Artichoke and Bergamot Phytosome Alliance: A Randomized Double Blind Clinical Trial in Mild Hypercholesterolemia
Abstract
1. Introduction
2. Materials and Methods
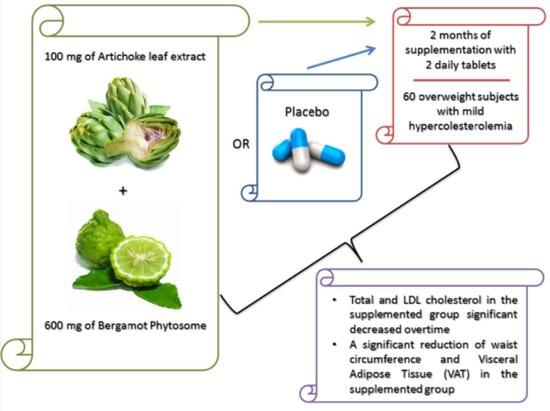
2.1. Experimental Design
2.2. Population
2.3. Dietary Supplement
2.4. Anthropometric Measurements
2.5. Dietary Intake
2.6. Body Composition
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anagnostis, P.; Selalmatzidou, D.; Polyzos, S.A.; Panagiotou, A.; Slavakis, A.; Panagiotidou, A.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P.; Kita, M. Comparative effects of rosuvastatin and atorvastatin on glucose metabolism and adipokine levels in non-diabetic patients with dyslipidaemia: A prospective randomised open-label study. Int. J. Clin. Pract. 2011, 65, 679–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Trompet, S.; Postmus, I.; Slagboom, P.E.; Heijmans, B.T.; Smit, R.A.J.; Maier, A.B.; Buckley, B.M.; Sattar, N.; Stott, D.J.; Ford, I.; et al. Non-response to (statin) therapy: The importance of distinguishing non-responders from non-adherers in pharmacogenetic studies. Eur. J. Clin. Pharmacol. 2016, 72, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Lansberg, P.; Lee, A.; Lee, Z.-V.; Subramaniam, K.; Setia, S. Vascular Health and Risk Management Dovepress Nonadherence to statins: Individualized intervention strategies outside the pill box. Vasc. Health Risk Manag. 2018, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.; Van Dijk, L.; Geers, H.C.J.; Winters, N.A.; Van Geffen, E.C.G.; Stiggelbout, A.M.; Bouvy, M.L. Understanding statin non-adherence: Knowing which perceptions and experiences matter to different patients. PLoS ONE 2016, 11, e0146272. [Google Scholar] [CrossRef]
- Gitt, A.K.; Drexel, H.; Feely, J.; Ferrières, J.; Gonzalez-Juanatey, J.R.; Thomsen, K.K.; Leiter, L.A.; Lundman, P.; Da Silva, P.M.; Pedersen, T.; et al. Persistent lipid abnormalities in statin-treated patients and predictors of LDL-cholesterol goal achievement in clinical practice in Europe and Canada. Eur. J. Prev. Cardiol. 2012, 19, 221–230. [Google Scholar] [CrossRef]
- Akyea, R.K.; Kai, J.; Qureshi, N.; Iyen, B.; Weng, S.F. Sub-optimal cholesterol response to initiation of statins and future risk of cardiovascular disease. Heart 2019, 105, 975–981. [Google Scholar] [CrossRef]
- Adorni, M.P.; Zimetti, F.; Lupo, M.G.; Ruscica, M.; Ferri, N. Naturally Occurring PCSK9 Inhibitors. Nutrients 2020, 12, 1440. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Opizzi, A.; Faliva, M.A.; Sala, P.; Perna, S.; Riva, A.; Morazzoni, P.; Bombardelli, E. Beneficial effects of artichoke leaf extract supplementation on increasing HDL-cholesterol in subjects with primary mild hypercholesterolaemia: A double-blind, randomized, placebo-controlled trial. Int. J. Food Sci. Nutr. 2013, 64, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Perna, S.; Spadaccini, D.; Botteri, L.; Girometta, C.; Riva, A.; Allegrini, P.; Petrangolini, G.; Infantino, V.; Rondanelli, M. Efficacy of bergamot: From anti-inflammatory and anti-oxidative mechanisms to clinical applications as preventive agent for cardiovascular morbidity, skin diseases, and mood alterations. Food Sci. Nutr. 2019, 7, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Mameghani, M.; Asghari-Jafarabadi, M.; Rezazadeh, K. TCF7L2-rs7903146 polymorphism modulates the effect of artichoke leaf extract supplementation on insulin resistance in metabolic syndrome: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2018, 16, 329–334. [Google Scholar] [CrossRef]
- Rezazadeh, K.; Rahmati-Yamchi, M.; Mohammadnejad, L.; Ebrahimi-Mameghani, M.; Delazar, A. Effects of artichoke leaf extract supplementation on metabolic parameters in women with metabolic syndrome: Influence of TCF7L2-rs7903146 and FTO-rs9939609 polymorphisms. Phyther. Res. 2018, 32, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Rundblad, A.; Larsen, S.V.; Myhrstad, M.C.; Ottestad, I.; Thoresen, M.; Holven, K.B.; Ulven, S.M. Differences in peripheral blood mononuclear cell gene expression and triglyceride composition in lipoprotein subclasses in plasma triglyceride responders and non-responders to omega-3 supplementation. Genes Nutr. 2019, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phyther. Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Calviello, G. Omega-3 pufa responders and non-responders and the prevention of lipid dysmetabolism and related diseases. Nutrients 2020, 12, 1363. [Google Scholar] [CrossRef]
- Nauman, M.C.; Johnson, J.J. Clinical application of bergamot (Citrus Bergamia) for reducing high cholesterol and cardiovascular disease markers. Integr. Food Nutr. Metab. 2019, 6, 10-15761. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Nobile, R. Citrus Bergamia, Risso: The peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emir. J. Food Agric. 2020, 32, 522–532. [Google Scholar]
- Gioffrè, G.; Ursino, D.; Labate, M.L.C.; Giuffrè, A.M. The peel essential oil composition of bergamot fruit (Citrus Bergamia, Risso) of Reggio Calabria (Italy): A review. Emir. J. Food Agric. 2020, 32, 835–845. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 2013, 33, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Kuczmannová, A.; Balažová, A.; Račanská, E.; Kameníková, M.; Fialová, S.; Majerník, J.; Nagy, M.; Gál, P.; Mučaji, P.; Atanasov, A.G.; et al. Agrimonia eupatoria L. and Cynara cardunculus L. Water Infusions: Comparison of Anti-Diabetic Activities. Molecules 2016, 21, 564. [Google Scholar] [CrossRef]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Gallucci, G.; Martello, E.; Fiorillo, M.; Dolce, V.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus Bergamia): “In vivo” studies. J. Funct. Foods 2014, 7, 558–568. [Google Scholar] [CrossRef]
- Cai, Y.; Xing, G.; Shen, T.; Zhang, S.; Rao, J.; Shi, R. Effects of 12-week supplementation of Citrus Bergamia extracts-based formulation CitriCholess on cholesterol and body weight in older adults with dyslipidemia: A randomized, double-blind, placebo-controlled trial. Lipids Health Dis. 2017, 16, 251. [Google Scholar] [CrossRef]
- Capomolla, A.S.; Janda, E.; Parafati, M.; Paone, S.; Sawicki, T.; Mollace, R.; Ragusa, S.; Mollace, V. Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients 2019, 11, 1271. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus Bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo- Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Formisano, C.; Rigano, D.; Lopatriello, A.; Sirignano, C.; Ramaschi, G.; Arnoldi, L.; Riva, A.; Sardone, N.; Taglialatela-Scafati, O. Detailed Phytochemical Characterization of Bergamot Polyphenolic Fraction (BPF) by UPLC-DAD-MS and LC-NMR. J. Agric. Food Chem. 2019, 67, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Raddino, R.; Scarabelli, T.M.; Pelà, G.; Sarasso, M.L.; Domenighini, D.; Fappani, A.; Visioli, O. Mild hypercolesterolaemia. Diagnosis and prognosis. Recenti Prog. Med. 1997, 88, 255–263. [Google Scholar] [PubMed]
- Rondanelli, M.; Peroni, G.; Riva, A.; Petrangolini, G.; Allegrini, P.; Fazia, T.; Bernardinelli, L.; Naso, M.; Faliva, M.A.; Tartara, A.; et al. Bergamot phytosome improved visceral fat and plasma lipid profiles in overweight and obese class I subject with mild hypercholesterolemia: A randomized placebo controlled trial. Phyther. Res. 2021, 35, 2045–2056. [Google Scholar] [CrossRef]
- Frisancho, A.R. New standards of weight and body composition by frame size and height for assessment of nutritional status of adults and the elderly. Am. J. Clin. Nutr. 1984, 40, 808–819. [Google Scholar] [CrossRef]
- Davis, U. Minimum Dietary Diversity for Women—A Guide to Measurement; FAO: Rome, Italy, 2016. [Google Scholar]
- Mohammad, A.; De Lucia Rolfe, E.; Sleigh, A.; Kivisild, T.; Behbehani, K.; Wareham, N.J.; Brage, S.; Mohammad, T. Validity of visceral adiposity estimates from DXA against MRI in Kuwaiti men and women. Nutr. Diabetes 2017, 7, e238. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.A. The Consolidated Standards of Reporting Trials (CONSORT): Guidelines for reporting randomized trials. Nurs. Res. 2005, 54, 128–132. [Google Scholar] [CrossRef]
- Lamiquiz-Moneo, I.; Giné-González, J.; Alisente, S.; Bea, A.M.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Jarauta, E.; Cenarro, A.; Civeira, F.; et al. Effect of bergamot on lipid profile in humans: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 60, 3133–3143. [Google Scholar] [CrossRef]
- Sahebkar, A.; Pirro, M.; Banach, M.; Mikhailidis, D.P.; Atkin, S.L.; Cicero, A.F.G. Lipid-lowering activity of artichoke extracts: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2018, 58, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Bairey Merz, C.N.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004, 110, 227–239. [Google Scholar] [CrossRef]
- Kottke, T.E.; Puska, P.; Salonen, J.T.; Tuomilehto, J.; Nissinen, A. Projected effects of high-risk versus population-based prevention strategies in coronary heart disease. Am. J. Epidemiol. 1985, 121, 697–704. [Google Scholar] [CrossRef]
- Calle, M.C.; Vega-López, S.; Segura-Pérez, S.; Volek, J.S.; Pérez-Escamilla, R.; Fernandez, M.L. Low Plasma Hdl Cholesterol and Elevated C Reactive Protein further Increase Cardiovascular Disease Risk in Latinos with Type 2 Diabetes. J. Diabetes Metab. 2010, 1, 109. [Google Scholar] [CrossRef]
- Cardenas, G.A.; Lavie, C.J.; Cardenas, V.; Milani, R.V.; McCullough, P.A. The importance of recognizing and treating low levels of high-density lipoprotein cholesterol: A new era in atherosclerosis management. Rev. Cardiovasc. Med. 2008, 9, 239–258. [Google Scholar]
- Nofer, J.R.; Walter, M.; Kehrel, B.; Wierwille, S.; Tepel, M.; Seedorf, U.; Assmann, G. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; La Du, B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J. Clin. Investig. 1998, 101, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.L.; Wang, S.H.; Peng, D.Q.; Zhao, S.P. HDL and immunomodulation: An emerging role of HDL against atherosclerosis. Immunol. Cell Biol. 2010, 88, 285–290. [Google Scholar] [CrossRef] [PubMed]


| Outcome | Control Group (n = 30) Mean ± SD | Supplemented Group (n = 30) Mean ± SD | Total Sample (n = 60, Female = 32, Male = 28) Mean ± SD | p-Value between Groups |
|---|---|---|---|---|
| Age (ys) | 59.69 ± 7.64 | 57.64 ± 11.14 | 58.65 ± 9.54 | 0.419 |
| Weight (Kg) | 76.40 ± 18.33 | 71.49 ± 14.83 | 73.82 ± 16.62 | 0.261 |
| BMI (Kg/m2) | 28.38 ± 3.07 | 27.38 ± 2.63 | 27.85 ± 2.87 | 0.184 |
| Waist Circumference (cm) | 96.63 ± 17.90 | 94.11 ± 13.00 | 95.31 ± 15.43 | 0.537 |
| Fat Mass (g) | 30,596.54 ± 14,237.25 | 28,364.42 ± 9271.64 | 29,423.73 ± 11,835.86 | 0.474 |
| Fat Free Mass (g) | 43,506.75 ± 7886.51 | 43,112.55 ± 9242.26 | 43,299.63 ± 8554.28 | 0.861 |
| Visceral Adipose Tissue (g) | 1100.21 ± 629.85 | 1108.19 ± 654.05 | 1104.41 ± 637.15 | 0.962 |
| Total Cholesterol (mg/dL) | 234.36 ± 17.18 | 236.97 ± 24.84 | 235.73 ± 21.41 | 0.640 |
| LDL Cholesterol (mg/dL) | 158.29 ± 19.57 | 155.83 ± 26.22 | 157.00 ± 23.14 | 0.688 |
| HDL Cholesterol (mg/dL) | 57.18 ± 15.16 | 59.42 ± 12.60 | 28.36 ± 13.80 | 0.538 |
| Total Cholesterol/HDL Cholesterol | 4.36 ± 1.14 | 4.14 ± 0.85 | 4.24 ± 1.00 | 0.392 |
| Triglycerides (mg/dL) | 112.36 ± 40.39 | 108.58 ± 34.91 | 110.37 ± 37.33 | 0.702 |
| Glycemia (mg/dL) | 87.18 ± 6.25 | 88.19 ± 10.83 | 87.71 ± 8.89 | 0.665 |
| Insulin (mcIU/mL) | 10.66 ± 5.94 | 10.10 ± 9.08 | 10.36 ± 7.69 | 0.783 |
| HOMA index | 2.34 ± 1.41 | 2.32 ± 2.44 | 2.33 ± 2.00 | 0.961 |
| Glycated Hemoglobin (%) | 5.95 ± 0.53 | 5.39 ± 0.34 | 5.66 ± 0.52 | 0.001 |
| Apolipoprotein A (mg/dL) | 159.39 ± 31.17 | 129.06 ± 25.29 | 143.46 ± 31.88 | 0.001 |
| Apolipoprotein B (mg/dL) | 133.36 ± 18.24 | 167.45 ± 29.25 | 151.27 ± 29.87 | 0.001 |
| Apolipoprotein B/Apolipoprotein A | 0.88 ± 0.25 | 1.36 ± 0.42 | 1.13 ± 0.42 | 0.001 |
| Aspartate Aminotransferase (UI/L) | 20.11 ± 5.95 | 19.65 ± 10.24 | 19.86 ± 8.41 | 0.835 |
| Alanine Aminotransferase (UI/L) | 22.11 ± 8.09 | 19.23 ± 13.29 | 20.59 ± 11.13 | 0.325 |
| Gamma Glutamyl Transferase (U/L) | 19.46 ± 6.69 | 21.52 ± 8.74 | 20.54 ± 7.84 | 0.320 |
| Creatinine (mg/dL) | 0.83 ± 0.11 | 0.82 ± 0.16 | 0.82 ± 0.14 | 0.877 |
| Outcome | Control Group (n = 30) Mean | Supplemented Group (n = 30) Mean | p-Value Between Groups Treatment Time |
|---|---|---|---|
| Total Cholesterol (mg/dL) | 0.009 | ||
| T0-baseline | 236 | 237 | |
| T1-30 days | 243 | 228 | |
| T2-60 days | 237 | 224 | |
| LDL Cholesterol (mg/dL) | 0.001 | ||
| T0-baseline | 157 | 156 | |
| T1-30 days | 165 | 143 | |
| T2-60 days | 156 | 139 | |
| HDL Cholesterol (mg/dL) | 0.001 | ||
| T0-baseline | 58.8 | 59.5 | |
| T1-30 days | 58.3 | 63.6 | |
| T2-60 days | 57.9 | 63.9 | |
| Total Cholesterol/HDL Cholesterol | 0.001 | ||
| T0-baseline | 4.29 | 4.13 | |
| T1-30 days | 4.5 | 3.69 | |
| T2-60 days | 4.4 | 3.60 | |
| Triglycerides (mg/dL) | 0.314 | ||
| T0-baseline | 111 | 108 | |
| T1-30 days | 114 | 106 | |
| T2-60 days | 119 | 106 | |
| Glycemia (mg/dL) | 0.834 | ||
| T0-baseline | 87.6 | 88.2 | |
| T1-30 days | 85.7 | 86.6 | |
| T2-60 days | 84.5 | 86.2 | |
| Insulin (mcIU/mL) | 0.111 | ||
| T0-baseline | 10.78 | 10.06 | |
| T1-30 days | 10.44 | 7.22 | |
| T2-60 days | 9.98 | 7.17 | |
| HOMA index | 0.279 | ||
| T0-baseline | 2.38 | 2.31 | |
| T1-30 days | 2.24 | 1.58 | |
| T2-60 days | 2.32 | 1.98 | |
| Glycated Hemoglobin (%) | 0.001 | ||
| T0-baseline | 5.98 | 5.39 | |
| T1-30 days | 5.88 | 5.4 | |
| T2-60 days | 5.73 | 5.47 | |
| Apolipoprotein A (mg/dL) | 0.423 | ||
| T0-baseline | 158 | 129 | |
| T1-30 days | 159 | 127 | |
| T2-60 days | 156 | 130 | |
| Apolipoprotein B (mg/dL) | 0.058 | ||
| T0-baseline | 133 | 168 | |
| T1-30 days | 137 | 162 | |
| T2-60 days | 133 | 161 | |
| Aspartate Aminotransferase (UI/L) | 0.862 | ||
| T0-baseline | 20.1 | 19.6 | |
| T1-30 days | 20.1 | 20 | |
| T2-60 days | 19.5 | 18.5 | |
| Alanine Aminotransferase (UI/L) | 0.943 | ||
| T0-baseline | 22.1 | 19.2 | |
| T1-30 days | 22.3 | 19.7 | |
| T2-60 days | 21.7 | 18.2 | |
| Gamma Glutamyl Transferase (U/L) | 0.416 | ||
| T0-baseline | 19.4 | 21.6 | |
| T1-30 days | 19.1 | 23 | |
| T2-60 days | 19.8 | 21 | |
| Creatinine (mg/dL) | 0.995 | ||
| T0-baseline | 0.827 | 0.821 | |
| T1-30 days | 0.815 | 0.808 | |
| T2-60 days | 0.821 | 0.813 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riva, A.; Petrangolini, G.; Allegrini, P.; Perna, S.; Giacosa, A.; Peroni, G.; Faliva, M.A.; Naso, M.; Rondanelli, M. Artichoke and Bergamot Phytosome Alliance: A Randomized Double Blind Clinical Trial in Mild Hypercholesterolemia. Nutrients 2022, 14, 108. https://doi.org/10.3390/nu14010108
Riva A, Petrangolini G, Allegrini P, Perna S, Giacosa A, Peroni G, Faliva MA, Naso M, Rondanelli M. Artichoke and Bergamot Phytosome Alliance: A Randomized Double Blind Clinical Trial in Mild Hypercholesterolemia. Nutrients. 2022; 14(1):108. https://doi.org/10.3390/nu14010108
Chicago/Turabian StyleRiva, Antonella, Giovanna Petrangolini, Pietro Allegrini, Simone Perna, Attilio Giacosa, Gabriella Peroni, Milena Anna Faliva, Maurizio Naso, and Mariangela Rondanelli. 2022. "Artichoke and Bergamot Phytosome Alliance: A Randomized Double Blind Clinical Trial in Mild Hypercholesterolemia" Nutrients 14, no. 1: 108. https://doi.org/10.3390/nu14010108
APA StyleRiva, A., Petrangolini, G., Allegrini, P., Perna, S., Giacosa, A., Peroni, G., Faliva, M. A., Naso, M., & Rondanelli, M. (2022). Artichoke and Bergamot Phytosome Alliance: A Randomized Double Blind Clinical Trial in Mild Hypercholesterolemia. Nutrients, 14(1), 108. https://doi.org/10.3390/nu14010108