Diet in Intestinal Fibrosis: A Double-Edged Sword
Abstract
:1. Intestinal Fibrosis
Western Diet
2. Obesity
3. High Salt
4. High Sugar
5. Beneficial Effects of Dietary Components
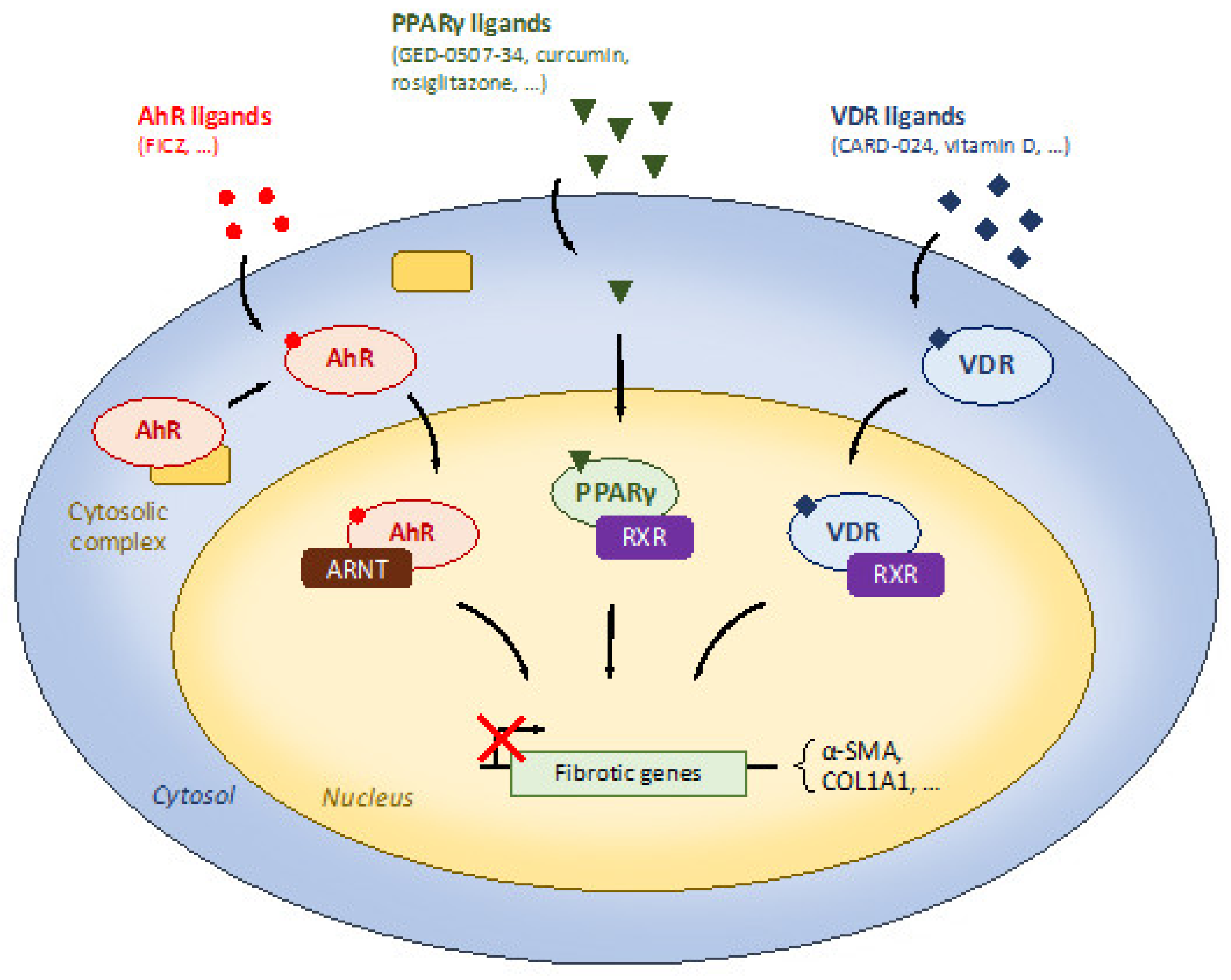
5.1. Dietary Modulation of Receptors with Anti-Fibrosis Properties
5.1.1. Peroxisome Proliferator-Activated Receptor γ (PPARγ)
5.1.2. Aryl Hydrocarbon Receptor (AhR)
5.1.3. Vitamin D Receptor
5.2. Dietary Modulation of Anti-Fibrosis Signaling
5.3. Inhibition of Pro-Fibrotic Molecules by Amino Acids
5.4. Anti-Fibrosis Properties of n-3 PUFA
5.5. Dietary Manipulation of the Gut Microbiota
5.6. Reduction of Myofibroblast Activation
5.7. Mucosal Healing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Mao, R.; Kurada, S.; Gordon, I.O.; Baker, M.E.; Gandhi, N.; McDonald, C.; Coffey, J.C.; Rieder, F. The Mesenteric Fat and Intestinal Muscle Interface: Creeping Fat Influencing Stricture Formation in Crohn’s Disease. Inflamm. Bowel Dis. 2019, 25, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Kugathasan, S.; Denson, L.A.; Walters, T.D.; Kim, M.-O.; Marigorta, U.M.; Schirmer, M.; Mondal, K.; Liu, C.; Griffiths, A.; Noe, J.D.; et al. Prediction of Complicated Disease Course for Children Newly Diagnosed with Crohn’s Disease: A Multicentre Inception Cohort Study. Lancet 2017, 389, 1710–1718. [Google Scholar] [CrossRef] [Green Version]
- Moran, G.W.; Dubeau, M.; Kaplan, G.G.; Yang, H.; Seow, C.H.; Fedorak, R.N.; Dieleman, L.A.; Barkema, H.W.; Ghosh, S.; Panaccione, R. Phenotypic Features of Crohn’s Disease Associated With Failure of Medical Treatment. Clin. Gastroenterol. Hepatol. 2014, 12, 434–442.e1. [Google Scholar] [CrossRef]
- Rieder, F.; Fiocchi, C.; Rogler, G. Mechanisms, Management, and Treatment of Fibrosis in Patients with Inflammatory Bowel Diseases. Gastroenterology 2017, 152, 340–350.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. IBD: In Food We Trust. J. Crohns Colitis 2016, 10, 1351–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion-Letellier, R.; Savoye, G.; Beck, P.L.; Panaccione, R.; Ghosh, S. Polyunsaturated Fatty Acids in Inflammatory Bowel Diseases: A Reappraisal of Effects and Therapeutic Approaches. Inflamm. Bowel Dis. 2013, 19, 650–661. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Guanghong, J.; Habibi, J.; DeMarco, V.G.; Martinez-Lemus, L.A.; Ma, L.; Whaley-Connell, A.T.; Aroor, A.R.; Domeier, T.L.; Zhu, Y.; Meininger, G.A.; et al. Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension 2015, 66, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Aroor, A.R.; Habibi, J.; Nistala, R.; Ramirez-Perez, F.I.; Martinez-Lemus, L.A.; Jaffe, I.Z.; Sowers, J.R.; Guanghong, J.; Whaley-Connell, A. Diet-Induced Obesity Promotes Kidney Endothelial Stiffening and Fibrosis Dependent on the Endothelial Mineralocorticoid Receptor. Hypertension 2019, 73, 849–858. [Google Scholar] [CrossRef]
- Taylor, L.; Almutairdi, A.; Shommu, N.; Fedorak, R.; Ghosh, S.; Reimer, R.A.; Panaccione, R.; Raman, M. Cross-Sectional Analysis of Overall Dietary Intake and Mediterranean Dietary Pattern in Patients with Crohn’s Disease. Nutrients 2018, 10, 1761. [Google Scholar] [CrossRef] [Green Version]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of Ultra-Processed Food Intake with Risk of Inflammatory Bowel Disease: Prospective Cohort Study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef] [PubMed]
- Moran, G.W.; Dubeau, M.-F.; Kaplan, G.G.; Panaccione, R.; Ghosh, S. The Increasing Weight of Crohn’s Disease Subjects in Clinical Trials: A Hypothesis-Generatings Time-Trend Analysis. Inflamm. Bowel Dis. 2013, 19, 2949–2956. [Google Scholar] [CrossRef]
- Grillot, J.; D’Engremont, C.; Parmentier, A.-L.; Lakkis, Z.; Piton, G.; Cazaux, D.; Gay, C.; De Billy, M.; Koch, S.; Borot, S.; et al. Sarcopenia and Visceral Obesity Assessed by Computed Tomography Are Associated with Adverse Outcomes in Patients with Crohn’s Disease. Clin. Nutr. 2020, 39, 3024–3030. [Google Scholar] [CrossRef]
- Braga Neto, M.B.; Gregory, M.H.; Ramos, G.P.; Bazerbachi, F.; Bruining, D.H.; Abu Dayyeh, B.K.; Kushnir, V.M.; Raffals, L.E.; Ciorba, M.A.; Loftus, E.V.; et al. Impact of Bariatric Surgery on the Long-Term Disease Course of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Mulet, C.; Saffarian, A.; Cavin, J.-B.; Ducroc, R.; Regnault, B.; Tan, C.K.; Duszka, K.; Burcelin, R.; Wahli, W.; et al. High-Fat Diet Modifies the PPAR-γ Pathway Leading to Disruption of Microbial and Physiological Ecosystem in Murine Small Intestine. Proc. Natl. Acad. Sci. USA 2016, 113, E5934–E5943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-C.; Lee, H.-Y.; Kim, T.K.; Kim, M.-S.; Park, Y.M.; Kim, J.; Park, K.; Kweon, M.-N.; Kim, S.-H.; Bae, J.-W.; et al. Obesogenic Diet-Induced Gut Barrier Dysfunction and Pathobiont Expansion Aggravate Experimental Colitis. PLoS ONE 2017, 12, e0187515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazur-Bialy, A.I.; Bilski, J.; Wojcik, D.; Brzozowski, B.; Surmiak, M.; Hubalewska-Mazgaj, M.; Chmura, A.; Magierowski, M.; Magierowska, K.; Mach, T.; et al. Beneficial Effect of Voluntary Exercise on Experimental Colitis in Mice Fed a High-Fat Diet: The Role of Irisin, Adiponectin and Proinflammatory Biomarkers. Nutrients 2017, 9, 410. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Torbenson, M.; Hamad, A.R.A.; Soloski, M.J.; Li, Z. High-Fat Diet Modulates Non-CD1d-Restricted Natural Killer T Cells and Regulatory T Cells in Mouse Colon and Exacerbates Experimental Colitis. Clin. Exp. Immunol. 2008, 151, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.-Y.; Pai, M.-H.; Chiang, E.-P.I. Consumption of High-Fat Diet Induces Tumor Progression and Epithelial–Mesenchymal Transition of Colorectal Cancer in a Mouse Xenograft Model. J. Nutr. Biochem. 2012, 23, 1302–1313. [Google Scholar] [CrossRef]
- Kwapisz, O.; Górka, J.; Korlatowicz, A.; Kotlinowski, J.; Waligórska, A.; Marona, P.; Pydyn, N.; Dobrucki, J.W.; Jura, J.; Miekus, K. Fatty Acids and a High-Fat Diet Induce Epithelial-Mesenchymal Transition by Activating TGFβ and β-Catenin in Liver Cells. Int. J. Mol. Sci. 2021, 22, 1272. [Google Scholar] [CrossRef]
- Ha, S.; Kim, M.J.; Kim, D.H.; Kim, B.M.; Chung, K.W.; Chung, H.Y. Short-Term Intake of High Fat Diet Aggravates Renal Fibrosis in Aged Sprague-Dawley Rats. Exp. Gerontol. 2020, 142, 111108. [Google Scholar] [CrossRef]
- Vieujean, S.; Hu, S.; Bequet, E.; Salee, C.; Massot, C.; Bletard, N.; Pierre, N.; Quesada Calvo, F.; Baiwir, D.; Mazzucchelli, G.; et al. Potential Role of Epithelial Endoplasmic Reticulum Stress and Anterior Gradient Protein 2 Homolog in Crohn’s Disease Fibrosis. J. Crohns Colitis 2021, jjab061. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.G.; Villalba, J.A.; Liang, X.; Xiong, K.; Tsoyi, K.; Ith, B.; Ayaub, E.A.; Tatituri, R.V.; Byers, D.E.; Hsu, F.-F.; et al. Palmitic Acid–Rich High-Fat Diet Exacerbates Experimental Pulmonary Fibrosis by Modulating Endoplasmic Reticulum Stress. Am. J. Respir. Cell Mol. Biol. 2019, 61, 737–746. [Google Scholar] [CrossRef]
- Latella, G.; Rieder, F. Intestinal Fibrosis: Ready to Be Reversed. Curr. Opin. Gastroenterol. 2017, 33, 239–245. [Google Scholar] [CrossRef]
- Xie, M.; Xiong, Z.; Yin, S.; Xiong, J.; Li, X.; Jin, L.; Zhang, F.; Chen, H.; Lan, P.; Lian, L. Adiponectin Alleviates Intestinal Fibrosis by Enhancing AMP-Activated Protein Kinase Phosphorylation. Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Schirbel, A.; Rebert, N.; Sadler, T.; West, G.; Rieder, F.; Wagener, C.; Horst, A.; Sturm, A.; de la Motte, C.; Fiocchi, C. Mutual Regulation of TLR/NLR and CEACAM1 in the Intestinal Microvasculature: Implications for IBD Pathogenesis and Therapy. Inflamm. Bowel Dis. 2019, 25, 294–305. [Google Scholar] [CrossRef]
- Borley, N.R.; Mortensen, N.J.; Jewell, D.P.; Warren, B.F. The Relationship between Inflammatory and Serosal Connective Tissue Changes in Ileal Crohn’s Disease: Evidence for a Possible Causative Link. J. Pathol. 2000, 190, 196–202. [Google Scholar] [CrossRef]
- Kredel, L.I.; Batra, A.; Stroh, T.; Kühl, A.A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from Local Fat Cells Shape the Macrophage Compartment of the Creeping Fat in Crohn’s Disease. Gut 2013, 62, 852–862. [Google Scholar] [CrossRef]
- Ha, C.W.Y.; Martin, A.; Sepich-Poore, G.D.; Shi, B.; Wang, Y.; Gouin, K.; Humphrey, G.; Sanders, K.; Ratnayake, Y.; Chan, K.S.L.; et al. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 2020, 183, 666–683.e17. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt Intakes around the World: Implications for Public Health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, I.; Marafini, I.; Dinallo, V.; Di Fusco, D.; Troncone, E.; Zorzi, F.; Laudisi, F.; Monteleone, G. Sodium Chloride-Enriched Diet Enhanced Inflammatory Cytokine Production and Exacerbated Experimental Colitis in Mice. J. Crohns Colitis 2017, 11, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Aguiar, S.L.F.; Miranda, M.C.G.; Guimarães, M.A.F.; Santiago, H.C.; Queiroz, C.P.; Cunha, P.D.S.; Cara, D.C.; Foureaux, G.; Ferreira, A.J.; Cardoso, V.N.; et al. High-Salt Diet Induces IL-17-Dependent Gut Inflammation and Exacerbates Colitis in Mice. Front. Immunol. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tubbs, A.L.; Liu, B.; Rogers, T.D.; Sartor, R.B.; Miao, E.A. Dietary Salt Exacerbates Experimental Colitis. J. Immunol. Baltim. Md 1950 2017, 199, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Amamou, A.; Rouland, M.; Yaker, L.; Goichon, A.; Guérin, C.; Aziz, M.; Savoye, G.; Marion-Letellier, R. Dietary Salt Exacerbates Intestinal Fibrosis in Chronic TNBS Colitis via Fibroblasts Activation. Sci. Rep. 2021, 11, 15055. [Google Scholar] [CrossRef]
- Laffin, M.; Fedorak, R.; Zalasky, A.; Park, H.; Gill, A.; Agrawal, A.; Keshteli, A.; Hotte, N.; Madsen, K.L. A High-Sugar Diet Rapidly Enhances Susceptibility to Colitis via Depletion of Luminal Short-Chain Fatty Acids in Mice. Sci. Rep. 2019, 9, 12294. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary Simple Sugars Alter Microbial Ecology in the Gut and Promote Colitis in Mice. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Gao, L.; Fan, Y.; Zhang, X.; Yang, L.; Huang, W.; Hang, T.; Li, M.; Du, S.; Ma, J. Zinc Supplementation Inhibits the High Glucose-induced EMT of Peritoneal Mesothelial Cells by Activating the Nrf2 Antioxidant Pathway. Mol. Med. Rep. 2019, 20, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, N.A.; Carpenter, A.J.; Belenchia, A.; Aroor, A.R.; Noda, M.; Siebenlist, U.; Chandrasekar, B.; DeMarco, V.G. Empagliflozin Reduces High Glucose-Induced Oxidative Stress and MiR-21-Dependent TRAF3IP2 Induction and RECK Suppression, and Inhibits Human Renal Proximal Tubular Epithelial Cell Migration and Epithelial-to-Mesenchymal Transition. Cell. Signal. 2020, 68, 109506. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Déchelotte, P.; Iacucci, M.; Ghosh, S. Dietary Modulation of Peroxisome Proliferator-Activated Receptor Gamma. Gut 2009, 58, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Speca, S.; Rousseaux, C.; Dubuquoy, C.; Rieder, F.; Vetuschi, A.; Sferra, R.; Giusti, I.; Bertin, B.; Dubuquoy, L.; Gaudio, E.; et al. Novel PPARγ Modulator GED-0507-34 Levo Ameliorates Inflammation-Driven Intestinal Fibrosis. Inflamm. Bowel Dis. 2016, 22, 279–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion-Letellier, R.; Butler, M.; Déchelotte, P.; Playford, R.J.; Ghosh, S. Comparison of Cytokine Modulation by Natural Peroxisome Proliferator–Activated Receptor γ Ligands with Synthetic Ligands in Intestinal-like Caco-2 Cells and Human Dendritic Cells—Potential for Dietary Modulation of Peroxisome Proliferator–Activated Receptor γ in Intestinal Inflammation. Am. J. Clin. Nutr. 2008, 87, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Jiang, B.; Wang, H.; Shen, C.; Chen, H.; Zeng, L. Curcumin Suppresses Intestinal Fibrosis by Inhibition of PPARγ-Mediated Epithelial-Mesenchymal Transition. Evid.-Based Complement. Altern. Med. ECAM 2017, 2017, 7876064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verginadis, I.I.; Kanade, R.; Bell, B.; Koduri, S.; Ben-Josef, E.; Koumenis, C. A Novel Mouse Model to Study Image-Guided, Radiation-Induced Intestinal Injury and Preclinical Screening of Radioprotectors. Cancer Res. 2017, 77, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Qiao, H.; Fang, D.; Chen, J.; Sun, Y.; Kang, C.; Di, L.; Li, J.; Chen, Z.; Chen, J.; Gao, Y. Orally Delivered Polycurcumin Responsive to Bacterial Reduction for Targeted Therapy of Inflammatory Bowel Disease. Drug Deliv. 2017, 24, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohno, M.; Nishida, A.; Sugitani, Y.; Nishino, K.; Inatomi, O.; Sugimoto, M.; Kawahara, M.; Andoh, A. Nanoparticle Curcumin Ameliorates Experimental Colitis via Modulation of Gut Microbiota and Induction of Regulatory T Cells. PLoS ONE 2017, 12, e0185999. [Google Scholar] [CrossRef] [Green Version]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR Signaling Pathways and Regulatory Functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The Aryl Hydrocarbon Receptor: An Environmental Sensor Integrating Immune Responses in Health and Disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl Hydrocarbon Receptor and Intestinal Immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.-P.; Michel, M.-L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 Impacts Colitis by Altering Gut Microbiota Metabolism of Tryptophan into Aryl Hydrocarbon Receptor Ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Monteleone, I.; Zorzi, F.; Marafini, I.; Di Fusco, D.; Dinallo, V.; Caruso, R.; Izzo, R.; Franzè, E.; Colantoni, A.; Pallone, F.; et al. Aryl Hydrocarbon Receptor-Driven Signals Inhibit Collagen Synthesis in the Gut. Eur. J. Immunol. 2016, 46, 1047–1057. [Google Scholar] [CrossRef]
- Dolivo, D.M.; Larson, S.A.; Dominko, T. Tryptophan Metabolites Kynurenine and Serotonin Regulate Fibroblast Activation and Fibrosis. Cell. Mol. Life Sci. CMLS 2018, 75, 3663–3681. [Google Scholar] [CrossRef]
- Nakai, R.; Fukuda, S.; Kawase, M.; Yamashita, Y.; Ashida, H. Curcumin and Its Derivatives Inhibit 2,3,7,8,-Tetrachloro-Dibenzo-p-Dioxin-Induced Expression of Drug Metabolizing Enzymes through Aryl Hydrocarbon Receptor-Mediated Pathway. Biosci. Biotechnol. Biochem. 2018, 82, 616–628. [Google Scholar] [CrossRef] [Green Version]
- Murai, M.; Tsuji, G.; Hashimoto-Hachiya, A.; Kawakami, Y.; Furue, M.; Mitoma, C. An Endogenous Tryptophan Photo-Product, FICZ, Is Potentially Involved in Photo-Aging by Reducing TGF-β-Regulated Collagen Homeostasis. J. Dermatol. Sci. 2018, 89, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, G.M.; Xi, X.; Kulkarni, A.A.; Olsen, K.C.; Pollock, S.J.; Baglole, C.J.; Gupta, S.; Casey, A.E.; Huxlin, K.R.; Sime, P.J.; et al. The Aryl Hydrocarbon Receptor Ligand ITE Inhibits TGFβ1-Induced Human Myofibroblast Differentiation. Am. J. Pathol. 2011, 178, 1556–1567. [Google Scholar] [CrossRef]
- Yan, J.; Tung, H.-C.; Li, S.; Niu, Y.; Garbacz, W.G.; Lu, P.; Bi, Y.; Li, Y.; He, J.; Xu, M.; et al. Aryl Hydrocarbon Receptor Signaling Prevents Activation of Hepatic Stellate Cells and Liver Fibrogenesis in Mice. Gastroenterology 2019, 157, 793–806.e14. [Google Scholar] [CrossRef] [PubMed]
- Suibhne, T.N.; Cox, G.; Healy, M.; O’Morain, C.; O’Sullivan, M. Vitamin D Deficiency in Crohn’s Disease: Prevalence, Risk Factors and Supplement Use in an Outpatient Setting. J. Crohns Colitis 2012, 6, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raftery, T.; Merrick, M.; Healy, M.; Mahmud, N.; O’Morain, C.; Smith, S.; McNamara, D.; O’Sullivan, M. Vitamin D Status Is Associated with Intestinal Inflammation as Measured by Fecal Calprotectin in Crohn’s Disease in Clinical Remission. Dig. Dis. Sci. 2015, 60, 2427–2435. [Google Scholar] [CrossRef]
- Kong, J.; Zhang, Z.; Musch, M.W.; Ning, G.; Sun, J.; Hart, J.; Bissonnette, M.; Li, Y.C. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G208–G216. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, Y.-G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal Epithelial Vitamin D Receptor Deletion Leads to Defective Autophagy in Colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Johnson, L.A.; Sauder, K.L.; Rodansky, E.S.; Simpson, R.U.; Higgins, P.D.R. CARD-024, a Vitamin D Analog, Attenuates the pro-Fibrotic Response to Substrate Stiffness in Colonic Myofibroblasts. Exp. Mol. Pathol. 2012, 93, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, H.; Wang, J.; Chen, X.; Pan, J.; Liu, P.; Zhang, J.; Chen, Y.; Zhu, W.; Tang, C.; et al. Vitamin D Receptor Inhibits EMT via Regulation of the Epithelial Mitochondrial Function in Intestinal Fibrosis. J. Biol. Chem. 2021, 296, 100531. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Ferrándiz, L.; Cosín-Roger, J.; Hernández, C.; Macias-Ceja, D.C.; Ortiz-Masiá, D.; Salvador, P.; Esplugues, J.V.; Hinojosa, J.; Navarro, F.; Calatayud, S.; et al. Diminished Vitamin D Receptor Protein Levels in Crohn’s Disease Fibroblasts: Effects of Vitamin D. Nutrients 2020, 12, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.N.; Panopoulos, M.; Neumann, W.L.; Turner, K.; Cantarel, B.L.; Thompson-Snipes, L.; Dassopoulos, T.; Feagins, L.A.; Souza, R.F.; Mills, J.C.; et al. Mitochondrial Dysfunction during Loss of Prohibitin 1 Triggers Paneth Cell Defects and Ileitis. Gut 2020, 69, 1928–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pompili, S.; Sferra, R.; Gaudio, E.; Viscido, A.; Frieri, G.; Vetuschi, A.; Latella, G. Can Nrf2 Modulate the Development of Intestinal Fibrosis and Cancer in Inflammatory Bowel Disease? Int. J. Mol. Sci. 2019, 20, 4061. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Gou, X.; Cai, P.; Xu, C.; Cao, L.; Zhao, Z.; Huang, M.; Jin, J. Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation. Oxid. Med. Cell. Longev. 2019, 2019, e2432416. [Google Scholar] [CrossRef]
- Senger, D.R.; Li, D.; Jaminet, S.-C.; Cao, S. Activation of the Nrf2 Cell Defense Pathway by Ancient Foods: Disease Prevention by Important Molecules and Microbes Lost from the Modern Western Diet. PLoS ONE 2016, 11, e0148042. [Google Scholar] [CrossRef]
- Cani, P.D.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids--at the Crossroads between the Gut Microbiota and Host Metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Mattace Raso, G.; Santoro, A.; Russo, R.; Simeoli, R.; Paciello, O.; Di Carlo, C.; Diano, S.; Calignano, A.; Meli, R. Palmitoylethanolamide Prevents Metabolic Alterations and Restores Leptin Sensitivity in Ovariectomized Rats. Endocrinology 2014, 155, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide Improves Colon Inflammation through an Enteric Glia/Toll like Receptor 4-Dependent PPAR-α Activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef]
- Ohara, M.; Ohnishi, S.; Hosono, H.; Yamamoto, K.; Fu, Q.; Maehara, O.; Suda, G.; Sakamoto, N. Palmitoylethanolamide Ameliorates Carbon Tetrachloride-Induced Liver Fibrosis in Rats. Front. Pharmacol. 2018, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Ye, S.; Chen, Q.; Jiang, N.; Liang, X.; Li, J.; Zong, R.; Huang, C.; Qiu, Y.; Ma, J.-X.; Liu, Z. PPARα-Dependent Effects of Palmitoylethanolamide Against Retinal Neovascularization and Fibrosis. Invest. Ophthalmol. Vis. Sci. 2020, 61, 15. [Google Scholar] [CrossRef] [Green Version]
- Coëffier, M.; Marion-Letellier, R.; Déchelotte, P. Potential for Amino Acids Supplementation during Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2010, 16, 518–524. [Google Scholar] [CrossRef]
- Bertrand, J.; Marion-Letellier, R.; Azhar, S.; Chan, P.; Legrand, R.; Goichon, A.; Ghouzali, I.; Aziz, M.; Vaudry, D.; Savoye, G.; et al. Glutamine Enema Regulates Colonic Ubiquitinated Proteins but Not Proteasome Activities during TNBS-Induced Colitis Leading to Increased Mitochondrial Activity. Proteomics 2015, 15, 2198–2210. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, B.; Crespo, I.; Kretzmann, N.A.; Mauriz, J.L.; Marroni, N.; Tuñón, M.J.; González-Gallego, J. Glutamine Prevents Fibrosis Development in Rats with Colitis Induced by 2,4,6-Trinitrobenzene Sulfonic Acid. J. Nutr. 2010, 140, 1065–1071. [Google Scholar] [CrossRef] [Green Version]
- Jensen, J.C.; Schaefer, R.; Nwokedi, E.; Bevans, D.W.; Baker, M.L.; Pappas, A.A.; Westbrook, K.C.; Klimberg, V.S. Prevention of Chronic Radiation Enteropathy by Dietary Glutamine. Ann. Surg. Oncol. 1994, 1, 157–163. [Google Scholar] [CrossRef]
- Rodrigo Goulart Pacheco, C.C.E.; Ller, M.T.C.-B. Use of Butyrate or Glutamine in Enema Solution Reduces Inflammation and Fibrosis in Experimental Diversion Colitis. World J. Gastroenterol. 2012, 18, 4278–4287. [Google Scholar] [CrossRef]
- Severo, J.S.; da Silva Barros, V.J.; Alves da Silva, A.C.; Luz Parente, J.M.; Lima, M.M.; Moreira Lima, A.Â.; dos Santos, A.A.; Matos Neto, E.M.; Tolentino, M. Effects of Glutamine Supplementation on Inflammatory Bowel Disease: A Systematic Review of Clinical Trials. Clin. Nutr. ESPEN 2021, 42, 53–60. [Google Scholar] [CrossRef]
- Lecleire, S.; Hassan, A.; Marion-Letellier, R.; Antonietti, M.; Savoye, G.; Bôle-Feysot, C.; Lerebours, E.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Combined Glutamine and Arginine Decrease Proinflammatory Cytokine Production by Biopsies from Crohn’s Patients in Association with Changes in Nuclear Factor-KappaB and P38 Mitogen-Activated Protein Kinase Pathways. J. Nutr. 2008, 138, 2481–2486. [Google Scholar] [CrossRef]
- Nozaki, Y.; Fujita, K.; Wada, K.; Yoneda, M.; Kessoku, T.; Shinohara, Y.; Imajo, K.; Ogawa, Y.; Nakamuta, M.; Saito, S.; et al. Deficiency of INOS-Derived NO Accelerates Lipid Accumulation-Independent Liver Fibrosis in Non-Alcoholic Steatohepatitis Mouse Model. BMC Gastroenterol. 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marion, R.; Coëffier, M.; Lemoulan, S.; Gargala, G.; Ducrotté, P.; Déchelotte, P. L-Arginine Modulates CXC Chemokines in the Human Intestinal Epithelial Cell Line HCT-8 by the NO Pathway. Biochimie 2005, 87, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S.; Binion, D.G.; Nelson, V.M.; Kanaa, Y.; Javadi, P.; Lazarova, Z.; Andrekopoulos, C.; Kalyanaraman, B.; Otterson, M.F.; Rafiee, P. Increased Arginase Activity and Endothelial Dysfunction in Human Inflammatory Bowel Disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1323–G1336. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Mbodji, K.; Hassan, A.; Aziz, M.; Boukhettala, N.; Coëffier, M.; Savoye, G.; Déchelotte, P.; Marion-Letellier, R. Anti-Inflammatory and Anti-Angiogenic Effect of Long Chain n-3 Polyunsaturated Fatty Acids in Intestinal Microvascular Endothelium. Clin. Nutr. Edinb. Scotl. 2011, 30, 678–687. [Google Scholar] [CrossRef]
- Hu, S.; Bae, M.; Park, Y.-K.; Lee, J.-Y. N-3 PUFAs Inhibit TGFβ1-Induced Profibrogenic Gene Expression by Ameliorating the Repression of PPARγ in Hepatic Stellate Cells. J. Nutr. Biochem. 2020, 85, 108452. [Google Scholar] [CrossRef]
- Maeshige, N.; Torii, K.; Tabuchi, H.; Imai, M.; Koga, Y.; Uemura, M.; Aoyama-Ishikawa, M.; Miyoshi, M.; Fujino, H.; Terashi, H.; et al. Inhibitory Effects of Short-Chain Fatty Acids and ω-3 Polyunsaturated Fatty Acids on Profibrotic Factors in Dermal Fibroblasts. Eplasty 2019, 19, e4. [Google Scholar] [PubMed]
- Zeng, Z.; Yang, H.; Wang, Y.; Ren, J.; Dai, Y.; Dai, C. Omega-3 Polyunsaturated Fatty Acids Attenuate Fibroblast Activation and Kidney Fibrosis Involving MTORC2 Signaling Suppression. Sci. Rep. 2017, 7, 46146. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, L.; Xia, H.; Chen, L.; Cui, S.; Wang, Y.; Zhou, T.; Xiong, W.; Song, L.; Li, S.; et al. Resolvin D1 Attenuates Mechanical Stretch-Induced Pulmonary Fibrosis via Epithelial-Mesenchymal Transition. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L1013–L1024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Dejanovic, D.; Yao, P.; Bhilocha, S.; Sadler, T.; Schirbel, A.; West, G.; Doyon, G.; Lopez, R.; Mao, R.; et al. Selective Deletion of MyD88 Signaling in α-SMA Positive Cells Ameliorates Experimental Intestinal Fibrosis via Post-Transcriptional Regulation. Mucosal Immunol. 2020. [Google Scholar] [CrossRef]
- Jacob, N.; Jacobs, J.P.; Kumagai, K.; Ha, C.W.Y.; Kanazawa, Y.; Lagishetty, V.; Altmayer, K.; Hamill, A.M.; Von Arx, A.; Sartor, R.B.; et al. Inflammation-Independent TL1A-Mediated Intestinal Fibrosis Is Dependent on the Gut Microbiome. Mucosal Immunol. 2018, 11, 1466–1476. [Google Scholar] [CrossRef] [Green Version]
- Imai, J.; Kitamoto, S.; Sugihara, K.; Nagao-Kitamoto, H.; Hayashi, A.; Morhardt, T.L.; Kuffa, P.; Higgins, P.D.R.; Barnich, N.; Kamada, N. Flagellin-Mediated Activation of IL-33-ST2 Signaling by a Pathobiont Promotes Intestinal Fibrosis. Mucosal Immunol. 2019, 12, 632–643. [Google Scholar] [CrossRef]
- Sivignon, A.; de Vallée, A.; Barnich, N.; Denizot, J.; Darcha, C.; Pignède, G.; Vandekerckove, P.; Darfeuille-Michaud, A. Saccharomyces Cerevisiae CNCM I-3856 Prevents Colitis Induced by AIEC Bacteria in the Transgenic Mouse Model Mimicking Crohn’s Disease. Inflamm. Bowel Dis. 2015, 21, 276–286. [Google Scholar] [CrossRef]
- Leccese, G.; Bibi, A.; Mazza, S.; Facciotti, F.; Caprioli, F.; Landini, P.; Paroni, M. Probiotic Lactobacillus and Bifidobacterium Strains Counteract Adherent-Invasive Escherichia Coli (AIEC) Virulence and Hamper IL-23/Th17 Axis in Ulcerative Colitis, but Not in Crohn’s Disease. Cells 2020, 9, 1824. [Google Scholar] [CrossRef]
- Ogawa, H.; Rafiee, P.; Fisher, P.J.; Johnson, N.A.; Otterson, M.F.; Binion, D.G. Sodium Butyrate Inhibits Angiogenesis of Human Intestinal Microvascular Endothelial Cells through COX-2 Inhibition. FEBS Lett. 2003, 554, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, X.-L.; Qi, F.-F.; Pang, Z.-L. Berberine Inhibits Epithelial-Mesenchymal Transition and Promotes Apoptosis of Tumour-Associated Fibroblast-Induced Colonic Epithelial Cells through Regulation of TGF-β Signalling. J. Cell Commun. Signal. 2020, 14, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de la Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-Induced Endothelial-to-Mesenchymal Transition: A Novel Mechanism of Intestinal Fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef] [PubMed]
- Binion, D.G.; Heidemann, J.; Li, M.S.; Nelson, V.M.; Otterson, M.F.; Rafiee, P. Vascular Cell Adhesion Molecule-1 Expression in Human Intestinal Microvascular Endothelial Cells Is Regulated by PI 3-Kinase/Akt/MAPK/NF-ΚB: Inhibitory Role of Curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G259–G268. [Google Scholar] [CrossRef] [Green Version]
- Rafiee, P.; Binion, D.G.; Wellner, M.; Behmaram, B.; Floer, M.; Mitton, E.; Nie, L.; Zhang, Z.; Otterson, M.F. Modulatory Effect of Curcumin on Survival of Irradiated Human Intestinal Microvascular Endothelial Cells: Role of Akt/MTOR and NF-{kappa}B. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G865–G877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, E.; Choi, Y.J.; Kim, C.H.; Fiocchi, C.; Pothoulakis, C.; Rhee, S.H. The Angiogenic Effect of Probiotic Bacillus Polyfermenticus on Human Intestinal Microvascular Endothelial Cells Is Mediated by IL-8. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G999–G1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont-Lucas, C.; Marion-Letellier, R.; Pala, M.; Guerin, C.; Amamou, A.; Jarbeau, M.; Bôle-Feysot, C.; Nicol, L.; David, A.; Aziz, M.; et al. A Polymeric Diet Rich in Transforming Growth Factor Beta 2 Does Not Reduce Inflammation in Chronic 2,4,6-Trinitrobenzene Sulfonic Acid Colitis in Pre-Pubertal Rats. BMC Gastroenterol. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Agista, A.Z.; Rusbana, T.B.; Islam, J.; Ohsaki, Y.; Sultana, H.; Hirakawa, R.; Watanabe, K.; Nochi, T.; Ardiansyah; Budijanto, S.; et al. Fermented Rice Bran Supplementation Prevents the Development of Intestinal Fibrosis Due to DSS-Induced Inflammation in Mice. Nutrients 2021, 13, 1869. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef] [Green Version]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, Pathophysiology and Management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, P.; Yilmaz, B.; Rossel, J.-B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or Gluten-Free Diets in Patients with Inflammatory Bowel Disease Are Associated with Lower Psychological Well-Being and a Different Gut Microbiota, but No Beneficial Effects on the Course of the Disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouvari, M.; Boutari, C.; Chrysohoou, C.; Fragkopoulou, E.; Antonopoulou, S.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B.; Mantzoros, C.S.; ATTICA study Investigators. Mediterranean Diet Is Inversely Associated with Steatosis and Fibrosis and Decreases Ten-Year Diabetes and Cardiovascular Risk in NAFLD Subjects: Results from the ATTICA Prospective Cohort Study. Clin. Nutr. Edinb. Scotl. 2021, 40, 3314–3324. [Google Scholar] [CrossRef] [PubMed]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marion-Letellier, R.; Leboutte, M.; Amamou, A.; Raman, M.; Savoye, G.; Ghosh, S. Diet in Intestinal Fibrosis: A Double-Edged Sword. Nutrients 2021, 13, 3148. https://doi.org/10.3390/nu13093148
Marion-Letellier R, Leboutte M, Amamou A, Raman M, Savoye G, Ghosh S. Diet in Intestinal Fibrosis: A Double-Edged Sword. Nutrients. 2021; 13(9):3148. https://doi.org/10.3390/nu13093148
Chicago/Turabian StyleMarion-Letellier, Rachel, Mathilde Leboutte, Asma Amamou, Maitreyi Raman, Guillaume Savoye, and Subrata Ghosh. 2021. "Diet in Intestinal Fibrosis: A Double-Edged Sword" Nutrients 13, no. 9: 3148. https://doi.org/10.3390/nu13093148
APA StyleMarion-Letellier, R., Leboutte, M., Amamou, A., Raman, M., Savoye, G., & Ghosh, S. (2021). Diet in Intestinal Fibrosis: A Double-Edged Sword. Nutrients, 13(9), 3148. https://doi.org/10.3390/nu13093148







