movement and intra-oral vacuum of term infants during breastfeeding and feeding from an experimental teat that released milk under vacuum only. Early Hum. Dev. 2012, 88, 443–449. [Google Scholar] [CrossRef]
- Wolff, P.H. The serial organization of sucking in the young infant. Pediatrics 1968, 42, 943–956. [Google Scholar]
- Sakalidis, V.S.; Kent, J.C.; Garbin, C.P.; Hepworth, A.R.; Hartmann, P.E.; Geddes, D.T. Longitudinal changes in suck-swallow-breathe, oxygen saturation, and heart rate patterns in term breastfeeding infants. J. Hum. Lact. 2013, 29, 236–245. [Google Scholar] [CrossRef]
- Bowen-Jones, A.; Thompson, C.; Drewett, R.F. Milk flow and sucking rates during breast-feeding. Dev. Med. Child Neurol. 1982, 24, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Rogers, B.; Arvedson, J. Assessment of infant oral sensorimotor and swallowing function. Ment. Retard. Disabil. Res. Rev. 2005, 11, 74–82. [Google Scholar] [CrossRef]
- Bu’Lock, F.; Woolridge, M.W.; Baum, J.D. Development of co-ordination of sucking, swallowing and breathing: Ultrasound study of term and preterm infants. Dev. Med. Child Neurol. 1990, 32, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, V.S.; McClellan, H.L.; Hepworth, A.R.; Kent, J.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Oxygen Saturation and Suck-Swallow-Breathe Coordination of Term Infants during Breastfeeding and Feeding from a Teat Releasing Milk Only with Vacuum. Int. J. Pediatr. 2012, 2012, 130769. [Google Scholar] [CrossRef] [Green Version]
- Sakalidis, V.S.; Geddes, D.T. Suck-Swallow-Breathe Dynamics in Breastfed Infants. J. Hum. Lact. 2016, 32, 201–211; quiz 393–205. [Google Scholar] [CrossRef] [PubMed]
- Watson-Genna, C. Supporting Sucking Skills in Breastfeeding Infants; Jones & Bartlett Learning: Burlington, MA, USA, 2017. [Google Scholar]
- Chapman, D.J.; Doughty, K.; Mullin, E.M.; Perez-Escamilla, R. Reliability of Lactation Assessment Tools Applied to Overweight and Obese Women. J. Hum. Lact. 2016, 32, 269–276. [Google Scholar] [CrossRef]
- Cote-Arsenault, D.; McCoy, T.P. Reliability and validity of swallows as a measure of breast milk intake in the first days of life. J. Hum. Lact. 2012, 28, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Gunther, M. Sore nipples; causes and prevention. Lancet 1945, 2, 590–593. [Google Scholar] [CrossRef]
- McClellan, H.L.; Hepworth, A.R.; Garbin, C.P.; Rowan, M.K.; Deacon, J.; Hartmann, P.E.; Geddes, D.T. Nipple pain during breastfeeding with or without visible trauma. J. Hum. Lact. 2012, 28, 511–521. [Google Scholar] [CrossRef]
- McClellan, H.; Geddes, D.; Kent, J.; Garbin, C.; Mitoulas, L.; Hartmann, P. Infants of mothers with persistent nipple pain exert strong sucking vacuums. Acta Paediatr. 2008, 97, 1205–1209. [Google Scholar] [CrossRef]
- McClellan, H.L.; Hepworth, A.R.; Kent, J.C.; Garbin, C.P.; Williams, T.M.; Hartmann, P.E.; Geddes, D.T. Breastfeeding frequency, milk volume, and duration in mother-infant dyads with persistent nipple pain. Breastfeed. Med. 2012, 7, 275–281. [Google Scholar] [CrossRef]
- Odom, E.C.; Li, R.; Scanlon, K.S.; Perrine, C.G.; Grummer-Strawn, L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics 2013, 131, e726–e732. [Google Scholar] [CrossRef] [Green Version]
- Kronborg, H.; Foverskov, E.; Nilsson, I.; Maastrup, R. Why do mothers use nipple shields and how does this influence duration of exclusive breastfeeding? Matern. Child Nutr. 2016, 13, e12251. [Google Scholar] [CrossRef] [PubMed]
- Eglash, A.; Ziemer, A.L.; Chevalier, A. Health professionals’ attitudes and use of nipple shields for breastfeeding women. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2010, 5, 147–151. [Google Scholar] [CrossRef]
- Woolridge, M.W.; Baum, J.D.; Drewett, R.F. Effect of a traditional and of a new nipple shield on sucking patterns and milk flow. Early Hum. Dev. 1980, 4, 357–364. [Google Scholar] [CrossRef]
- Auerbach, K.G. The effect of nipple shields on maternal milk volume. J. Obstet. Gynecol. Neonatal Nurs. 1990, 19, 419–427. [Google Scholar] [CrossRef]
- Chertok, I.R.; Schneider, J.; Blackburn, S. A pilot study of maternal and term infant outcomes associated with ultrathin nipple shield use. J. Obstet. Gynecol. Neonatal Nurs. JOGNN/NAACOG 2006, 35, 265–272. [Google Scholar] [CrossRef]
- Amir, L.H.; Jones, L.E.; Buck, M.L. Nipple pain associated with breastfeeding: Incorporating current neurophysiology into clinical reasoning. Aust. Fam. Physician 2015, 44, 127–132. [Google Scholar]
- Haeberle, H.; Lumpkin, E.A. Merkel Cells in Somatosensation. Chemosens. Percept. 2008, 1, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Lipchock, S.V.; Reed, D.R.; Mennella, J.A. The gustatory and olfactory systems during infancy: Implications for development of feeding behaviors in the high-risk neonate. Clin. Perinatol. 2011, 38, 627–641. [Google Scholar] [CrossRef] [Green Version]
- Mobbs, E.J.; Mobbs, G.A.; Mobbs, A.E. Imprinting, latchment and displacement: A mini review of early instinctual behaviour in newborn infants influencing breastfeeding success. Acta Paediatr. 2016, 105, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Melzack, R. The McGill Pain Questionnaire: Major properties and scoring methods. Pain 1975, 1, 277–299. [Google Scholar] [CrossRef]
- Chertok, I.R. Reexamination of ultra-thin nipple shield use, infant growth and maternal satisfaction. J. Clin. Nurs. 2009, 18, 2949–2955. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Marchesan, I.Q.; Gusmão, R.J.; Rodrigues, A.C.; Berretin-Felix, G. Histological characteristics of altered human lingual frenulum. Int. J. Pediatr. Child Health 2014, 2, 5–9. [Google Scholar]
- Daly, S.E.; Kent, J.C.; Huynh, D.Q.; Owens, R.A.; Alexander, B.F.; Ng, K.C.; Hartmann, P.E. The determination of short-term breast volume changes and the rate of synthesis of human milk using computerized breast measurement. Exp. Physiol. 1992, 77, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, P.P.; Brown, L.P.; Hurst, N.M.; Spatz, D.L.; Engstrom, J.L.; Borucki, L.C.; Krouse, A.M. Nipple shields for preterm infants: Effect on milk transfer and duration of breastfeeding. J. Hum. Lact. 2000, 16, 106–114; quiz 129–131. [Google Scholar] [CrossRef] [PubMed]
- Geddes, D.; Kok, C.; Nancarrow, K.; Hepworth, A.; Simmer, K. Preterm Infant Feeding: A Mechanistic Comparison between a Vacuum Triggered Novel Teat and Breastfeeding. Nutrients 2018, 10, 376. [Google Scholar] [CrossRef] [Green Version]
- Simmer, K.; Kok, C.; Nancarrow, K.; Hepworth, A.R.; Geddes, D.T. Novel feeding system to promote establishment of breastfeeds after preterm birth: A randomized controlled trial. J. Perinatol. 2016, 36, 210–215. [Google Scholar] [CrossRef] [Green Version]
- Perrella, S.L.; Nancarrow, K.; Trevenen, M.; Murray, K.; Geddes, D.T.; Simmer, K.N. Effect of vacuum-release teat versus standard teat use on feeding milestones and breastfeeding outcomes in very preterm infants: A randomized controlled trial. PLoS ONE 2019, 14, e0214091. [Google Scholar] [CrossRef]
- Dharel, D.; Singhal, N.; Wood, C.; Cieslak, Z.; Bacchini, F.; Shah, P.S.; Ye, X.Y.; Alshaikh, B.; Canadian Neonatal Network; Canadian Preterm Birth Network Investigators. Rates and Determinants of Mother’s Own Milk Feeding in Infants Born Very Preterm. J. Pediatr. 2021, 236, 21–27.e4. [Google Scholar] [CrossRef]
- Perrella, S.L.; Nancarrow, K.; Rea, A.; Murray, K.; Simmer, K.; Geddes, D.T. Longitudinal follow-up of preterm breastfeeding to 12 weeks corrected gestational age. Adv. Neonatal Care 2021, in press. [Google Scholar]
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human milk composition promotes optimal infant growth, development and health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Netting, M.J.; Middleton, P.F.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, A.B.S. The impact of maternal obesity on human milk macronutrient composition: A systematic review and meta-analysis. Nutrients 2020, 12, 934. [Google Scholar] [CrossRef] [Green Version]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between Maternal Body Composition and Appetite Hormones and Macronutrients in Human Milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Kugananthan, S.; Lai, C.T.; Gridneva, Z.; Mark, P.J.; Geddes, D.T.; Kakulas, F. Leptin Levels Are Higher in Whole Compared to Skim Human Milk, Supporting a Cellular Contribution. Nutrients 2016, 8, 711. [Google Scholar] [CrossRef]
- Houseknecht, K.L.; McGuire, M.K.; Portocarrero, C.P.; McGuire, M.A.; Beerman, K. Leptin is present in human milk and is related to maternal plasma leptin concentration and adiposity. Biochem. Biophys. Res. Commun. 1997, 240, 742–747. [Google Scholar] [CrossRef]
- Miralles, O.; Sanchez, J.; Palou, A.; Pico, C. A physiological role of breast milk leptin in body weight control in developing infants. Obesity 2006, 14, 1371–1377. [Google Scholar] [CrossRef]
- De Luca, A.; Frasquet-Darrieux, M.; Gaud, M.A.; Christin, P.; Boquien, C.Y.; Millet, C.; Herviou, M.; Darmaun, D.; Robins, R.J.; Ingrand, P.; et al. Higher Leptin but Not Human Milk Macronutrient Concentration Distinguishes Normal-Weight from Obese Mothers at 1-Month Postpartum. PLoS ONE 2016, 11, e0168568. [Google Scholar] [CrossRef]
- Gridneva, Z.; Kugananthan, S.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human Milk Adiponectin and Leptin and Infant Body Composition over the First 12 Months of Lactation. Nutrients 2018, 10, 1125. [Google Scholar] [CrossRef] [Green Version]
- Pundir, S.; Gridneva, Z.; Pillai, A.; Thorstensen, E.B.; Wall, C.R.; Geddes, D.T.; Cameron-Smith, D. Human Milk Glucocorticoid Levels Are Associated with Infant Adiposity and Head Circumference over the First Year of Life. Front. Nutr. 2020, 7, 166. [Google Scholar] [CrossRef]
- Bjorntorp, P.; Rosmond, R. Obesity and cortisol. Nutrition 2000, 16, 924–936. [Google Scholar] [CrossRef]
- Gridneva, Z.; Tie, W.J.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human Milk Casein and Whey Protein and Infant Body Composition over the First 12 Months of Lactation. Nutrients 2018, 10, 1332. [Google Scholar] [CrossRef] [Green Version]
- Leghi, G.E.; Lai, C.T.; Narayanan, A.; Netting, M.J.; Dymock, M.; Rea, A.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. Daily variation of macronutrient concentrations in mature human milk over 3 weeks. Sci. Rep. 2021, 11, 10224. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Prime, D.K.; Hepworth, A.R.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Investigation of short-term variations in term breast milk composition during repeated breast expression sessions. J. Hum. Lact. 2013, 29, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Leghi, G.E.; Netting, M.J.; Lai, C.T.; Narayanan, A.; Dymock, M.; Rea, A.; Wlodek, M.E.; Geddes, D.T.; Muhlhausler, B.S. Reduction in Maternal Energy Intake during Lactation Decreased Maternal Body Weight and Concentrations of Leptin, Insulin and Adiponectin in Human Milk without Affecting Milk Production, Milk Macronutrient Composition or Infant Growth. Nutrients 2021, 13, 1892. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in Human Milk and Body Composition of Term Infants during the First 12 Months of Lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martinez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Hellstrom, P.M.; Gryback, P.; Jacobsson, H. The physiology of gastric emptying. Best Pract. Res. Clin. Anaesthesiol. 2006, 20, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.L.; Hepworth, A.R.; Simmer, K.N.; Geddes, D.T. Validation of ultrasound methods to monitor gastric volume changes in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2013, 57, 741–749. [Google Scholar] [CrossRef]
- Gridneva, Z.; Kugananthan, S.; Hepworth, A.R.; Tie, W.J.; Lai, C.T.; Ward, L.C.; Hartmann, P.E.; Geddes, D.T. Effect of Human Milk Appetite Hormones, Macronutrients, and Infant Characteristics on Gastric Emptying and Breastfeeding Patterns of Term Fully Breastfed Infants. Nutrients 2016, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Cannon, A.M.; Gridneva, Z.; Hepworth, A.R.; Lai, C.T.; Tie, W.J.; Khan, S.; Hartmann, P.E.; Geddes, D.T. The relationship of human milk leptin and macronutrients with gastric emptying in term breastfed infants. Pediatr. Res. 2017, 82, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Gridneva, Z.; Rea, A.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Relationships between Breastfeeding Patterns and Maternal and Infant Body Composition over the First 12 Months of Lactation. Nutrients 2018, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Wells, J.C.; Chomtho, S.; Fewtrell, M.S. Programming of body composition by early growth and nutrition. Proc. Nutr. Soc. 2007, 66, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Orsso, C.E.; Silva, M.I.B.; Gonzalez, M.C.; Rubin, D.A.; Heymsfield, S.B.; Prado, C.M.; Haqq, A.M. Assessment of body composition in pediatric overweight and obesity: A systematic review of the reliability and validity of common techniques. Obes. Rev. 2020, 21, e13041. [Google Scholar] [CrossRef]
- Perrella, S.L.; Hepworth, A.R.; Simmer, K.N.; Hartmann, P.E.; Geddes, D.T. Repeatability of gastric volume measurements and intragastric content using ultrasound in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.L.; Hepworth, A.R.; Gridneva, Z.; Simmer, K.N.; Hartmann, P.E.; Geddes, D.T. Gastric Emptying and Curding of Pasteurized Donor Human Milk and Mother’s Own Milk in Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 125–129. [Google Scholar] [CrossRef]
- Cavell, B. Gastric emptying in infants fed human milk or infant formula. Acta Paediatr. Scand. 1981, 70, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Ewer, A.K.; Durbin, G.M.; Morgan, M.E.; Booth, I.W. Gastric emptying in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1994, 71, F24–F27. [Google Scholar] [CrossRef] [Green Version]
- Moore, T.A.; Wilson, M.E. Feeding intolerance: A concept analysis. Adv. Neonatal Care 2011, 11, 149–154. [Google Scholar] [CrossRef]
- Boyd, C.A.; Quigley, M.A.; Brocklehurst, P. Donor breast milk versus infant formula for preterm infants: Systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F169–F175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrella, S.L.; Hepworth, A.R.; Simmer, K.N.; Geddes, D.T. Influences of breast milk composition on gastric emptying in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Perrella, S.L.; Hepworth, A.R.; Gridneva, Z.; Simmer, K.N.; Hartmann, P.E.; Geddes, D.T. Gastric emptying of different meal volumes of identical composition in preterm infants: A time series analysis. Pediatr. Res. 2018, 83, 778–783. [Google Scholar] [CrossRef]
- Henderson, T.R.; Fay, T.N.; Hamosh, M. Effect of pasteurization on long chain polyunsaturated fatty acid levels and enzyme activities of human milk. J. Pediatr. 1998, 132, 876–878. [Google Scholar] [CrossRef]
- Lindquist, S.; Hernell, O. Lipid digestion and absorption in early life: An update. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 314–320. [Google Scholar] [CrossRef]
- Feltrin, K.L.; Little, T.J.; Meyer, J.H.; Horowitz, M.; Smout, A.J.; Wishart, J.; Pilichiewicz, A.N.; Rades, T.; Chapman, I.M.; Feinle-Bisset, C. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R524–R533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, A.; Wong, W.W.; Shulman, R.J. Factors regulating gastric emptying in preterm infants. J. Pediatr. 2006, 149, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Cobb, B.A.; Carlo, W.A.; Ambalavanan, N. Gastric residuals and their relationship to necrotizing enterocolitis in very low birth weight infants. Pediatrics 2004, 113, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Vasbinder, A.J.; de Kruif, C.G. Casein–whey protein interactions in heated milk: The influence of pH. Int. Dairy J. 2003, 13, 669–677. [Google Scholar] [CrossRef]
- Little, T.J.; Russo, A.; Meyer, J.H.; Horowitz, M.; Smyth, D.R.; Bellon, M.; Wishart, J.M.; Jones, K.L.; Feinle-Bisset, C. Free fatty acids have more potent effects on gastric emptying, gut hormones, and appetite than triacylglycerides. Gastroenterology 2007, 133, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Lebenthal, E.; Krantz, B. Effect of caloric density on gastric emptying in premature infants. J. Pediatr. 1984, 104, 118–122. [Google Scholar] [CrossRef]
- Ellis, Z.M.; Tan, H.S.G.; Embleton, N.D.; Sangild, P.T.; van Elburg, R.M. Milk feed osmolality and adverse events in newborn infants and animals: A systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F333–F340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewer, A.K.; Yu, V.Y. Gastric emptying in pre-term infants: The effect of breast milk fortifier. Acta Paediatr. 1996, 85, 1112–1115. [Google Scholar] [CrossRef]
- Chen, S.S.; Tzeng, Y.L.; Gau, B.S.; Kuo, P.C.; Chen, J.Y. Effects of prone and supine positioning on gastric residuals in preterm infants: A time series with cross-over study. Int. J. Nurs. Stud. 2013, 50, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Mandel, D.; Mimouni, F.B.; Solovkin, L.; Dollberg, S. Gastric residual in growing preterm infants: Effect of body position. Am. J. Perinatol. 2004, 21, 163–166. [Google Scholar] [CrossRef]
- Malhotra, A.K.; Deorari, A.K.; Paul, V.K.; Bagga, A.; Singh, M. Gastric residuals in preterm babies. J. Trop. Pediatr. 1992, 38, 262–264. [Google Scholar] [CrossRef]
- Erickson, T.; Gill, G.; Chan, G.M. The effects of acidification on human milk’s cellular and nutritional content. J. Perinatol. 2013, 33, 371–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, L.C.; Poston, L.; Godfrey, K.M.; Koletzko, B. Assessing early growth and adiposity: Report from an EarlyNutrition Academy workshop. Ann. Nutr. Metab. 2013, 63, 120–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggero, P.; Gianni, M.L.; Amato, O.; Orsi, A.; Piemontese, P.; Morlacchi, L.; Mosca, F. Is term newborn body composition being achieved postnatally in preterm infants? Early Hum. Dev. 2009, 85, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Gray, H.L.; Ode, K.L.; Younge, N.; Georgieff, M.K.; Demerath, E.W. Body composition changes in preterm infants following hospital discharge: Comparison with term infants. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Wootton, S.A.; Leaf, A.A.; Jackson, A.A. Preterm birth and body composition at term equivalent age: A systematic review and meta-analysis. Pediatrics 2012, 130, e640–e649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, G.; Simmer, K.; Sherriff, J.; Nathan, E.; Geddes, D.; Hartmann, P. Feasibility study: Assessing the influence of macronutrient intakes on preterm body composition, using air displacement plethysmography. J. Paediatr. Child Health 2015, 51, 862–869. [Google Scholar] [CrossRef] [PubMed]
- McLeod, G.; Geddes, D.; Nathan, E.; Sherriff, J.; Simmer, K.; Hartmann, P. Feasibility of using ultrasound to measure preterm body composition and to assess macronutrient influences on tissue accretion rates. Early Hum. Dev. 2013, 89, 577–582. [Google Scholar] [CrossRef]
- Ay, L.; Van Houten, V.A.; Steegers, E.A.; Hofman, A.; Witteman, J.C.; Jaddoe, V.W.; Hokken-Koelega, A.C. Fetal and postnatal growth and body composition at 6 months of age. J. Clin. Endocrinol. Metab. 2009, 94, 2023–2030. [Google Scholar] [CrossRef]
- Gao, X.; McMahon, R.J.; Woo, J.G.; Davidson, B.S.; Morrow, A.L.; Zhang, Q. Temporal changes in milk proteomes reveal developing milk functions. J. Proteome Res. 2012, 11, 3897–3907. [Google Scholar] [CrossRef]
- Goldman, A.S. Modulation of the gastrointestinal tract of infants by human milk. Interfaces and interactions. An evolutionary perspective. J. Nutr. 2000, 130, 426S–431S. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Demmelmair, H.; Prell, C.; Timby, N.; Lonnerdal, B. Benefits of Lactoferrin, Osteopontin and Milk Fat Globule Membranes for Infants. Nutrients 2017, 9, 817. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.B.; Sugahara, H.; Odamaki, T.; Xiao, J.Z. Different physiological properties of human-residential and non-human-residential bifidobacteria in human health. Benef. Microbes 2018, 9, 111–122. [Google Scholar] [CrossRef]
- Gridneva, Z.; Lai, C.T.; Rea, A.; Tie, W.J.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk immunomodulatory proteins are related to development of infant body composition during the first year of lactation. Pediatr. Res. 2020, 89, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, P. Clinical Benefits of Lactoferrin for Infants and Children. J. Pediatr. 2016, 173, S43–S52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, M.P.; Sherman, J.; Arcinue, R.; Niklas, V. Randomized Control Trial of Human Recombinant Lactoferrin: A Substudy Reveals Effects on the Fecal Microbiome of Very Low Birth Weight Infants. J. Pediatr. 2016, 173, S37–S42. [Google Scholar] [CrossRef] [Green Version]
- Koleva, P.T.; Kim, J.S.; Scott, J.A.; Kozyrskyj, A.L. Microbial programming of health and disease starts during fetal life. Birth Defects Res. C Embryo Today 2015, 105, 265–277. [Google Scholar] [CrossRef]
- Lonnerdal, B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Am. J. Clin. Nutr. 2017, 106, 1681S–1687S. [Google Scholar] [CrossRef]
- Bol’shakova, A.M.; Shcherbakova, E.G.; Ivanova, S.D.; Medvedeva, M.M.; Zhuravleva, T.P. Lysozyme in the feeding of premature infants with mixed pathology. Antibiotiki 1984, 29, 784–790. [Google Scholar]
- Prescott, S.L. Early nutrition as a major determinant of ‘immune health’: Implications for allergy, obesity and other noncommunicable diseases. In Preventive Aspects of Early Nutrition; Fewtrell, M.S., Haschke, F., Prescott, S.L., Eds.; Vevey/S. Kager AG: Basel, Switzerland, 2016; Volume 85, pp. 1–17. [Google Scholar]
- Pribylova, J.; Krausova, K.; Kocourkova, I.; Rossmann, P.; Klimesova, K.; Kverka, M.; Tlaskalova-Hogenova, H. Colostrum of healthy mothers contains broad spectrum of secretory IgA autoantibodies. J. Clin. Immunol. 2012, 32, 1372–1380. [Google Scholar] [CrossRef]
- Hassiotou, F.; Geddes, D.T. Programming of appetite control during breastfeeding as a preventative strategy against the obesity epidemic. J. Hum. Lact. 2014, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chandrasekera, P.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Hollanders, J.J.; Heijboer, A.C.; van der Voorn, B.; Rotteveel, J.; Finken, M.J.J. Nutritional programming by glucocorticoids in breast milk: Targets, mechanisms and possible implications. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 397–408. [Google Scholar] [CrossRef]
- Westerbacka, J.; Yki-Jarvinen, H.; Vehkavaara, S.; Hakkinen, A.M.; Andrew, R.; Wake, D.J.; Seckl, J.R.; Walker, B.R. Body fat distribution and cortisol metabolism in healthy men: Enhanced 5beta-reductase and lower cortisol/cortisone metabolite ratios in men with fatty liver. J. Clin. Endocrinol. Metab. 2003, 88, 4924–4931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjorntorp, P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 1997, 13, 795–803. [Google Scholar] [CrossRef]
- Hahn-Holbrook, J.; Le, T.B.; Chung, A.; Davis, E.P.; Glynn, L.M. Cortisol in human milk predicts child BMI. Obesity 2016, 24, 2471–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rollins, J.D.; Collins, J.S.; Holden, K.R. United States head circumference growth reference charts: Birth to 21 years. J. Pediatr. 2010, 156, 907–913.e2. [Google Scholar] [CrossRef]
- Lebel, C.; Deoni, S. The development of brain white matter microstructure. Neuroimage 2018, 182, 207–218. [Google Scholar] [CrossRef]
- Deoni, S.C.L. Neuroimaging of the Developing Brain and Impact of Nutrition. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Obel, C.; Hedegaard, M.; Henriksen, T.B.; Secher, N.J.; Olsen, J. Stressful life events in pregnancy and head circumference at birth. Dev. Med. Child Neurol. 2003, 45, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef]
- Koleva, P.T.; Bridgman, S.L.; Kozyrskyj, A.L. The infant gut microbiome: Evidence for obesity risk and dietary intervention. Nutrients 2015, 7, 2237–2260. [Google Scholar] [CrossRef] [Green Version]
- Sims, C.R.; Lipsmeyer, M.E.; Turner, D.E.; Andres, A. Human milk composition differs by maternal BMI in the first 9 months postpartum. Am. J. Clin. Nutr. 2020, 112, 548–557. [Google Scholar] [CrossRef]
- Kon, I.Y.; Shilina, N.M.; Gmoshinskaya, M.V.; Ivanushkina, T.A. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann. Nutr. Metab. 2014, 65, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M.; Smith, E.B.; Ellis, K.J. Infant feeding mode affects early growth and body composition. Pediatrics 2000, 106, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Daly, S.E.; Di Rosso, A.; Owens, R.A.; Hartmann, P.E. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp. Physiol. 1993, 78, 741–755. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Murray, K.; Muhlhausler, B.S.; Wlodek, M.E.; Geddes, D.T. Human Milk Sampling Protocols Affect Estimation of Infant Lipid Intake. J. Nutr. 2020, 150, 2924–2930. [Google Scholar] [CrossRef]
- Du, J.; Gay, M.C.L.; Lai, C.T.; Trengove, R.D.; Hartmann, P.E.; Geddes, D.T. Comparison of gravimetric, creamatocrit and esterified fatty acid methods for determination of total fat content in human milk. Food Chem. 2017, 217, 505–510. [Google Scholar] [CrossRef]
- Tie, W.J.; Kent, J.C.; Tat Lai, C.; Rea, A.; Hepworth, A.R.; Murray, K.; Geddes, D.T. Reproducibility of the creamatocrit technique for the measurement of fat content in human milk. Food Chem. 2021, 356, 129708. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.P.; Engstrom, J.L.; Zuleger, J.L.; Motykowski, J.E.; Vasan, U.; Meier, W.A.; Hartmann, P.E.; Williams, T.M. Accuracy of a user-friendly centrifuge for measuring creamatocrits on mothers’ milk in the clinical setting. Breastfeed. Med. 2006, 1, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Goldman, A.S.; Chheda, S. The Immune System in Human Milk: A Historic Perspective. Ann. Nutr. Metab. 2021, 1–8. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Trengove, R.D.; Geddes, D.T. Human Milk Lipidomics: Current Techniques and Methodologies. Nutrients 2018, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Wlodek, M.E.; Trengove, R.D.; Murray, K.; Geddes, D.T. Untargeted lipidomics using liquid chromatography-ion mobility-mass spectrometry reveals novel triacylglycerides in human milk. Sci. Rep. 2020, 10, 9255. [Google Scholar] [CrossRef]
- Gardner, A.S.; Rahman, I.A.; Lai, C.T.; Hepworth, A.; Trengove, N.; Hartmann, P.E.; Geddes, D.T. Changes in Fatty Acid Composition of Human Milk in Response to Cold-Like Symptoms in the Lactating Mother and Infant. Nutrients 2017, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- George, A.D.; Gay, M.C.L.; Wlodek, M.E.; Geddes, D.T. The importance of infants’ lipid intake in human milk research. Nutr. Rev. 2021, nuaa141. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.R.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.E.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2013, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Franco, L.; Regal, P.; Lamas, A.; Cepeda, A.; Fente, C. Breast Milk: A Source of Functional Compounds with Potential Application in Nutrition and Therapy. Nutrients 2021, 13, 1026. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F. Establishment of the early-life microbiome: A DOHaD perspective. J. Dev. Orig. Health Dis. 2019, 201–210, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Sindi, A.S.M.; Cheema, A.S.; Lai, C.T.; Muhlhausler, B.S.; Wlodek, M.E.; Payne, M.S.; Geddes, D.T. The human milk microbiome: Who, what, when, where, why, and how? Nutr. Rev. 2021, 79, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Asnicar, F.; Manara, S.; Zolfo, M.; Truong, D.T.; Scholz, M.; Armanini, F.; Ferretti, P.; Gorfer, V.; Pedrotti, A.; Tett, A.; et al. Studying Vertical Microbiome Transmission from Mothers to Infants by Strain-Level Metagenomic Profiling. mSystems 2017, 2, e00164-16. [Google Scholar] [CrossRef] [Green Version]
- Jost, T.; Lacroix, C.; Braegger, C.P.; Rochat, F.; Chassard, C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ. Microbiol. 2014, 16, 2891–2904. [Google Scholar] [CrossRef]
- Duranti, S.; Lugli, G.A.; Mancabelli, L.; Armanini, F.; Turroni, F.; James, K.; Ferretti, P.; Gorfer, V.; Ferrario, C.; Milani, C.; et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 2017, 5, 66. [Google Scholar] [CrossRef]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V.; et al. Exploring Vertical Transmission of Bifidobacteria from Mother to Child. Appl. Environ. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sindi, A.S.; Geddes, D.T.; Wlodek, M.E.; Muhlhausler, B.S.; Payne, M.S.; Stinson, L.F. Can we modulate the breastfed infant gut microbiota through maternal diet? FEMS Microbiol. Rev. 2021, fuab011. [Google Scholar] [CrossRef]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk Derived Antimicrobial Bioactive Peptides: A Review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Moossavi, S.; Fehr, K.; Derakhshani, H.; Sbihi, H.; Robertson, B.; Bode, L.; Brook, J.; Turvey, S.E.; Moraes, T.J.; Becker, A.B.; et al. Human milk fungi: Environmental determinants and inter-kingdom associations with milk bacteria in the CHILD Cohort Study. BMC Microbiol. 2020, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [Green Version]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Stinson, L.F.; Gay, M.C.L.; Koleva, P.T.; Eggesbo, M.; Johnson, C.C.; Wegienka, G.; du Toit, E.; Shimojo, N.; Munblit, D.; Campbell, D.E.; et al. Human Milk From Atopic Mothers Has Lower Levels of Short Chain Fatty Acids. Front. Immunol. 2020, 11, 1427. [Google Scholar] [CrossRef]
- Benn, C.S.; Wohlfahrt, J.; Aaby, P.; Westergaard, T.; Benfeldt, E.; Michaelsen, K.F.; Bjorksten, B.; Melbye, M. Breastfeeding and risk of atopic dermatitis, by parental history of allergy, during the first 18 months of life. Am. J. Epidemiol. 2004, 160, 217–223. [Google Scholar] [CrossRef]
- Lodge, C.J.; Tan, D.J.; Lau, M.X.; Dai, X.; Tham, R.; Lowe, A.J.; Bowatte, G.; Allen, K.J.; Dharmage, S.C. Breastfeeding and asthma and allergies: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Prentice, P.M.; Schoemaker, M.H.; Vervoort, J.; Hettinga, K.; Lambers, T.T.; van Tol, E.A.F.; Acerini, C.L.; Olga, L.; Petry, C.J.; Hughes, I.A.; et al. Human Milk Short-Chain Fatty Acid Composition is Associated with Adiposity Outcomes in Infants. J. Nutr. 2019, 149, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.S.; Lai, C.T.; Dymock, M.; Rae, A.; Geddes, D.T.; Payne, M.S.; Stinson, L.F. Impact of expression mode and timing of sample collection, relative to milk ejection, on human milk bacterial DNA profiles. J. Appl. Microbiol. 2021, 131, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.S.; Stinson, L.F.; Lai, C.T.; Geddes, D.T.; Payne, M.S. DNA extraction method influences human milk bacterial profiles. J. Appl. Microbiol. 2021, 130, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Stinson, L.F.; Ma, J.; Rea, A.; Dymock, M.; Geddes, D.T. Centrifugation does not remove bacteria from the fat fraction of human milk. Sci. Rep. 2021, 11, 572. [Google Scholar] [CrossRef]
- Douellou, T.; Galia, W.; Kerangart, S.; Marchal, T.; Milhau, N.; Bastien, R.; Bouvier, M.; Buff, S.; Montel, M.C.; Sergentet-Thevenot, D. Milk Fat Globules Hamper Adhesion of Enterohemorrhagic Escherichia coli to Enterocytes: In Vitro and in Vivo Evidence. Front. Microbiol. 2018, 9, 947. [Google Scholar] [CrossRef]
- Haiden, N.; Ziegler, E.E. Human Milk Banking. Ann. Nutr. Metab. 2016, 69 (Suppl. S2), 8–15. [Google Scholar] [CrossRef]
- Yang, R.; Chen, D.; Deng, Q.; Xu, X. The effect of donor human milk on the length of hospital stay in very low birthweight infants: A systematic review and meta-analysis. Int. Breastfeed. J. 2020, 15, 89. [Google Scholar] [CrossRef]
- Zanganeh, M.; Jordan, M.; Mistry, H. A systematic review of economic evaluations for donor human milk versus standard feeding in infants. Matern. Child Nutr. 2021, 17, e13151. [Google Scholar] [CrossRef]
- Hartmann, B.T.; Pang, W.W.; Keil, A.D.; Hartmann, P.E.; Simmer, K.; Australian Neonatal Clinical Care, U. Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Hum. Dev. 2007, 83, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.A.; O’Connor, D.L.; McGeer, A.J.; Unger, S. The impact of thermal pasteurization on viral load and detectable live viruses in human milk and other matrices: A rapid review. Appl. Physiol. Nutr. Metab. 2021, 46, 10–26. [Google Scholar] [CrossRef]
- Almutawif, Y.; Hartmann, B.; Lloyd, M.; Erber, W.; Geddes, D. A retrospective audit of bacterial culture results of donated human milk in Perth, Western Australia. Early Hum. Dev. 2017, 105, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, A.; Sinkiewicz-Darol, E.; Barbarska, O.; Bernatowicz-Lojko, U.; Borszewska-Kornacka, M.K.; van Goudoever, J.B. Innovative Techniques of Processing Human Milk to Preserve Key Components. Nutrients 2019, 11, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czank, C.; Prime, D.K.; Hartmann, B.; Simmer, K.; Hartmann, P.E. Retention of the immunological proteins of pasteurized human milk in relation to pasteurizer design and practice. Pediatr. Res. 2009, 66, 374–379. [Google Scholar] [CrossRef] [Green Version]
- Czank, C.; Simmer, K.; Hartmann, P.E. Simultaneous pasteurization and homogenization of human milk by combining heat and ultrasound: Effect on milk quality. J. Dairy Res. 2010, 77, 183–189. [Google Scholar] [CrossRef]
- Christen, L.; Lai, C.T.; Hartmann, P.E. Ultrasonication and the quality of human milk: Variation of power and time of exposure. J. Dairy Res. 2012, 79, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, L.; Lai, C.T.; Hartmann, B.; Hartmann, P.E.; Geddes, D.T. Ultraviolet-C Irradiation: A Novel Pasteurization Method for Donor Human Milk. PLoS ONE 2013, 8, e68120. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, M.L.; Hod, N.; Jayaraman, J.; Marchant, E.A.; Christen, L.; Chiang, P.; Hartmann, P.; Shellam, G.R.; Simmer, K. Inactivation of Cytomegalovirus in Breast Milk Using Ultraviolet-C Irradiation: Opportunities for a New Treatment Option in Breast Milk Banking. PLoS ONE 2016, 11, e0161116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, L.; Lai, C.T.; Hartmann, B.; Hartmann, P.E.; Geddes, D.T. The effect of UV-C pasteurization on bacteriostatic properties and immunological proteins of donor human milk. PLoS ONE 2013, 8, e85867. [Google Scholar] [CrossRef] [Green Version]
- Almutawif, Y.; Hartmann, B.; Lloyd, M.; Lai, C.T.; Rea, A.; Geddes, D. Staphylococcus aureus Enterotoxin Production in Raw and Pasteurized Milk: The Effect of Selected Different Storage Durations and Temperatures. Breastfeed. Med. 2019, 14, 256–261. [Google Scholar] [CrossRef]
- Almutawif, Y.; Hartmann, B.; Lloyd, M.; Lai, C.T.; Rea, A.; Geddes, D. Staphylococcus aureus Enterotoxin Production in Raw, Holder-Pasteurized, and Ultraviolet-C-Treated Donated Human Milk. Breastfeed. Med. 2019, 14, 262–270. [Google Scholar] [CrossRef]
- Alsaweed, M.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci. Rep. 2016, 6, 20680. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.R.; Williams, T.M.; Twigger, A.J.; Perrella, S.; Lai, C.T.; Filgueira, L.; Geddes, D.T.; Hartmann, P.E. Breastmilk cell and fat contents respond similarly to removal of breastmilk by the infant. PLoS ONE 2013, 8, e78232. [Google Scholar] [CrossRef] [Green Version]
- Schultz-Pernice, I.; Engelbrecht, L.K.; Petricca, S.; Scheel, C.H.; Twigger, A.J. Morphological Analysis of Human Milk Membrane Enclosed Structures Reveals Diverse Cells and Cell-like Milk Fat Globules. J. Mammary Gland Biol. Neoplasia 2020, 25, 397–408. [Google Scholar] [CrossRef]
- Aydin, M.S.; Yigit, E.N.; Vatandaslar, E.; Erdogan, E.; Ozturk, G. Transfer and Integration of Breast Milk Stem Cells to the Brain of Suckling Pups. Sci. Rep. 2018, 8, 14289. [Google Scholar] [CrossRef] [Green Version]
- Cregan, M.D.; Fan, Y.; Appelbee, A.; Brown, M.L.; Klopcic, B.; Koppen, J.; Mitoulas, L.R.; Piper, K.M.; Choolani, M.A.; Chong, Y.S.; et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007, 329, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Zeps, N.; Cregan, M.; Hartmann, P.; Martin, T. 14-3-3 sigma (sigma) regulates proliferation and differentiation of multipotent p63-positive cells isolated from human breastmilk. Cell Cycle 2011, 10, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Gascard, P.; Dumont, N.; Zhao, J.; Pan, D.; Petrie, S.; Margeta, M.; Tlsty, T.D. Rare somatic cells from human breast tissue exhibit extensive lineage plasticity. Proc. Natl. Acad. Sci. USA 2013, 110, 4598–4603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, A.; Nissen, N. Mammary gland-derived nestin-positive cell populations can be isolated from human male and female donors. Stem Cell Res. Ther. 2013, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef]
- Lloyd-Lewis, B.; Harris, O.B.; Watson, C.J.; Davis, F.M. Mammary Stem Cells: Premise, Properties, and Perspectives. Trends Cell Biol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Watson, C.J. How should we define mammary stem cells? Trends Cell Biol. 2021. [Google Scholar] [CrossRef]
- Twigger, A.J.; Khaled, W.T. Mammary gland development from a single cell ’omics view. Semin. Cell Dev. Biol. 2021, 114, 171–185. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Pervolarakis, N.; Blake, K.; Ma, D.; Davis, R.T.; James, N.; Phung, A.T.; Willey, E.; Kumar, R.; Jabart, E.; et al. Profiling human breast epithelial cells using single cell RNA sequencing identifies cell diversity. Nat. Commun. 2018, 9, 2028. [Google Scholar] [CrossRef]
- Martin Carli, J.F.; Trahan, G.D.; Jones, K.L.; Hirsch, N.; Rolloff, K.P.; Dunn, E.Z.; Friedman, J.E.; Barbour, L.A.; Hernandez, T.L.; MacLean, P.S.; et al. Single Cell RNA Sequencing of Human Milk-Derived Cells Reveals Sub-Populations of Mammary Epithelial Cells with Molecular Signatures of Progenitor and Mature States: A Novel, Non-invasive Framework for Investigating Human Lactation Physiology. J. Mammary Gland Biol. Neoplasia 2020, 25, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Twigger, A.-J.; Engelbrecht, L.K.; Bach, K.; Schultz-Pernice, I.; Petricca, S.; Scheel, C.H.; Khaled, W. Transcriptional changes in the mammary gland during lactation revealed by single cell sequencing of cells from human milk. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wenham, C.; Smith, J.; Morgan, R.; Gender and COVID-19 Working Group. COVID-19: The gendered impacts of the outbreak. Lancet 2020, 395, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulou, D.; Triantafyllidou, P.; Daskalaki, A.; Syridou, G.; Papaevangelou, V. Breastfeeding during the novel coronavirus (COVID-19) pandemic: Guidelines and challenges. J. Matern. Fetal Neonatal Med. 2020, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Spatz, D.L.; Froh, E.B. Birth and Breastfeeding in the Hospital Setting during the COVID-19 Pandemic. MCN Am. J. Matern. Child Nurs. 2021, 46, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Snyder, K.; Worlton, G. Social Support During COVID-19: Perspectives of Breastfeeding Mothers. Breastfeed. Med. 2021, 16, 39–45. [Google Scholar] [CrossRef]
- Chambers, C.; Krogstad, P.; Bertrand, K.; Contreras, D.; Tobin, N.H.; Bode, L.; Aldrovandi, G. Evaluation for SARS-CoV-2 in Breast Milk from 18 Infected Women. JAMA 2020, 324, 1347–1348. [Google Scholar] [CrossRef]
- Dumitriu, D.; Emeruwa, U.N.; Hanft, E.; Liao, G.V.; Ludwig, E.; Walzer, L.; Arditi, B.; Saslaw, M.; Andrikopoulou, M.; Scripps, T.; et al. Outcomes of Neonates Born to Mothers With Severe Acute Respiratory Syndrome Coronavirus 2 Infection at a Large Medical Center in New York City. JAMA Pediatr. 2021, 175, 157–167. [Google Scholar] [CrossRef]
- Gross, R.; Conzelmann, C.; Muller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Munch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef]
- Costa, S.; Posteraro, B.; Marchetti, S.; Tamburrini, E.; Carducci, B.; Lanzone, A.; Valentini, P.; Buonsenso, D.; Sanguinetti, M.; Vento, G.; et al. Excretion of SARS-CoV-2 in human breast milk. Clin. Microbiol. Infect. 2020, 26, 1430–1432. [Google Scholar] [CrossRef]
- Tam, P.C.K.; Ly, K.M.; Kernich, M.L.; Spurrier, N.; Lawrence, D.; Gordon, D.L.; Tucker, E.C. Detectable Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Human Breast Milk of a Mildly Symptomatic Patient With Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2021, 72, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, C.; Dong, L.; Zhang, C.; Chen, Y.; Liu, J.; Zhang, C.; Duan, C.; Zhang, H.; Mol, B.W.; et al. Viral shedding of COVID-19 in pregnant women. SSRN 2020. [Google Scholar] [CrossRef]
- Van Keulen, B.J.; Romijn, M.; Bondt, A.; Dingess, K.A.; Kontopodi, E.; van der Straten, K.; den Boer, M.A.; Burger, J.A.; Poniman, M.; Bosch, B.J.; et al. Human Milk from Previously COVID-19-Infected Mothers: The Effect of Pasteurization on Specific Antibodies and Neutralization Capacity. Nutrients 2021, 13, 1645. [Google Scholar] [CrossRef]
- Pace, R.M.; Williams, J.E.; Jarvinen, K.M.; Belfort, M.B.; Pace, C.D.W.; Lackey, K.A.; Gogel, A.C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio 2021, 12, e03192-20. [Google Scholar] [CrossRef]
- Fox, A.; Marino, J.; Amanat, F.; Krammer, F.; Hahn-Holbrook, J.; Zolla-Pazner, S.; Powell, R.L. Robust and Specific Secretory IgA Against SARS-CoV-2 Detected in Human Milk. iScience 2020, 23, 101735. [Google Scholar] [CrossRef]
- Perl, S.H.; Uzan-Yulzari, A.; Klainer, H.; Asiskovich, L.; Youngster, M.; Rinott, E.; Youngster, I. SARS-CoV-2-Specific Antibodies in Breast Milk After COVID-19 Vaccination of Breastfeeding Women. JAMA 2021, 325, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: A cohort study. Am. J. Obstet. Gynecol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Sakalidis, V.S.; Rea, A.; Perrella, S.L.; McEachran, J.; Collis, G.; Miraudo, J.; Prosser, S.A.; Gibson, L.Y.; Silva, D.; Geddes, D.T. Wellbeing of Breastfeeding Women in Australia and New Zealand during the COVID-19 Pandemic: A Cross-Sectional Study. Nutrients 2021, 13, 1831. [Google Scholar] [CrossRef]
- Brown, A.; Shenker, N. Experiences of breastfeeding during COVID-19: Lessons for future practical and emotional support. Matern. Child Nutr. 2021, 17, e13088. [Google Scholar] [CrossRef]
- Zanardo, V.; Manghina, V.; Giliberti, L.; Vettore, M.; Severino, L.; Straface, G. Psychological impact of COVID-19 quarantine measures in northeastern Italy on mothers in the immediate postpartum period. Int. J. Gynaecol. Obstet. 2020, 150, 184–188. [Google Scholar] [CrossRef]
- Davenport, M.H.M.; Meah, V.L.S.; Strynadka, M.C.; Khurana, R. Moms Are Not OK: COVID-19 and Maternal Mental Health. Front. Glob. Women’s Health 2020, 1, 1. [Google Scholar] [CrossRef]
- Ceulemans, M.; Verbakel, J.Y.; van Calsteren, K.; Eerdekens, A.; Allegaert, K.; Foulon, V. SARS-CoV-2 Infections and Impact of the COVID-19 Pandemic in Pregnancy and Breastfeeding: Results from an Observational Study in Primary Care in Belgium. Int. J. Environ. Res. Public Health 2020, 17, 6766. [Google Scholar] [CrossRef] [PubMed]
- Guvenc, G.; Yesilcinar, I.; Ozkececi, F.; Oksuz, E.; Ozkececi, C.F.; Konukbay, D.; Kok, G.; Karasahin, K.E. Anxiety, depression, and knowledge level in postpartum women during the COVID-19 pandemic. Perspect. Psychiatr. Care 2021, 57, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Dawel, A.; Shou, Y.; Smithson, M.; Cherbuin, N.; Banfield, M.; Calear, A.L.; Farrer, L.M.; Gray, D.; Gulliver, A.; Housen, T.; et al. The Effect of COVID-19 on Mental Health and Wellbeing in a Representative Sample of Australian Adults. Front. Psychiatry 2020, 11, 579985. [Google Scholar] [CrossRef] [PubMed]
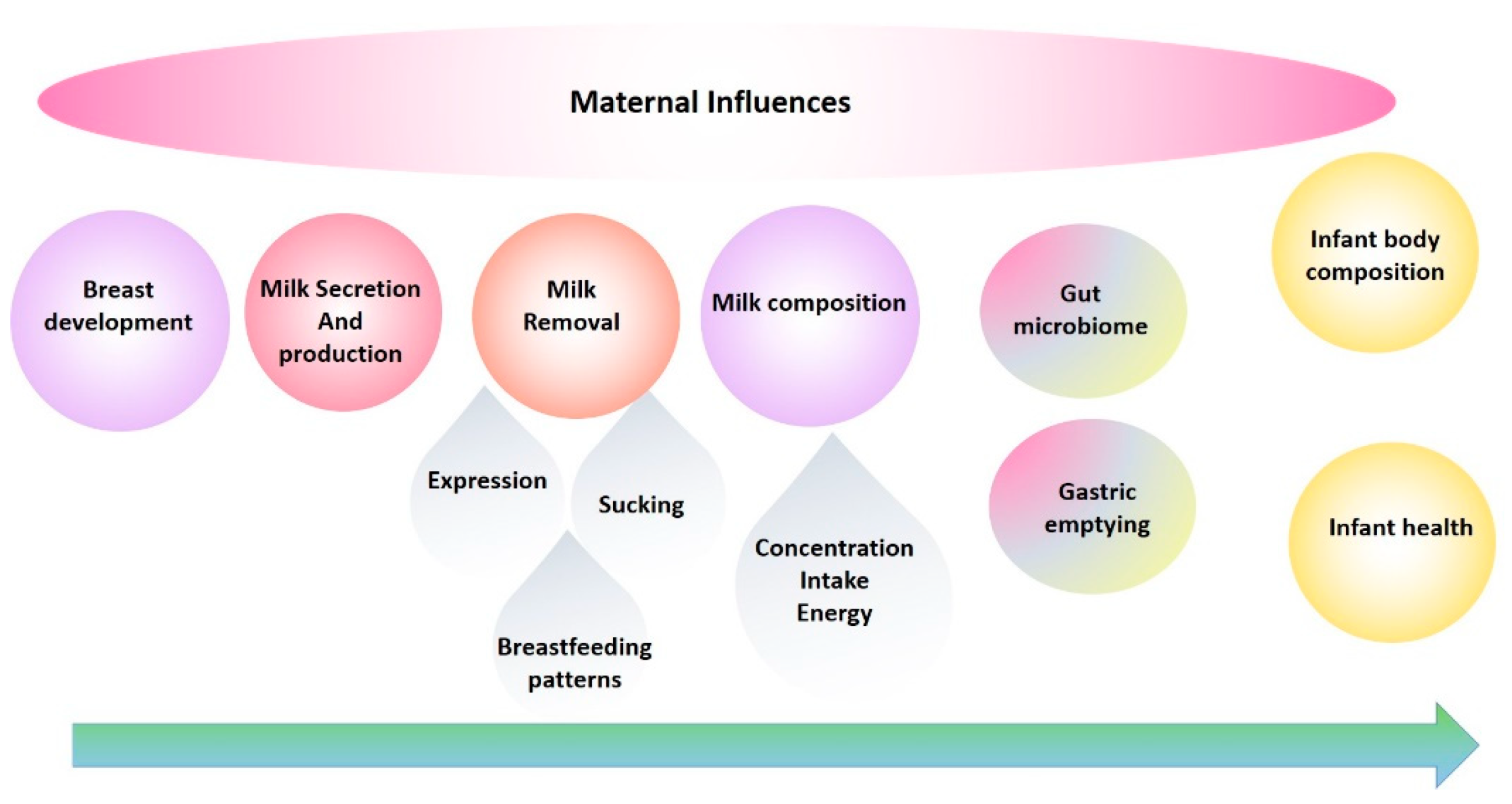


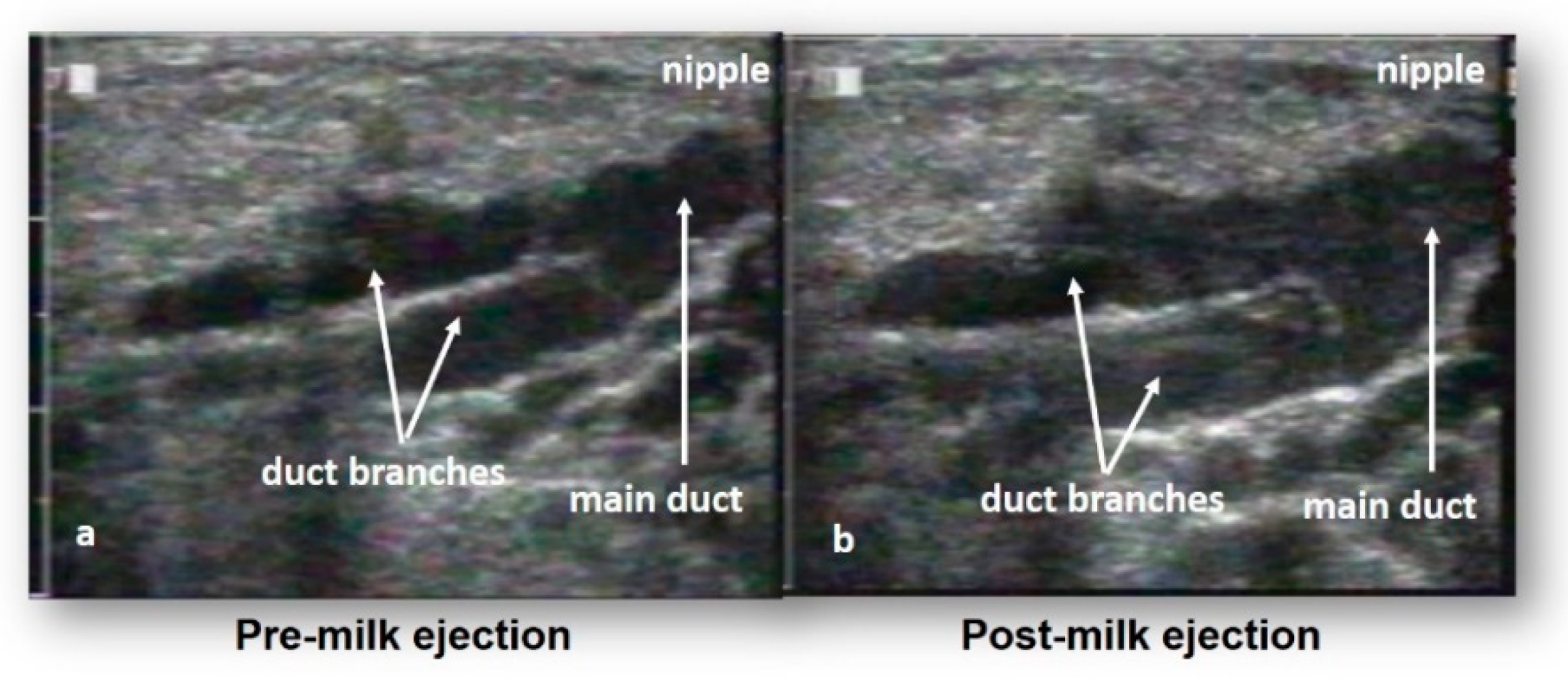
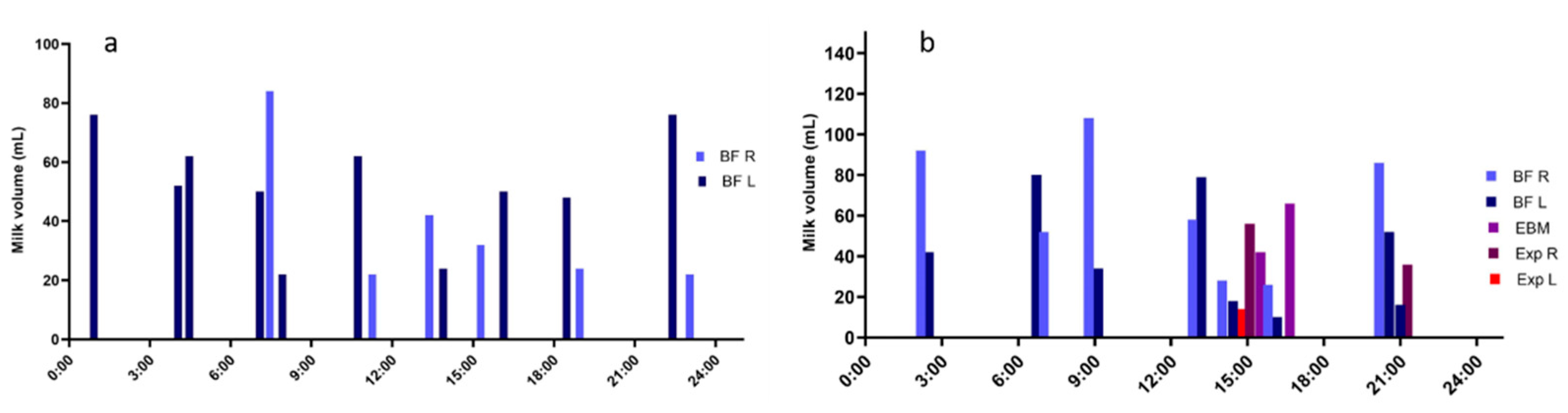
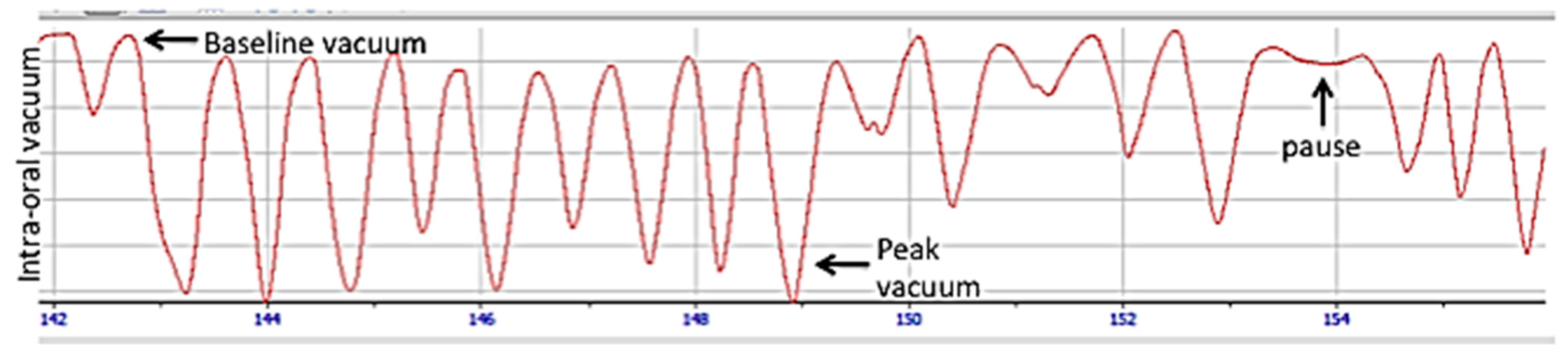
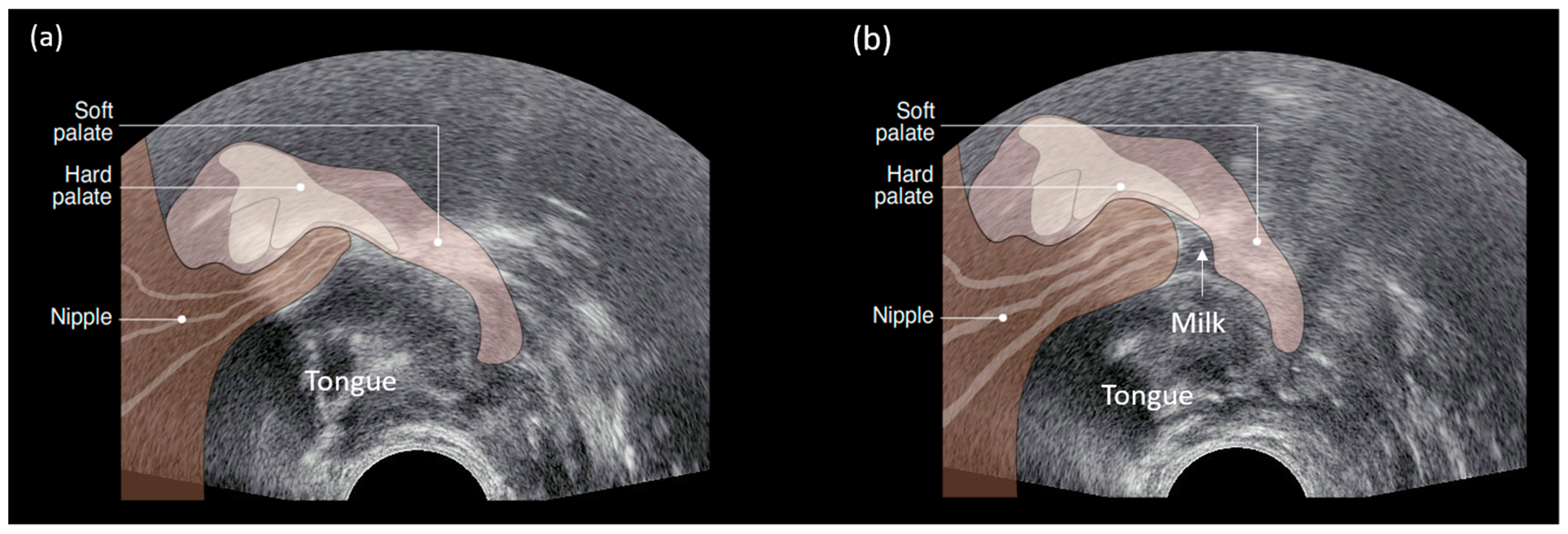


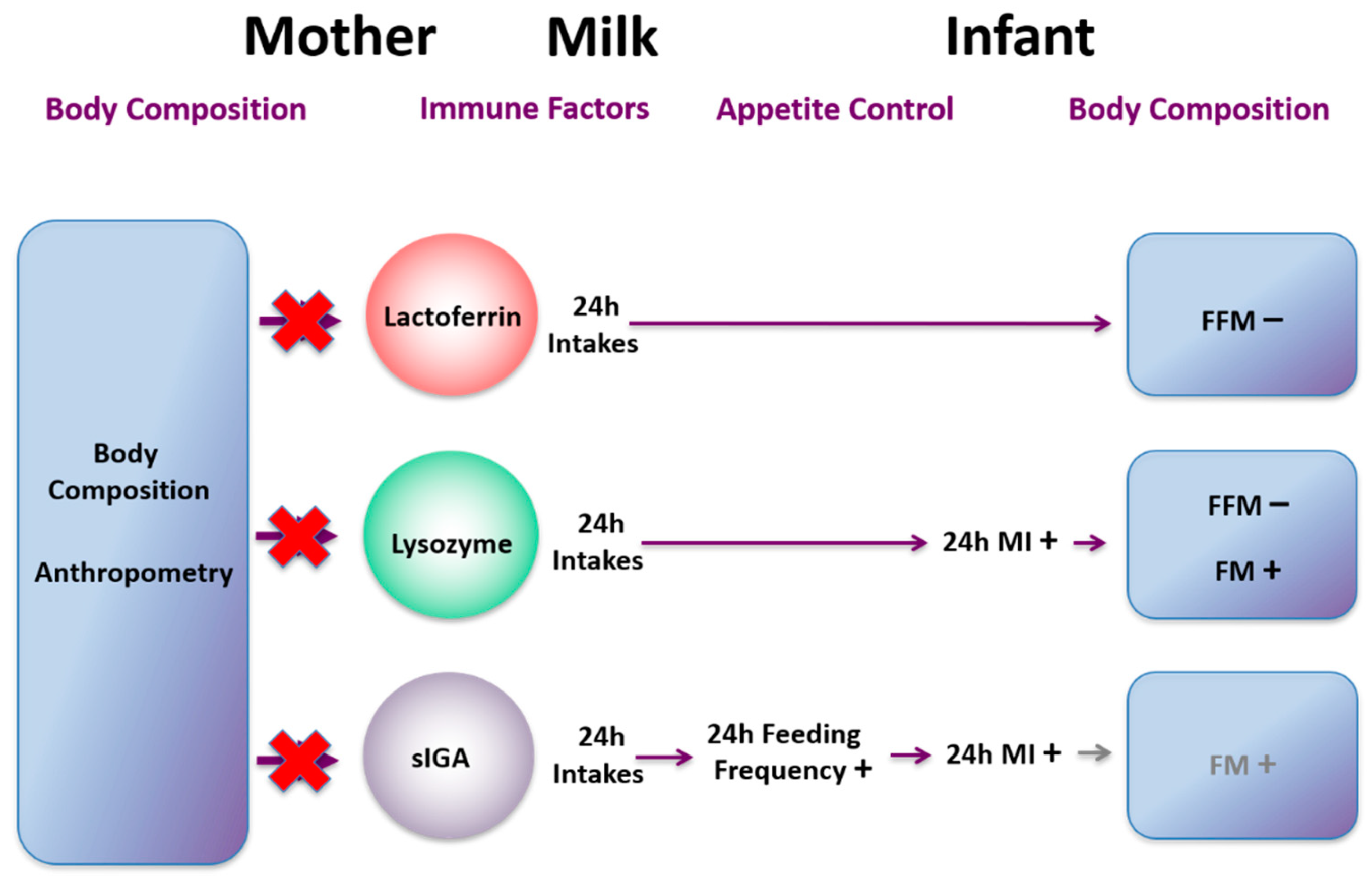
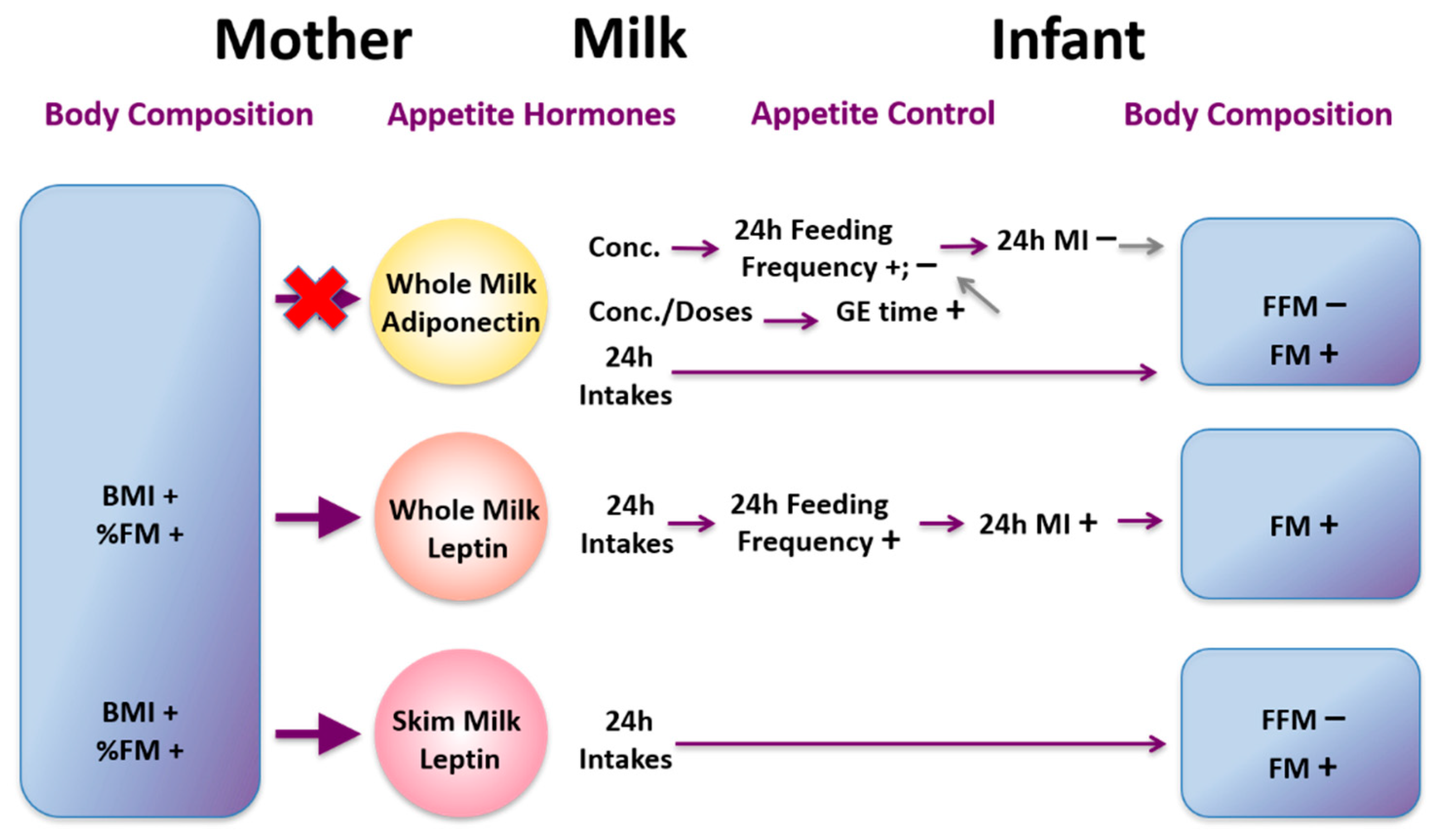
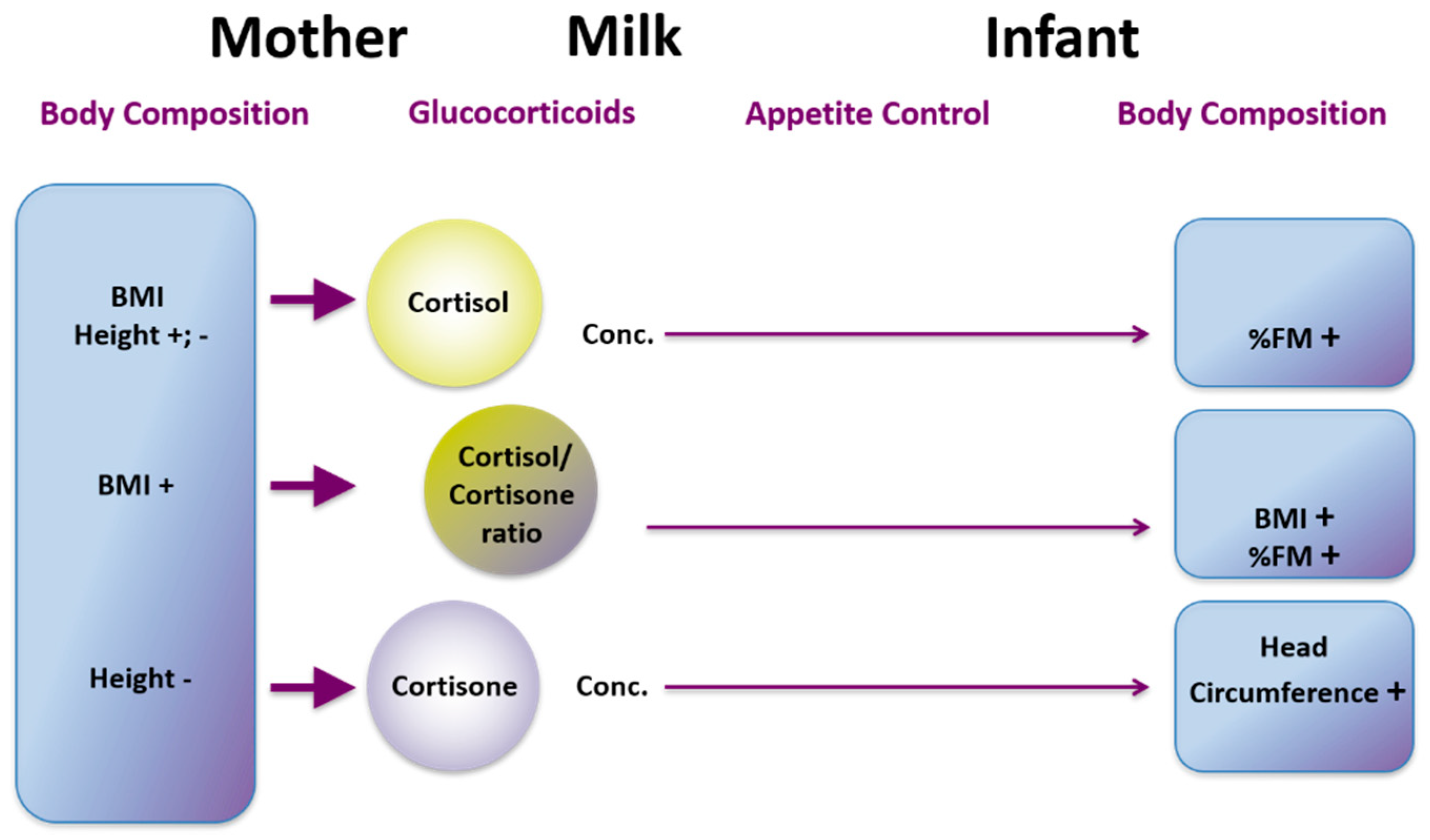


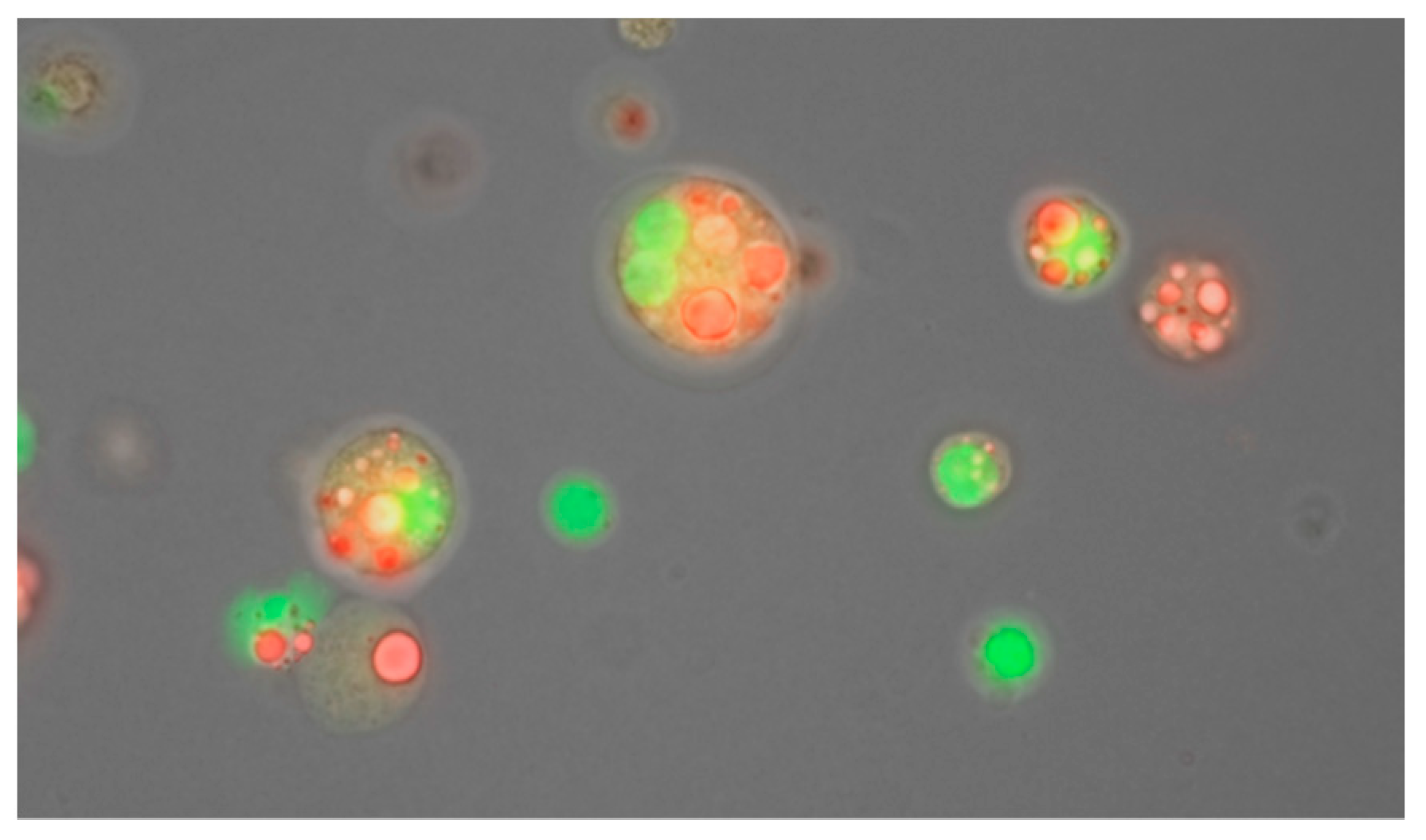
| Mean (SD) | Range | |
|---|---|---|
| 24 h milk intake | 788 (169) | 478–1356 |
| Single breastfeed | ||
| Frequency (feeds/24 h) | 11 (3) | 6–18 |
| Volume transferred (mL) | 76 (13) | 0–240 |
| Breastfeeding session | ||
| Frequency (feeds/24 h) | 8 (2) | 4–13 |
| Total volume transferred (mL) | 101 (16) | 0–350 |
| Fat content | ||
| Mean fat content (g/L) | 41.1 (7.8) | 22.3–61.6 |
| Total fat consumed (g) | 32.0 (7.7) | 5.4–49.5 |
| Breast Anatomy/Genetics | Breast Physiology | Milk Removal (Autocrine Control) | |
|---|---|---|---|
| Infant | Pump | ||
| No breast growth in pregnancy | Separation from the infant | Infrequent and ineffective breast emptying | Infrequent and ineffective breast emptying |
| Breast hypoplasia | Pregnancy complications | Oral anomalies-ankyloglossia | Slow stimulation of ME |
| Breast surgery | Maternal endocrine disorders | Prematurity | Incorrect pump settings (vacuum, vacuum pattern) |
| Nipple piercing | Mastitis | Infants exerting strong vacuums and causing pain | Cold conditions-reduce efficacy of milk removal |
| Zinc transporter mutations | Blocked ducts (temporary) | Low tone, Down’s syndrome | Pump shield not fitted correctly to maternal anatomy |
| Medications | Cleft lip and/or palate | ||
| Infants ≥ 34/40 PMA n = 902 | Infants < 34/40 PMA n = 284 | |
|---|---|---|
| Minimal or no milk transfer, give full supplement | 98% | 99% |
| Partial feed transferred, give 50% supplement | 29% | 16% |
| Full feed transferred, no supplement needed | 47% | 91% |
| Sucking | Milk Removal | |
|---|---|---|
| Nipple pain | Strong intra-oral vacuums (baseline and peak) Compressive tongue motion | May or may not be impacted Most women can attain a full milk production with support |
| Nipple shield | Sucking characteristics do not differ significantly | Duration of feeds were longer reducing efficiency however this is not clinically significant Women using shields for persistent pain in the first week of lactation experienced no impact on milk transfer and effectiveness of breast emptying Most women can attain a full milk production with support |
| Ankyloglossia | Anterior tongue tie associated with tongue motion compressing either the nipple base or tip | Frenotomy for anterior tongue tie ‘normalised’ tongue movement and reduced pain improved milk production Frenotomy for posterior tongue tie reduced pain but did not improve milk production |
| Prematurity | Weak intra-oral vacuums (both baseline and peak) Tongue action during breastfeeding similar to term infants Tongue action with nipple shields similar to breastfeeding | Low milk volumes removed from the breast with or without a nipple shield |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.-J.; Hartmann, P.E. 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients 2021, 13, 3071. https://doi.org/10.3390/nu13093071
Geddes DT, Gridneva Z, Perrella SL, Mitoulas LR, Kent JC, Stinson LF, Lai CT, Sakalidis V, Twigger A-J, Hartmann PE. 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients. 2021; 13(9):3071. https://doi.org/10.3390/nu13093071
Chicago/Turabian StyleGeddes, Donna Tracy, Zoya Gridneva, Sharon Lisa Perrella, Leon Robert Mitoulas, Jacqueline Coral Kent, Lisa Faye Stinson, Ching Tat Lai, Vanessa Sakalidis, Alecia-Jane Twigger, and Peter Edwin Hartmann. 2021. "25 Years of Research in Human Lactation: From Discovery to Translation" Nutrients 13, no. 9: 3071. https://doi.org/10.3390/nu13093071
APA StyleGeddes, D. T., Gridneva, Z., Perrella, S. L., Mitoulas, L. R., Kent, J. C., Stinson, L. F., Lai, C. T., Sakalidis, V., Twigger, A.-J., & Hartmann, P. E. (2021). 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients, 13(9), 3071. https://doi.org/10.3390/nu13093071






