A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Subject Selection
2.3. Product Intervention and Hp Eradication Treatment
2.4. Clinical Outcomes
2.5. Biological Outcomes
2.6. Procedure
2.7. Sample Size
2.8. Randomisation
2.9. Statistical Analysis
2.9.1. Clinical Outcomes
2.9.2. SCFA, Calprotectin, and Quantification of Test Product Strains in Feces
2.9.3. Gut Microbiota
3. Results
3.1. Subject Enrollment, Population at Baseline, and Compliance
3.2. Clinical Outcomes
3.3. Test Product Strains Viability in Feces
3.4. Global Gut Microbiota Response to Hp Treatment and Product Intervention
3.5. Taxonomical Analysis by Quantitative Microbiome Profiling
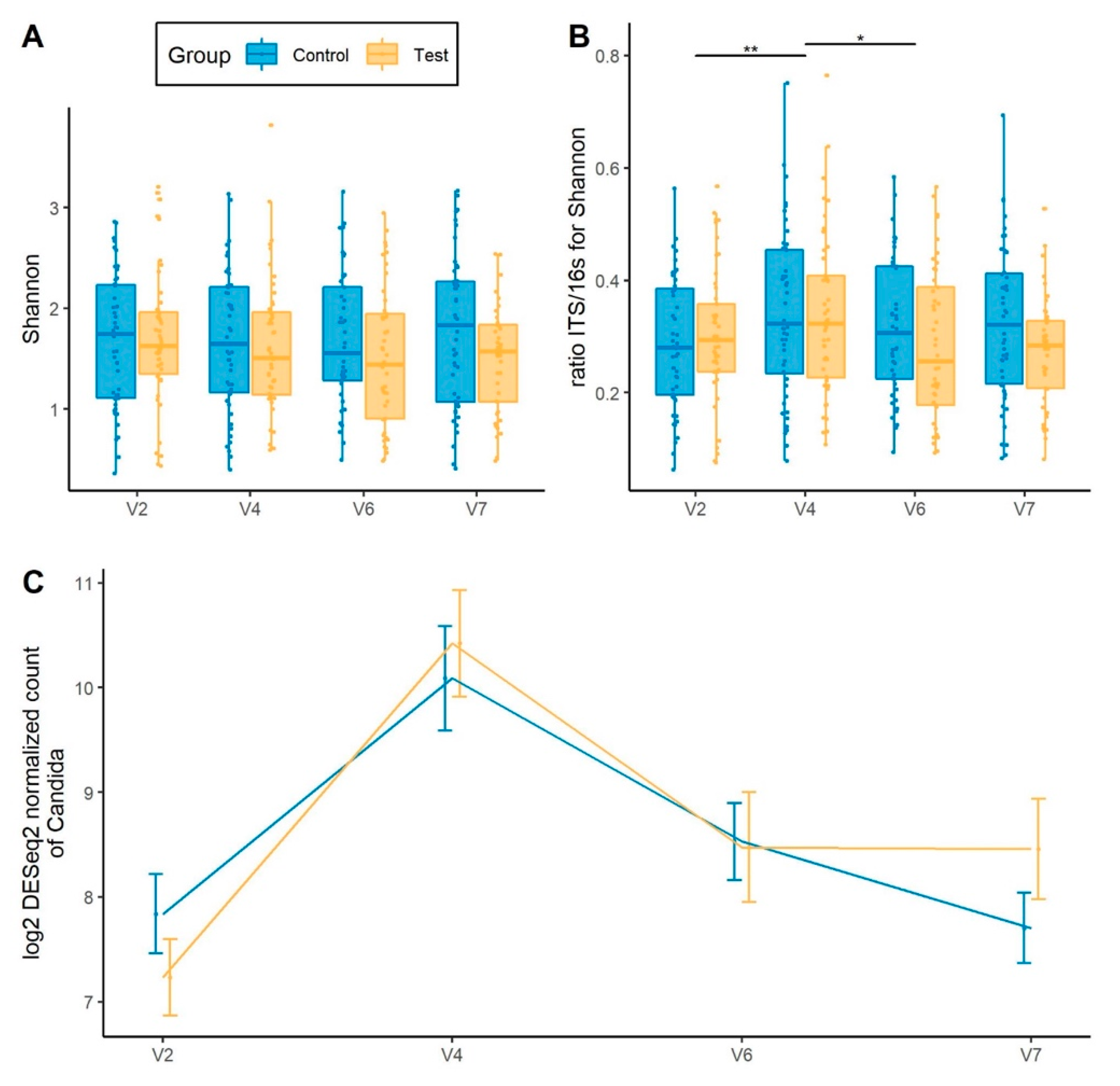
3.6. Gut Mycobiota Response to Hp Treatment and Product Intervention
3.7. SCFA and Calprotectin Response to H. pylori Treatment and Product Intervention
3.8. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Macke, L.; Schulz, C.; Koletzko, L.; Malfertheiner, P. Systematic review: The effects of proton pump inhibitors on the microbiome of the digestive tract-evidence from next-generation sequencing studies. Aliment. Pharmacol. Ther. 2020, 51, 505–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef] [Green Version]
- Malfertheiner, P.; Link, A.; Selgrad, M. Helicobacter pylori: Perspectives and time trends. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 628–638. [Google Scholar] [CrossRef]
- Fallone, C.A.; Moss, S.F.; Malfertheiner, P. Reconciliation of recent Helicobacter pylori treatment guidelines in a time of increasing resistance to antibiotics. Gastroenterology 2019, 157, 44–53. [Google Scholar] [CrossRef]
- Ye, Q.; Shao, X.; Shen, R.; Chen, D.; Shen, J. Changes in the human gut microbiota composition caused by Helicobacter pylori eradication therapy: A systematic review and meta-analysis. Helicobacter 2020, 25, e12713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Huang, Y. Effect of Lactobacillus acidophilus and Bifidobacterium bifidum supplementation to standard triple therapy on Helicobacter pylori eradication and dynamic changes in intestinal flora. World J. Microbiol. Biotechnol. 2014, 30, 847–853. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Sun, G.; Peng, L.; Lu, Z.; Yan, B.; Huang, K.; Yang, Y. Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in duodenal ulcer. Sci. Rep. 2019, 9, 12874. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, D.; Kathagen, G.; D’hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Wullt, M.; Johansson Hagslätt, M.-L.; Odenholt, I.; Berggren, A. Lactobacillus plantarum 299v enhances the concentrations of fecal short-chain fatty acids in patients with recurrent clostridium difficile-associated diarrhea. Dig. Dis. Sci. 2007, 52, 2082–2086. [Google Scholar] [CrossRef] [PubMed]
- Koning, C.J.; Jonkers, D.M.; Stobberingh, E.E.; Mulder, L.; Rombouts, F.M.; Stockbrügger, R.W. The effect of a multispecies probiotic on the intestinal microbiota and bowel movements in healthy volunteers taking the antibiotic amoxycillin. Am. J. Gastroenterol. 2008, 103, 178–189. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.M.; Qian, W.; Qin, Y.Y.; He, J.; Zhou, Y.H. Probiotics in Helicobacter pylori eradication therapy: A systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 4345–4357. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Kołodziej, M. Systematic review with meta-analysis: Lactobacillus rhamnosus GG in the prevention of antibiotic-associated diarrhoea in children and adults. Aliment. Pharmacol. Ther. 2015, 42, 1149–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McFarland, L.V.; Huang, Y.; Wang, L.; Malfertheiner, P. Systematic review and meta-analysis: Multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United Eur. Gastroenterol. J. 2015, 4, 546–561. [Google Scholar] [CrossRef] [Green Version]
- Hickson, M.; D’Souza, A.L.; Muthu, N.; Rogers, T.R.; Want, S.; Rajkumar, C.; Bulpitt, C.J. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: Randomised double blind placebo controlled trial. BMJ 2007, 335, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, C.G.; Kottmann, T.; Alavi, M. Commercially available probiotic drinks containing Lactobacillus casei DN-114001 reduce antibiotic-associated diarrhea. World J. Gastroenterol. 2014, 20, 15837–15844. [Google Scholar] [CrossRef] [PubMed]
- Sýkora, J.; Valecková, K.; Amlerová, J.; Siala, K.; Dedek, P.; Watkins, S.; Varvarovská, J.; Stožický, F.; Pazdiora, P.; Schwarz, J. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: A prospective randomized double-blind study. J. Clin. Gastroenterol. 2005, 39, 692–698. [Google Scholar] [CrossRef]
- Alvarez, A.; Tap, J.; Chambaud, I.; Cools-Portier, S.; Quinquis, L.; Bourlioux, P.; Marteau, P.; Guillemard, E.; Schrezenmeir, J.; Derrien, M. Consumption of a mix of strains in a fermented milk product is safe and functionally enriches the human gut microbiome of healthy subjects. Sci. Rep. 2020, 10, 15974. [Google Scholar] [CrossRef]
- World Health Organization. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers, 4th Rev.; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Duman, D.G.; Bor, S.; Ozütemiz, O.; Sahin, T.; Oğuz, D.; Iştan, F.; Vural, T.; Sandkci, M.; Işksal, F.; Simşek, I.; et al. Efficacy and safety of Saccharomyces boulardii in prevention of antibiotic-associated diarrhoea due to Helicobacter pylori eradication. Eur. J. Gastroenterol. Hepatol. 2005, 17, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Emara, M.H.; Mohamed, S.Y.; Abdel-Aziz, H.R. Lactobacillus reuteri in management of Helicobacter pylori infection in dyspeptic patients: A double-blind placebo-controlled randomized clinical trial. Ther. Adv. Gastroenterol. 2014, 7, 4–13. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, C.; Wilks, M.; Islam, J.; Ali, K.; Raftery, J.; Davies, K.A.; Timeyin, J.; Cheek, E.; Cohen, J.; Wright, J.; et al. Do probiotics prevent antibiotic-associated diarrhoea? Results of a multicentre randomized placebo-controlled trial. J. Hosp. Infect. 2020, 105, 280–288. [Google Scholar] [CrossRef]
- Yuan, Y.; Ford, A.C.; Khan, K.J.; Gisbert, J.P.; Forman, D.; Leontiadis, G.I.; Tse, F.; Calvet, X.; Fallone, C.; Fischbach, L.; et al. Optimum duration of regimens for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2013, CD008337. [Google Scholar] [CrossRef]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Bang, C.; Franke, A.; Zimmermann, K.; Nauck, M.; Völker, U.; Völzke, H.; Biffar, R.; et al. Helicobacter pylori infection associates with fecal microbiota composition and diversity. Sci. Rep. 2019, 9, 20100. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sun, J.; Luo, H.; Ren, H.; Zhou, H.; Lin, Y.; Han, M.; Chen, B.; Liao, H.; Brix, S.; et al. Assessment of fecal DNA extraction protocols for metagenomic studies. GigaScience 2020, 9, giaa071. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.-M.; Chen, C.-C.; Chang, C.-M.; Fang, Y.-J.; Bair, M.-J.; Chen, P.-Y.; Chang, C.-Y.; Hsu, Y.-C.; Chen, M.-J.; Chen, C.-C.; et al. Long-term changes of gut microbiota, antibiotic resistance, and metabolic parameters after Helicobacter pylori eradication: A multicentre, open-label, randomised trial. Lancet Infect. Dis. 2019, 19, 1109–1120. [Google Scholar] [CrossRef]
- Yanagi, H.; Tsuda, A.; Matsushima, M.; Takahashi, S.; Ozawa, G.; Koga, Y.; Takagi, A. Changes in the gut microbiota composition and the plasma ghrelin level in patients with Helicobacter pylori-infected patients with eradication therapy. BMJ Open Gastroenterol. 2017, 4, e000182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.K.; Kim, M.; Bakshi, U.; Cunningham, K.Y.; Davis, J.M.; Lazaridis, K.N.; Nelson, H.; Chia, N.; Sung, J. A predictive index for health status using species-level gut microbiome profiling. Nat. Commun. 2020, 11, 4635. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423.e1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tap, J.; Ruppé, E.; Derrien, M. The human gut microbiota in all its states: From disturbance to resilience. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Crouzet, L.; Derrien, M.; Cherbuy, C.; Plancade, S.; Foulon, M.; Chalin, B.; van Hylckama Vlieg, J.E.T.; Grompone, G.; Rigottier-Gois, L.; Serror, P. Lactobacillus paracasei CNCM I-3689 reduces vancomycin-resistant Enterococcus persistence and promotes Bacteroidetes resilience in the gut following antibiotic challenge. Sci. Rep. 2018, 8, 5098. [Google Scholar] [CrossRef] [Green Version]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.-L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Bühling, A.; Radun, D.; Müller, W.A.; Malfertheiner, P. Influence of anti-Helicobacter triple-therapy with metronidazole, omeprazole and clarithromycin on intestinal microflora. Aliment. Pharmacol. Ther. 2001, 15, 1445–1452. [Google Scholar] [CrossRef]
- Lino, T.; Mori, K.; Tanaka, K.; Suzuki, K.I.; Harayama, S. Oscillibacter valericigenes gen. nov., sp. nov., a valerate-producing anaerobic bacterium isolated from the alimentary canal of a Japanese corbicula clam. Int. J. Syst. Evol. Microbiol. 2007, 57, 1840–1845. [Google Scholar] [CrossRef] [Green Version]
- Djouzi, Z.; Andrieux, C.; Degivry, M.-C.; Bouley, C.; Szylit, O. The association of yogurt starters with Lactobacillus casei DN 114.001 in fermented milk alters the composition and metabolism of intestinal microflora in germ-free rats and in human flora–associated rats. J. Nutr. 1997, 127, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Veiga, P.; Gallini, C.A.; Beal, C.; Michaud, M.; Delaney, M.L.; DuBois, A.; Khlebnikov, A.; van Hylckama Vlieg, J.E.T.; Punit, S.; Glickman, J.N.; et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc. Natl. Acad. Sci. USA 2010, 107, 18132–18137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myllyluoma, E.; Veijola, L.; Ahlroos, T.; Tynkkynen, S.; Kankuri, E.; Vapaatalo, H.; Rautelin, H.; Korpela, R. Probiotic supplementation improves tolerance to Helicobacter pylori eradication therapy--a placebo-controlled, double-blind randomized pilot study. Aliment. Pharmacol. Ther. 2005, 21, 1263–1272. [Google Scholar] [CrossRef]
- Cox, A.J.; Makino, H.; Cripps, A.W.; West, N.P. Recovery of Lactobacillus casei strain Shirota (LcS) from faeces with 14 days of fermented milk supplementation in healthy Australian adults. Asia Pac. J. Clin. Nutr. 2019, 28, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Martín, R.; Chamignon, C.; Mhedbi-Hajri, N.; Chain, F.; Derrien, M.; Escribano-Vázquez, U.; Garault, P.; Cotillard, A.; Pham, H.P.; Chervaux, C.; et al. The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response. Sci. Rep. 2019, 9, 5398. [Google Scholar] [CrossRef] [Green Version]
- Shastri, Y.M.; Bergis, D.; Povse, N.; Schäfer, V.; Shastri, S.; Weindel, M.; Ackermann, H.; Stein, J. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am. J. Med. 2008, 121, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Barbut, F.; Gouot, C.; Lapidus, N.; Suzon, L.; Syed-Zaidi, R.; Lalande, V.; Eckert, C. Faecal lactoferrin and calprotectin in patients with Clostridium difficile infection: A case-control study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2423–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundgren, D.; Eklöf, V.; Palmqvist, R.; Hultdin, J.; Karling, P. Proton pump inhibitor use is associated with elevated faecal calprotectin levels. A cross-sectional study on subjects referred for colonoscopy. Scand. J. Gastroenterol. 2019, 54, 152–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.; Camilli, G.; Griffiths, J.S.; Richardson, J.P.; Kichik, N.; Naglik, J.R. Candida albicans and candidalysin in inflammatory disorders and cancer. Immunology 2021, 162, 11–16. [Google Scholar] [CrossRef] [PubMed]




| Test (N = 68) | Control (N = 68) | ||
|---|---|---|---|
| Age (years) | Mean (SD) | 42.1 (10.1) | 42.6 (11.3) |
| Min; Max | 26; 65 | 23; 64 | |
| Sex, n (%) | Male | 34 (50.0) | 35 (51.5) |
| Female | 34 (50.0) | 33 (48.5) | |
| BMI (kg/m²) | Mean (SD) | 24.8 (2.9) | 25.0 (2.6) |
| Min; Max | 19.6; 30.0 | 19.7; 29.5 | |
| Alcohol Consumer, n (%) | No | 17 (25.0) | 24 (35.3) |
| Yes | 51 (75.0) | 44 (64.7) | |
| Alcohol units/week 1 | Mean (SD) | 2.2 (1.8) | 2.3 (1.4) |
| Min; Max | 1; 8 | 1; 7 | |
| Smoking status, n (%) | Current smoker | 19 (27.9) | 14 (20.6) |
| Ex-smoker | 20 (29.4) | 20 (29.4) | |
| Never a smoker | 29 (42.6) | 34 (50.0) | |
| Number of cigarettes/week 2 | Mean (SD) | 41.2 (39.9) | 58.1 (40.8) |
| Min; Max | 1; 140 | 1; 105 | |
| Physical activity IPAQ, n (%) | Low | 8 (11.8) | 8 (11.8) |
| Moderate | 24 (35.3) | 22 (32.4) | |
| High | 36 (52.9) | 38 (55.9) | |
| Medical/Surgical history, n (%) | 49 (72.1) | 47 (69.1) | |
| Concomitant medication or nutritional supplements, n (%) | 38 (55.9) | 27 (39.7) | |
| Product restricted during the study, n (%) | 0 | 0 |
| Test (N = 68) | Control (N = 68) | |
|---|---|---|
| AAD occurrence—definition 1, n (%) | 2 (2.9) | 1 (1.5) |
| AAD occurrence—definition 2, n (%) | 5 (7.4) | 3 (4.4) |
| CDAD occurrence—definition 1 or 2, n (%) | 0 | 0 |
| Cd positive test at baseline (V2), n (%) | 0 | 0 |
| Cd positive test between V2 and V6, n (%) | 2 (2.9) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillemard, E.; Poirel, M.; Schäfer, F.; Quinquis, L.; Rossoni, C.; Keicher, C.; Wagner, F.; Szajewska, H.; Barbut, F.; Derrien, M.; et al. A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy. Nutrients 2021, 13, 3171. https://doi.org/10.3390/nu13093171
Guillemard E, Poirel M, Schäfer F, Quinquis L, Rossoni C, Keicher C, Wagner F, Szajewska H, Barbut F, Derrien M, et al. A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy. Nutrients. 2021; 13(9):3171. https://doi.org/10.3390/nu13093171
Chicago/Turabian StyleGuillemard, Eric, Marion Poirel, Florent Schäfer, Laurent Quinquis, Caroline Rossoni, Christian Keicher, Frank Wagner, Hania Szajewska, Frédéric Barbut, Muriel Derrien, and et al. 2021. "A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy" Nutrients 13, no. 9: 3171. https://doi.org/10.3390/nu13093171
APA StyleGuillemard, E., Poirel, M., Schäfer, F., Quinquis, L., Rossoni, C., Keicher, C., Wagner, F., Szajewska, H., Barbut, F., Derrien, M., & Malfertheiner, P. (2021). A Randomised, Controlled Trial: Effect of a Multi-Strain Fermented Milk on the Gut Microbiota Recovery after Helicobacter pylori Therapy. Nutrients, 13(9), 3171. https://doi.org/10.3390/nu13093171






