Abstract
Breastfeeding (BF) is considered the normative standard of feeding for all infants. However, the impact of BF in patients with cystic fibrosis (CF) is not completely defined. Therefore, we conducted a systematic review to evaluate BF prevalence in the CF population and its impact on anthropometric and pulmonary outcomes. We searched MEDLINE, Embase and the Cochrane Library for original articles published in English up to 4 December 2020 that report the prevalence of BF and/or any measure of association between BF and anthropometric or pulmonary outcomes. Nine observational studies were identified (six retrospective cohort studies, one prospective cohort study, one survey and one case–control study within a retrospective cohort). The BF rate in CF patients is lower than that of the healthy population (approximately 50–60% of infants were breastfed at any time). The benefits in anthropometric outcomes of BF for >2 months in this at-risk population are unclear. A few relatively small studies suggest a potential benefit of BF in reducing lung infections, although data are inconsistent. The currently available data are insufficient to draw definite conclusions on the benefits of exclusive BF in anthropometric and pulmonary outcomes in CF. Clinical trials evaluating well-defined BF promotion interventions are needed.
1. Introduction
Cystic fibrosis (CF) is a life-shortening autosomal recessive genetic condition affecting more than 90,000 people worldwide [1]. It is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which alters the physiological sodium and chloride transport, resulting in viscous secretions. CF is a multisystem disease affecting the respiratory, gastrointestinal and reproductive tracts, and the sweat glands.
For many years, breastfeeding (BF) for infants with CF was discouraged, mainly due to concerns regarding reports of hypoproteinemia, hyponatremia and vitamin D and E deficiency. Due to the associated limitations in the absorption of fats and fat-soluble vitamins, BF was considered inadequate, in terms of energy supply, protein and sodium content, to meet the increased requirements of infants with CF, particularly for those with pancreatic insufficiency (PI) and meconium ileus (MI) [2,3]. However, these events are unlikely to be related to BF per se and, in the modern era of CF nutritional care, can be easily prevented with close monitoring of nutritional status, adequate sodium and vitamin supplementation, and pancreatic enzyme replacement therapy (PERT).
Starting from the 1990s, the increasing evidence of several benefits of BF in healthy infants led to a change in perspective, with 77% of CF centers recommending BF alone or in any combination with hydrolyzed formula and PERT, if required. However, it was only in 2002 that the CF Foundation Consensus approved BF as the recommended primary source of nutrition in the first year of life for patients with CF [4]. Accordingly, the 2016 ESPEN-ESPGHAN-ECFS guidelines [5] recommended exclusive BF for newly diagnosed infants with CF, highlighting the need for specific advice regarding PERT, salt supplements and nutritional intake. However, the optimal duration of exclusive BF was not indicated, leaving the WHO general recommendations [6] as the main indication.
Therefore, collecting evidence on the potential benefits of BF for infants with CF would be important to give evidence-based recommendations to parents in order to provide infants with CF with the best nutritional support from early life.
To this aim, we conducted a systematic review on BF prevalence in CF and its relationship with anthropometric and pulmonary outcomes.
2. Materials and Methods
We searched MEDLINE, Embase and the Cochrane Library databases for original articles published in English up to 4 December 2020 that report the prevalence of BF and/or any measure of association between BF and anthropometric or pulmonary outcomes. Citations were exported from the databases and then imported to Rayyan for title and abstract screening [7]. Weight, length/height and BMI (expressed as raw values or z-scores for age and sex), as well as weight-for-length z-scores, were considered as anthropometric outcomes, while the number of pulmonary exacerbations, IV antibiotic treatments, hospitalizations, lung infections, radiological scores of lung disease and forced expiratory volume in one second (FEV1) were considered among pulmonary outcomes.
We included articles reporting randomized clinical trials and observational studies, while reviews, opinion papers, conference proceedings and studies where the analysis was not based on individual data were excluded. The following search terms were used for the search: “cystic fibrosis” AND (“breastfeeding” OR “breastfed”). Titles and abstracts were screened, and the full texts of the eligible articles were obtained. The references included in the full text of the eligible articles were manually searched for studies that could have been missed.
From the included articles, we extracted the following data: year of publication, country where the study was conducted, study design, number of enrolled patients, prevalence and duration of BF, groups compared, measured outcomes and corresponding results.
Since we did not find any randomized clinical trial related to the objectives of our systematic review, we used the Newcastle–Ottawa Scale to assess the risk of bias in each cohort or case–control study [8]. The risk of bias for studies reporting results from surveys was evaluated trough the response rate.
The study findings were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [9].
3. Results
3.1. Selected Articles
Figure 1 shows the flow diagram of the studies included in this review.
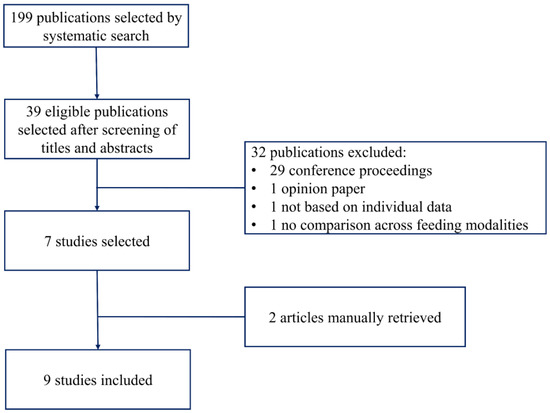
Figure 1.
Flow diagram of the studies selection.
The systematic search yielded 199 unique items; 39 were considered eligible after screening of the title and abstract. Thirty-two publications were excluded: 29 were published as conference proceedings, one article was an opinion paper [10], one article was a survey among CF center directors that did not report individual data [11] and one article [12] reported preintervention and postintervention values of growth parameters after a project to improve BF in CF, but it did not show a comparison between breastfed and formula-fed infants. Thus, seven studies were identified by the systematic search [13,14,15,16,17,18,19], and two studies were identified by the manual search [20,21].
Table 1 gives a summary overview of the nine selected articles: six retrospective cohort studies, one prospective cohort study, one survey and one case–control study within a retrospective cohort.

Table 1.
Summary overview of the selected studies.
The risk of bias for the cohort and case–control studies was generally low, although some comparability issues emerged (Table 2). In fact, the majority of the studies contained no or only partial control for important confounders, including PI, MI and socioeconomic status. With regard to the only survey [13] included in this systematic review, the response rate was very low (27%), with only 868 questionnaires returned out of 3200 sent, leaving doubts about the representativeness of the survey population.

Table 2.
Risk of bias for the included case–control and cohort studies according to the Newcastle–Ottawa scale.
3.2. Prevalence and Duration of Breastfeeding
All nine identified studies reported the prevalence of BF in CF (Table 3).

Table 3.
Summary overview of the studies reporting on prevalence of breastfeeding in cystic fibrosis.
The prevalence of BF was approximately 50–60%. The BF rate was found to be higher among patients with PS than among patients with PI and MI. Overall, BF prevalence was consistently lower compared to that of the general population, and this was also the case for exclusive BF rate. However, the figures provided by the included studies are difficult to compare since they were collected at different time points.
3.3. Breastfeeding and Anthropometric Outcomes
Six studies investigated the relationship between BF and anthropometric outcomes in CF (Table 4).

Table 4.
Summary overview of the studies evaluating the association between breastfeeding and anthropometric outcomes in cystic fibrosis.
The association between BF and anthropometric outcomes failed to reach statistical significance in the majority of the studies. The multi-center retrospective cohort study by Jadin et al. showed a rapid decline in weight-for-age z-scores from birth to six months of age only in children who had been exclusively breastfed for more than two months [15]. In contrast, Leung et al. found a positive association between exclusive BF and weight-for-age z-scores at 3 months of age, but this result was not confirmed at 6 and 12 months of age [17]. A positive association between BF and weight-for-age z-scores at 12 and 24 months of age was also found in a small subgroup (N = 16) of patients with MI [20].
3.4. Breastfeeding and Pulmonary Outcomes
Seven studies investigated the relationship between BF and pulmonary outcomes in CF (Table 5).

Table 5.
Summary overview of the studies evaluating the association between breastfeeding and pulmonary outcomes in cystic fibrosis.
All studies evaluated the association between BF and lung infections, while only two studies also considered respiratory function indicators [13,14], one of which showed a positive association [14]. Colombo et al. reported a lower number of infections over the first three years of life among infants who had been breastfed for more than four months as compared to those who had been formula-fed or breastfed for a shorter period [14]. Parker et al. found lower IV antibiotic use over two years before the enrollment in their survey in patients who had been exclusively breastfed for more than six months as compared to formula-fed patients [13]. Among the three studies investigating P. aeruginosa acquisition or colonization [15,16,18], one study found an inverse association [15], and two studies found null associations [16,18], although one of them [18] reported a trend toward a later acquisition among exclusively or partially breastfed infants.
However, only one study evaluated the association between BF and a radiological score, showing higher scores among infants who had been breastfed for at least 2 months as compared to those breastfed for a shorter period [15].
One study on CF patients with MI evaluated a composite outcome including P. aeruginosa infection and faltering growth, reporting a higher risk of negative outcome among infants who were never breastfed as compared to breastfed infants, but the result was not statistically significant [19].
4. Discussion
The studies identified for the present systematic review are consistent in reporting a lower prevalence of BF among infants with CF than among healthy infants. Based on these studies, the impact of BF on anthropometric and pulmonary outcomes remains uncertain because data are based on relatively small studies that are largely heterogeneous in terms of design and findings.
BF prevalence is much lower than that reported in healthy infants, and it is initiated on average in 50% of newborn infants with CF. Data on BF duration are even scarcer, but seem to indicate that it is also shorter, particularly in patients with PI and MI.
The decision of mothers to discontinue BF is based on several factors, including personal perceptions of the adequacy of breast milk (BM), poor social and family support, and difficulties for women in reconciling work with child’s care [22]. All these barriers play a more important role for mothers of children with CF, who have to face many challenges soon after diagnosis, including the psychological stress associated with the newly diagnosed disease, the frequent failure to thrive of their infants and the multiple therapies prescribed to prevent the progression of the disease [12]. Moreover, the management of CF therapy in breastfed children requires further arrangements to ensure proper PERT and adequate supplementations of sodium, iron and vitamins; this may lead both the parents and the physician to decide on the early discontinuation of BF. Notably, iron supplementation can paradoxically limit the highly efficient iron absorption from BM, by “blocking” lactoferrin [23,24,25].
On the other hand, initiatives to promote and support BF among mothers of children with CF have recently shown promising results in reducing the discontinuation of BF. In a recent study by Miller et al. [12], an International Board-Certified Lactation Consultant (IBCLC) was incorporated into the initial CF-diagnosis visit in order to support mothers who were already BF. After the intervention, 94% of mothers (16 out of 17 mothers) continued BF vs. 57% of the pre-intervention group (8 out of 14 mothers). Duration of BF and exclusive BF was increased, although not significantly, to an average of 7.7 and 4.5 months, respectively, compared to the 6.4 and 3.6 months in the pre-intervention group.
With regard to the relationship among BF, anthropometric and pulmonary outcomes, the available data are limited, based on a relatively small number of patients, and heterogeneous in terms of measured outcomes, comparisons and results. Overall, the results on anthropometric outcomes are controversial, while there is some evidence that BF may reduce or delay lung infections in CF.
Most studies did not find significant differences in anthropometric outcomes across different feeding modalities. Nevertheless, two studies suggested some benefits of BF for patients with MI, possibly impacting the later expression of the individual growth potential [19,20]. Accordingly, one study documented a positive association between BF and growth rate in 16 infants [20] and the other, based on 85 patients, reported a reduced risk of faltering growth and/or chronic P. aeruginosa infection at 1 year of age (evaluated as a composite outcome) among breastfed compared to never breastfed infants with CF [19]. It is well known that human milk contains high concentrations of antimicrobial proteins, including lactoferrin, lysozyme, lactadherin and HMO, and growth factors, such as transforming growth factor beta, epidermal growth factor and insulin-like growth factor, which may improve the gut microbiome in these patients [26].
Since only a few relatively small studies suggest a potential benefit of BF in reducing lung infections, the relationship between BF and pulmonary outcomes remains unclear. Most studies did not consider potential confounding variables (e.g., higher socio-economic status and maternal level of education in the BF group), and the majority had a retrospective design with possible recall bias.
When interpreting the results of the studies reported in this systematic review, some important issues related to the different periods in which they were carried out should be considered. First, the aggressive market campaigns in the 1970s and 1980s strongly influenced maternal feeding choices, and at that time, formula feeding was more frequent than in recent years. Second, the composition of formula milk has been improved over time to mimic BM, both in terms of nutritional and functional effects. Finally, the small amount of included studies, the short follow-up for some of them, and the high heterogeneity in the timing of the measurements, comparisons and outcomes should also be considered. This last issue prevented us from providing a quantitative summary measure of the association between BF and CF outcomes through a meta-analysis.
5. Conclusions
In conclusion, our systematic review indicates that BF can be recommended in CF, since infants who are breastfed even for a prolonged period of time do not show a compromised growth, provided that they are monitored in specialized centers according to the Standards of Care [27]. MI does not seem to represent a contraindication to BF, even if only a few small studies considered the effect of BF in this subgroup of infants, who are at increased risk of growth faltering.
Large randomized clinical trials evaluating interventions of BF promotion with well-defined feeding strategies are needed to draw definitive conclusions on the positive effects of BF on CF outcomes.
Author Contributions
Conceptualization, C.C., M.L.G. and G.A.; methodology, G.A.; writing—original draft preparation, C.C., M.L.G., G.A. and A.C.; writing—review and editing, C.C., G.A., V.D., A.C., F.M., C.A., M.L.G.; supervision, C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.A. Hypoproteinemia and anemia in infants with cystic fibrosis. A presenting symptom complex often misdiagnosed. JAMA J. Am. Med. Assoc. 1974, 228, 585–588. [Google Scholar] [CrossRef]
- Sokol, R.J.; Reardon, M.C.; Accurso, F.J.; Stall, C.; Narkewicz, M.; Abman, S.H.; Hammond, K.B. Fat-soluble-vitamin status during the first year of life in infants with cystic fibrosis identified by screening of newborns. Am. J. Clin. Nutr. 1989, 50, 1064–1071. [Google Scholar] [CrossRef]
- Borowitz, D.; Baker, R.D.; Stallings, V.; Bachrach, L.K.; Beall, R.J.; Ph, D.; Campbell, P.W.; Casey, S.C.; Cohen, M.B.; Corey, M.; et al. Consensus report on nutrition for pediatric patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 2002, 35, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef] [Green Version]
- WHO. Exclusive Breastfeeding for Six Months Best for Babies Everywhere. Available online: http://www.who.int/mediacentre/news/statements/2011/breastfeeding_20110115/en/ (accessed on 27 January 2021).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.A.; Shea, B.; O’connell, D.; Petersen, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Department of Epidemiology and Community Medicine, University of Ottawa: Ottawa, ON, Canada, 2012; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 27 January 2021).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Smith, C. Supporting Optimal Growth in Infants with Chronic Conditions: How Are We Doing and What Can We Do? Breastfeed. Med. 2019, 14, S18–S19. [Google Scholar] [CrossRef] [Green Version]
- Luder, E.; Kattan, M.; Tanzer-Torres, G.; Bonforte, R.J. Current Recommendations for Breast-feeding in Cystic Fibrosis Centers. Am. J. Dis. Child. 1990, 144, 1153–1156. [Google Scholar] [CrossRef]
- Miller, T.; Antos, N.J.; Brock, L.A.; Wade, T.; Goday, P.S. Lactation Consultation Sustains Breast Milk Intake in Infants with Cystic Fibrosis. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 358–362. [Google Scholar] [CrossRef]
- Parker, E.M.; O’Sullivan, B.P.; Shea, J.C.; Regan, M.M.; Freedman, S.D. Survey of Breast-Feeding Practices and Outcomes in the Cystic Fibrosis Population. Pediatr. Pulmonol. 2004, 37, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Costantini, D.; Zazzeron, L.; Faelli, N.; Russo, M.C.; Ghisleni, D.; Gatelli, I.; Giovannini, M.; Riva, E.; Zetterström, R.; et al. Benefits of breastfeeding in cystic fibrosis: A single-centre follow-up survey. Acta Paediatr. 2007, 96, 1228–1232. [Google Scholar] [CrossRef]
- Jadin, S.A.; Wu, G.S.; Zhang, Z.; Shoff, S.M.; Tippets, B.M.; Farrell, P.M.; Miller, T.; Rock, M.J.; Levy, H.; Lai, H.J. Growth and pulmonary outcomes during the first 2 y of life of breastfed and formula-fed infants diagnosed with cystic fibrosis through the Wisconsin Routine Newborn Screening Program. Am. J. Clin. Nutr. 2011, 93, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, M.; Emerson, J.; McNamara, S.; Thompson, V.; Ramsey, B.W.; Morgan, W.; Gibson, R.L. Risk factors for age at initial Pseudomonas acquisition in the cystic fibrosis epic observational cohort. J. Cyst. Fibros. 2012, 11, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, D.H.; Heltshe, S.L.; Borowitz, D.; Gelfond, D.; Kloster, M.; Heubi, J.E.; Stalvey, M.; Ramsey, B.W.; Stecenko, A.; Schechter, M.; et al. Effects of diagnosis by newborn screening for cystic fibrosis on weight and length in the first year of life. JAMA Pediatr. 2017, 171, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Munck, A.; Boulkedid, R.; Weiss, L.; Foucaud, P.; Wizla-Derambure, N.; Reix, P.; Bremont, F.; Derelle, J.; Schroedt, J.; Alberti, C. Nutritional Status in the First 2 Years of Life in Cystic Fibrosis Diagnosed by Newborn Screening. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 123–130. [Google Scholar] [CrossRef]
- Padoan, R.; Cirilli, N.; Falchetti, D.; Cesana, B.M. Risk factors for adverse outcome in infancy in meconium ileus cystic fibrosis infants: A multicentre Italian study. J. Cyst. Fibros. 2019, 18, 863–868. [Google Scholar] [CrossRef]
- Holliday, K.E.; Allen, J.R.; Waters, D.L.; Gruca, M.A.; Thompson, S.M.; Gaskin, K.J. Growth of human milk-fed and formula-fed infants with cystic fibrosis. J. Pediatr. 1991, 118, 77–79. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Linnane, B.; George, S.; Ni Chroinin, M.; Mullane, D.; Herzig, M.; Greally, P.; Elnazir, B.; Healy, F.; Mc Nally, P.; et al. Neonatal screening programme for CF: Results from the Irish Comparative Outcomes Study (ICOS). Pediatr. Pulmonol. 2020, 55, 2323–2329. [Google Scholar] [CrossRef]
- Brown, C.R.L.; Dodds, L.; Legge, A.; Bryanton, J.; Semenic, S. Factors influencing the reasons why mothers stop breastfeeding. Can. J. Public Health 2014, 105. [Google Scholar] [CrossRef]
- Cerami, C. Iron Nutriture of the Fetus, Neonate, Infant, and Child. Ann. Nutr. Metab. 2017, 71, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Domellöf, M.; Lönnerdal, B.; Abrams, S.A.; Hernell, O. Iron absorption in breast-fed infants: Effects of age, iron status, iron supplements, and complementary foods. Am. J. Clin. Nutr. 2002, 76, 198–204. [Google Scholar] [CrossRef]
- Lönnerdal, B. Excess iron intake as a factor in growth, infections, and development of infants and young children. Proc. Am. J. Clin. Nutr. 2017, 106, 1681S–1687S. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition: Nutrients and Bioactive Factors. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Castellani, C.; Duff, A.J.A.; Bell, S.C.; Heijerman, H.G.M.; Munck, A.; Ratjen, F.; Sermet-Gaudelus, I.; Southern, K.W.; Barben, J.; Flume, P.A.; et al. ECFS best practice guidelines: The 2018 revision. J. Cyst. Fibros. 2018, 17, 153–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).