Vitamin B6 in Health and Disease
Abstract
:1. Introduction
2. Some Metabolic Functions of PLP in Health
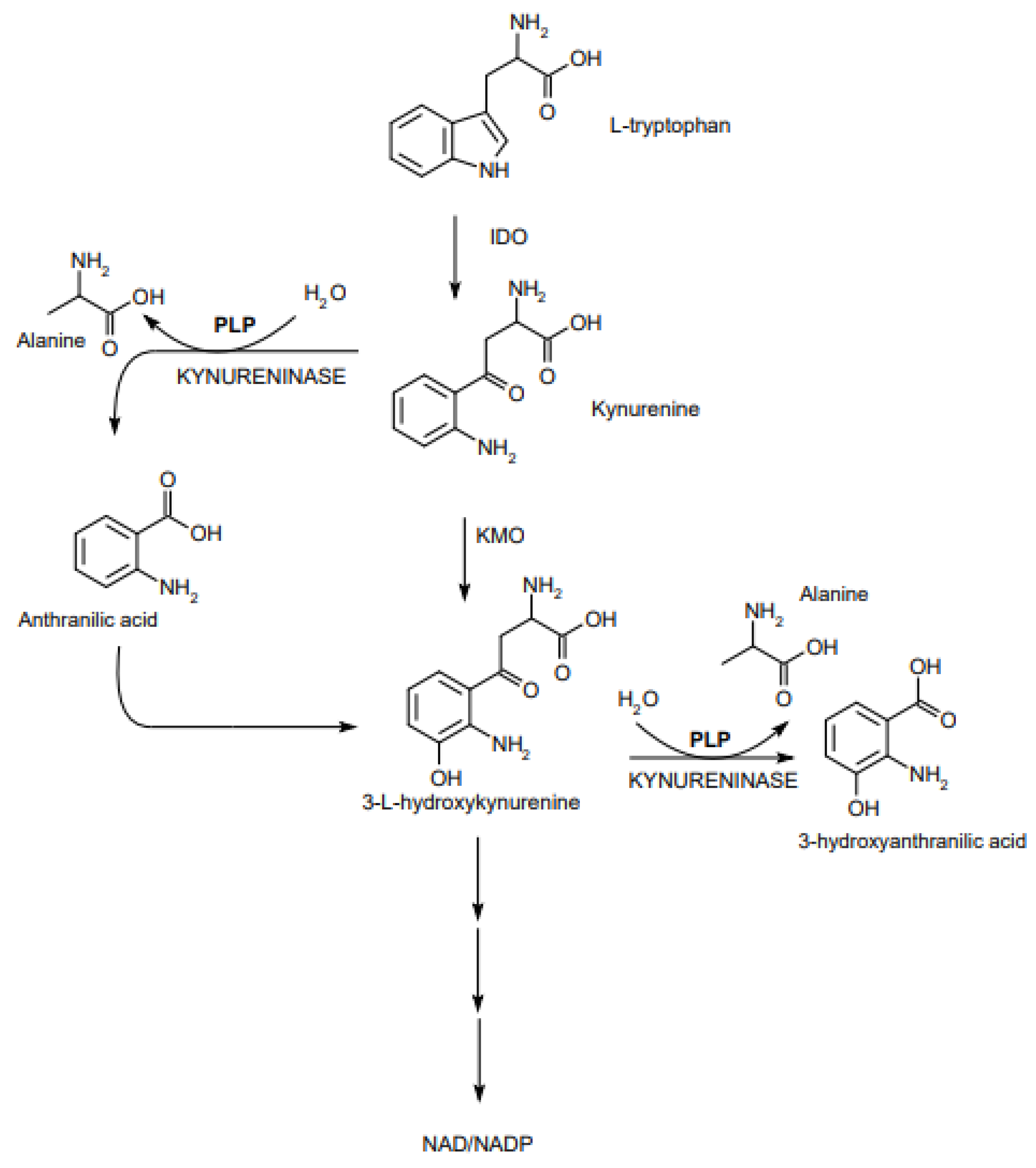
2.1. Role of PLP in Metabolism of S1P and Tryptophan
2.2. Role of PLP in Other Pathways
3. Vitamin B6 and Diseases
3.1. Vitamin B6 and Diabetes
3.2. Vitamin B6, Immunity and Inflammation
3.3. Vitamin B6 and Cancer
3.4. Vitamin B6 and Cardiovascular Diseases and Pneumonia
3.5. Vitamin B6 and COVID-19
3.6. Some Other Aspects of Vitamin B6
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hellmann, H.; Mooney, S. Vitamin B6: A Molecule for Human Health? Molecules 2010, 15, 442–459. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.K.; Christen, P. The molecular evolution of pyridoxal-5′-phosphate-dependent enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 2000, 74, 129–184. [Google Scholar] [CrossRef]
- Di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin B6 salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2011, 1814, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; McCann, A.; Midttun, Ø.; Ulvik, A. Inflammation, vitamin B6 and related pathways. Mol. Asp. Med. 2016, 53, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Salata, D.; Budkowska, M.; Dolegowska, B. Sphingosine-1-phosphate--molecular maestro. Postepy Biochem. 2012, 58, 281–291. [Google Scholar]
- Desbarats, J. Pyridoxal 5′-phosphate to mitigate immune dysregulation and coagulopathy in COVID-19. Preprints 2020, 2. [Google Scholar] [CrossRef]
- Zhang, P.; Tsuchiya, K.; Kinoshita, T.; Kushiyama, H.; Suidasari, S.; Hatakeyama, M.; Imura, H.; Kato, N.; Suda, T. Vitamin B6 Prevents IL-1β Protein Production by Inhibiting NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 24517–24527. [Google Scholar] [CrossRef] [Green Version]
- Hajsl, M.; Hlavackova, A.; Broulikova, K.; Sramek, M.; Maly, M.; Dyr, J.E.; Suttnar, J. Tryptophan Metabolism, Inflammation, and Oxidative Stress in Patients with Neurovascular Disease. Metabolites 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Cervenka, I.; Agudelo, L.Z.; Ruas, J.L. Kynurenines: Tryptophan’s metabolites in exercise, inflammation, and mental health. Science 2017, 357, 357–369. [Google Scholar] [CrossRef] [Green Version]
- Bessede, A.; Gargaro, M.; Pallotta, M.T.; Matino, D.; Brunacci, C.; Bicciato, S.; Mazza, E.M.C.; Macchiarulo, A.; Vacca, C.; Iannitti, R.; et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 2015, 511, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Walden, M.; Tian, L.; Ross, R.; Sykora, U.M.; Byrne, D.P.; Hesketh, E.L.; Masandi, S.K.; Cassel, J.; George, R.; Ault, J.R.; et al. Metabolic control of BRISC-SHMT2 assembly regulates immune signalling. Nature 2019, 570, 194–199. [Google Scholar] [CrossRef]
- Snell, L.M.; Brooks, D.G. New insights into type I interferon and the immunopathogenesis of persistent viral infections. Curr. Opin. Immunol. 2015, 34, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Martí-Carvajal, A.J.; Solà, I.; Lathyris, D.; Salanti, G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst. Rev. 2009, CD006612. [Google Scholar] [CrossRef] [Green Version]
- Keenan, H.A.; Costacou, T.; Sun, J.K.; Doria, A. Cinical Factors associated with resistance to Microvascular Complications in Diabetic. Diabetes Care 2007, 30, 1995–1997. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, S.; Li, H.; Adijiang, A.; Pischetsrieder, M.; Niwa, T. Pyridoxal phosphate prevents progression of diabetic nephropathy. Nephrol. Dial. Transplant. 2007, 22, 2165–2174. [Google Scholar] [CrossRef] [Green Version]
- Nix, W.A.; Zirwes, R.; Bangert, V.; Kaiser, R.P.; Schilling, M.; Hostalek, U.; Obeid, R. Vitamin B status in patients with type 2 diabetes mellitus with and without incipient nephropathy. Diabetes Res. Clin. Pract. 2015, 107, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Satyanarayana, A.; Balakrishna, N.; Pitla, S.; Reddy, P.Y.; Mudili, S.; Lopamudra, P.; Suryanarayana, P.; Viswanath, K.; Ayyagari, R.; Reddy, G.B. Status of B-Vitamins and homocysteine in diabetic retinopathy: Association with Vitamin-B12 deficiency and hyperhomocysteinemia. PLoS ONE 2011, 6, e26747. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.; Nakamura, Y.; Fukui, T.; Fukuwatari, T.; Ugi, S.; Maegawa, H.; Doi, Y.; Shibata, K. Concentrations of Water-Soluble Vitamins in Blood and Urinary Excretion in Patients with Diabetes Mellitus. Nutr. Metab. Insights 2016, 9, 85. [Google Scholar] [CrossRef]
- Rogers, K.; Higgins, E.; Kline, E. Experimental diabetes causes mitochondrial loss and cytoplasmic enrichment of pyridoxal phosphate and aspartate aminotransferase activity. Biochem. Med. Metab. Biol. 1986, 36, 91–97. [Google Scholar] [CrossRef]
- Okada, M.; Shibuya, M.; Yamamoto, E.; Murakami, Y. Effect of diabetes on vitamin B6 requirement in experimental animals. Diabetes Obes. Metab. 1999, 1, 221–225. [Google Scholar] [CrossRef]
- Leklem, J.E.; Hollenbeck, C.B. Acute ingestion of glucose decreases plasma pyridoxal 5′-phosphate and total vitamin B-6 concentration. Am. J. Clin. Nutr. 1990, 51, 832–836. [Google Scholar] [CrossRef] [Green Version]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory linking obesity and metabolic disease and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Toyota, T.; Kai, Y.; Kakizaki, M.; Ohtsuka, H.; Shibata, Y.; Goto, Y. The Endocrine Pancreas in Pyridoxine Deficient Rats. Tohoku J. Exp. Med. 1981, 134, 331–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubí, B. Pyridoxal 5′-phosphate (PLP) deficiency might contribute to the onset of type I diabetes. Med. Hypotheses 2012, 78, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G. Insuline resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol. Neurobiol. 2013, 48, 294–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, A.M.; Welle, K.; Ho, E.S.; Mesaros, C.; Susiarjo, M. Vitamin B6 deficiency disrupts serotonin signaling in pancreatic islets and induces gestational diabetes in mice. Commun. Biol. 2021, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Kumrungsee, T.; Zhang, P.; Chartkul, M.; Yanaka, N.; Kato, N. Potential Role of Vitamin B6 in Ameliorating the Severity of COVID-19 and Its Complications. Front. Nutr. 2020, 7, 562051. [Google Scholar] [CrossRef]
- Qian, B.; Shen, S.; Zhang, J.; Jing, P. Effects of Vitamin B6 Deficiency on the Composition and Functional Potential of T Cell Populations. J. Immunol. Res. 2017, 2017, 2197975. [Google Scholar] [CrossRef] [Green Version]
- Biesalski, H.K. Nutrition meets the microbiome: Micronutrients and the microbiota. Ann. N. Y. Acad. Sci. 2016, 1372, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Hosomi, K.; Sawane, K.; Kunisawa, J. Metabolism of dietary and microbial vitamin b family in the regulation of host immunity. Front. Nutr. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakakeeny, L.; Roubenoff, R.; Obin, M.; Fontes, J.D.; Benjamin, E.J.; Bujanover, Y.; Jacques, P.F.; Selhub, J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. Adults. J. Nutr. 2012, 142, 1280–1285. [Google Scholar] [CrossRef] [Green Version]
- Elmadfa, I.; Meyer, A.L. The Role of the Status of Selected Micronutrients in Shaping the Immune Function. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 1100–1115. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkelsen, K.; Prakash, M.D.; Kuol, N.; Nurgali, K.; Stojanovska, L.; Apostolopoulos, V. Anti-tumor effects of vitamin B2, B6 and B9 in promonocytic lymphoma cells. Int. J. Mol. Sci. 2019, 20, 3763. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Suda, T.; Suidasari, S.; Kumrungsee, T.; Yanaka, N.; Kato, N. Novel Preventive Mechanisms of Vitamin B6 against Inflammation, Inflammasome, and Chronic Diseases; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128119075. [Google Scholar]
- Wei, D.H.; Mao, Q.Q. Vitamin B6, vitamin B12 and methionine and risk of pancreatic cancer: A metaanalysis. Nutr. J. 2020, 19, 111. [Google Scholar] [CrossRef]
- Mocellin, S.; Briarava, M.; Pilati, P. Vitamin B6 and cancer risk: A field synopsis and meta-analysis. J. Natl. Cancer Inst. 2017, 109, djw230. [Google Scholar] [CrossRef]
- Friso, S.; Jacques, P.F.; Wilson, P.W.F.; Rosenberg, I.H.; Selhub, J. Low circulating vitamin B6 is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 2001, 103, 2788–2791. [Google Scholar] [CrossRef] [Green Version]
- Deluyker, D.; Ferferieva, V.; Driesen, R.B.; Verboven, M.; Lambrichts, I.; Bito, V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci. Rep. 2017, 7, 16010. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.; Park, K. Dietary vitamin B6 intake associated with a decreased risk of cardiovascular disease: A prospective cohort study. Nutrients 2019, 11, 1484. [Google Scholar] [CrossRef] [Green Version]
- Page, J.H.; Ma, J.; Chiuve, S.E.; Stampfer, M.J.; Selhub, J.; Manson, J.E.; Rimm, E.B. Plasma Vitamin B6 and Risk of Myocardial Infarction in Women. Circulation 2009, 120, 649. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.C.; Wei, J.C.C.; Wu, D.J.; Huang, Y.C. Vitamin B6 supplementation improves pro-inflammatory responses in patients with rheumatoid arthritis. Eur. J. Clin. Nutr. 2010, 64, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dakshinamurti, K. Vitamins and their derivatives in the prevention and treatment of metabolic syndrome diseases (Diabetes)1,2. Can. J. Physiol. Pharmacol. 2015, 93, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Suidasari, S.; Hasegawa, T.; Yanaka, N.; Kato, N. Dietary supplemental vitamin B6 increases carnosine and anserine concentrations in the heart of rats. Springerplus 2015, 4, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suidasari, S.; Stautemas, J.; Uragami, S.; Yanaka, N.; Derave, W.; Kato, N. Carnosine Content in Skeletal Muscle Is Dependent on Vitamin B6 Status in Rats. Front. Nutr. 2016, 2, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumrungsee, T.; Zhang, P.; Yanaka, N.; Suda, T.; Kato, N. Emerging cardioprotective mechanisms of vitamin B6: A narrative review. Eur. J. Nutr. 2021. published online. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.R.; Zhou, S.N.; Fu, C.N.; Song, J.W.; Wang, X.Q.; Bai, W.W.; Li, P.; Song, P.; Zhu, M.L.; Ma, Z.M.; et al. Vitamin B6 inhibits macrophage activation to prevent lipopolysaccharide-induced acute pneumonia in mice. J. Cell. Mol. Med. 2020, 24, 3139–3148. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Lengyel, C.O.; Whiting, S.J.; Zello, G.A. Nutrient inadequacies among elderly residents of long-term care facilities. Can. J. Diet. Pract. Res. 2008, 69, 82–88. [Google Scholar] [CrossRef]
- Merigliano, C.; Mascolo, E.; Burla, R.; Saggio, I.; Vernì, F. The Relationship between Vitamin B6, Diabetes and Cancer. Front. Genet. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Mikkelsen, K.; Dhaheri, A.; Ali, I.H.; Platat, C.; Ismail, C.L.; Stojanovska, L.; Apostolopoulous, V. Be well: A potential role for vitamin B in COVID-19. Maturitas 2021, 144, 108–111. [Google Scholar] [CrossRef]
- Cámara, M.; Sánchez-Mata, M.C.; Fernández-Ruiz, V.; Cámara, R.M.; Cebadera, E.; Domínguez, L. A Review of the Role of Micronutrients and Bioactive Compounds on Immune System Supporting to Fight against the COVID-19 Disease. Foods 2021, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Schorgg, P.; Bärnighausen, T.; Rohrmann, S.; Cassidy, A.; Karavasiloglou, N.; Kühn, T. Vitamin B6 Status among Vegetarians: Findings from a Population-Based Survey. Nutrients 2021, 13, 1627. [Google Scholar] [CrossRef]
- Berkins, S.; Schiöth, H.B.; Rukh, G. Depression and Vegetarians: Association between Dietary Vitamin B6, B12 and Folate Intake and Global and Subcortical Brain Volumes. Nutrients 2021, 13, 1790. [Google Scholar] [CrossRef] [PubMed]
- Nemazannikova, N.; Mikkelsen, K.; Stojanovska, L.; Blatch, G.L.; Apostolopoulos, V. Is there a Link between Vitamin B and Multiple Sclerosis? Med. Chem. 2018, 14, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Hallam, K.; Stojanovska, L.; Apostolopoulos, V. Yeast based spreads improve anxiety and stress. J. Funct. Foods 2018, 40, 471–476. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. https://doi.org/10.3390/nu13093229
Stach K, Stach W, Augoff K. Vitamin B6 in Health and Disease. Nutrients. 2021; 13(9):3229. https://doi.org/10.3390/nu13093229
Chicago/Turabian StyleStach, Kamilla, Wojciech Stach, and Katarzyna Augoff. 2021. "Vitamin B6 in Health and Disease" Nutrients 13, no. 9: 3229. https://doi.org/10.3390/nu13093229
APA StyleStach, K., Stach, W., & Augoff, K. (2021). Vitamin B6 in Health and Disease. Nutrients, 13(9), 3229. https://doi.org/10.3390/nu13093229




