Evaluation of 2’-Fucosyllactose and Bifidobacterium longum Subspecies infantis on Growth, Organ Weights, and Intestinal Development of Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Experimental Diets
2.3. Probiotic Treatment
2.4. Sample Collection
2.5. Intestine Histomorphology
2.6. Disaccharidase Activities
2.7. Dry Matter of Luminal Contents
2.8. DNA Extraction from Luminal Contents
2.9. qPCR for Bifidobacterium spp. and B. infantis
2.10. Statistical Analysis
3. Results
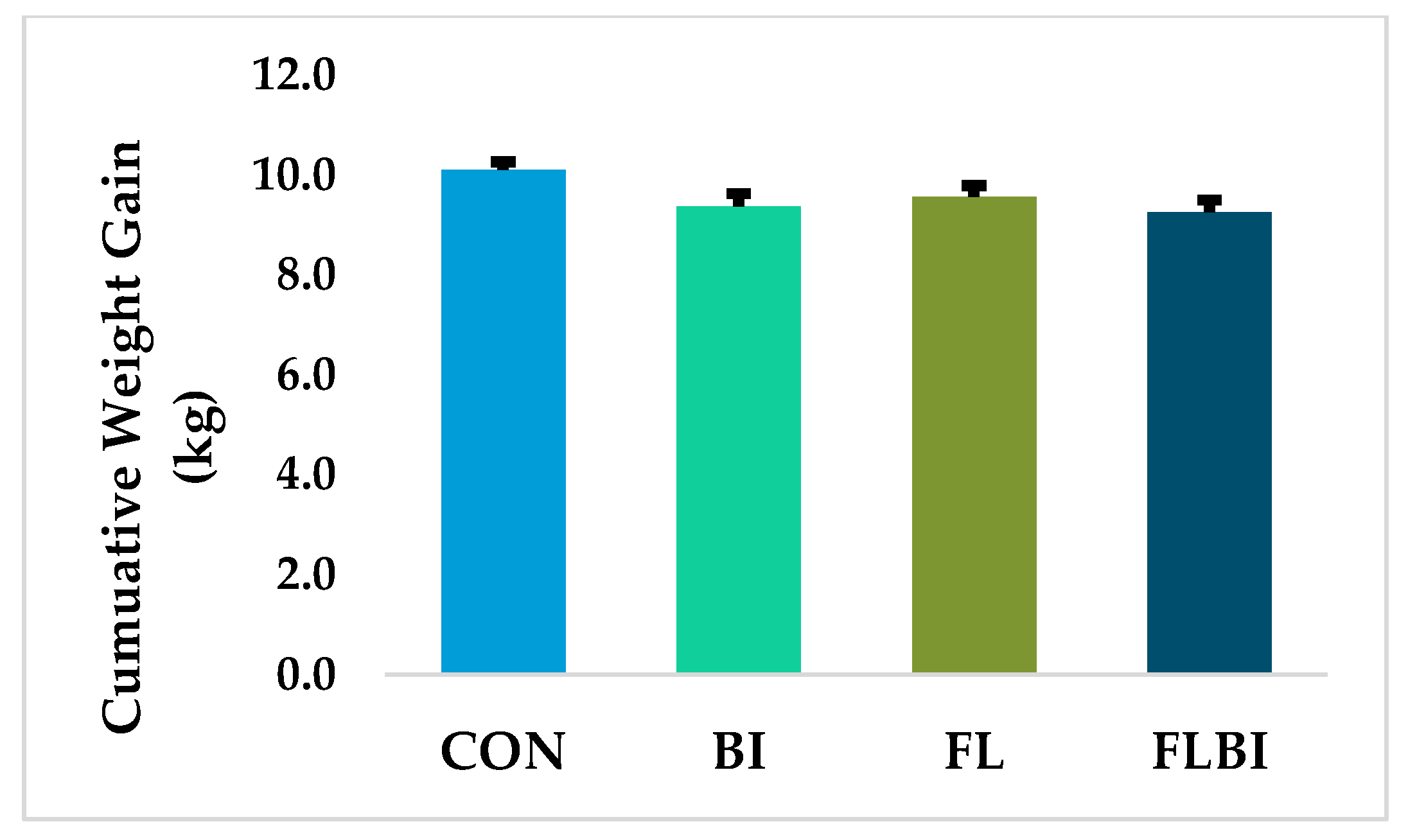
3.1. Tolerance, Weight Gain, Organ Weights and Intestinal Length and Weight
3.2. Intestine Histomorphology and Disaccharidase Activity
3.3. Dry Matter of Luminal Contents and qPCR for Bifidobacterium spp. and B. infantis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carr, L.E.; Virmani, M.D.; Rosa, F.; Munblit, D.; Matazel, K.S.; Elolimy, A.A.; Yeruva, L. Role of human milk bioactives on infants’ gut and immune health. Front. Immunol. 2021, 12, 604080. [Google Scholar] [CrossRef] [PubMed]
- Salamone, M.; Di Nardo, V. Effects of human milk oligosaccharides (HMOs) on gastrointestinal health. Front. Biosci. 2020, 12, 183–198. [Google Scholar] [CrossRef]
- Donovan, S.M.; Comstock, S.S. Human milk oligosaccharides influence neonatal mucosal and systemic immunity. Ann. Nutr. Metab. 2016, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a source of beneficial microbes and associated benefits for infant health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Engfer, M.B.; Stahl, B.; Finke, B.; Sawatzki, G.; Daniel, H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am. J. Clin. Nutr. 2000, 71, 1589–1596. [Google Scholar] [CrossRef] [Green Version]
- Gnoth, M.J.; Kunz, C.; Kinne-Saffran, E.; Rudloff, S. Human milk oligosaccharides are minimally digested in vitro. J. Nutr. 2000, 130, 3014–3020. [Google Scholar] [CrossRef]
- Holscher, H.D.; Bode, L.; Tappenden, K.A. Human milk oligosaccharides influence intestinal epithelial cell maturation in vitro. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Davis, S.R.; Tappenden, K.A. Human milk oligosaccharides influence maturation of human intestinal Caco-2Bbe and HT-29 cell lines. J. Nutr. 2014, 144, 586–591. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Goodson, M.L.; Vang, W.; Rutkowsky, J.; Kalanetra, K.; Bhattacharya, M.; Barile, D.; Raybould, H.E. Human milk oligosaccharide 2’-fucosyllactose supplementation improves gut barrier function and signaling in the vagal afferent pathway in mice. Food Funct. 2021, 12, 8507–8521. [Google Scholar] [CrossRef]
- Underwood, M.A.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Bifidobacterium longum subspecies infantis: Champion colonizer of the infant gut. Pediatr. Res. 2015, 77, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and butyrate-producing colon bacteria: Importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [Green Version]
- Thomson, P.; Medina, D.A.; Garrido, D. Human milk oligosaccharides and infant gut bifidobacteria: Molecular strategies for their utilization. Food Microbiol. 2018, 75, 37–46. [Google Scholar] [CrossRef]
- Garrido, D.; Kim, J.H.; German, J.B.; Raybould, H.E.; Mills, D.A. Oligosaccharide binding proteins from Bifidobacterium longum subsp. infantis reveal a preference for host glycans. PLoS ONE 2011, 6, e17315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaburi, G.; Duar, R.M.; Brown, H.; Mitchell, R.D.; Kazi, S.; Chew, S.; Cagney, O.; Flannery, R.L.; Sylvester, K.G.; Frese, S.A.; et al. Metagenomic insights of the infant microbiome community structure and function across multiple sites in the United States. Sci. Rep. 2021, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Yao, X.-D.; Nasser, L.; Roozrogousheh, A.; Rosenthal, K.L. Breastfeeding behaviors and the innate immune system of human Milk: Working together to protect infants against inflammation, HIV-1, and other infections. Front. Immunol. 2017, 8, 1631. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; Moya, J.; Breck, M.A.; Cook, C.; Fineberg, A.; Angkustsiri, K.; Underwood, M.A. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: A phase I clinical trial. BMC Pediatr. 2017, 17, 133. [Google Scholar] [CrossRef]
- Frese, S.A.; Hutton, A.A.; Contreras, L.N.; Shaw, C.A.; Palumbo, M.C.; Casaburi, G.; Xu, G.; Davis, J.C.C.; Lebrilla, C.B.; Henrick, B.M.; et al. Persistence of supplemented Bifidobacterium longum subsp. infantis EVC001 in breastfed infants. mSphere 2017, 2, 00501–005017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef]
- Zabel, B.; Yde, C.C.; Roos, P.; Marcussen, J.; Jensen, H.M.; Salli, K.; Hirvonen, J.; Ouwehand, A.C.; Morovic, W. Novel genes and metabolite trends in Bifidobacterium longum subsp. infantis Bi-26 metabolism of human milk oligosaccharide 2’-fucosyllactose. Sci. Rep. 2019, 9, 7983. [Google Scholar] [CrossRef]
- Zabel, B.E.; Gerdes, S.; Evans, K.C.; Nedveck, D.; Singles, S.K.; Volk, B.; Budinoff, C. Strain-specific strategies of 2’-fucosyllactose, 3-fucosyllactose, and difucosyllactose assimilation by Bifidobacterium longum subsp. infantis Bi-26 and ATCC 15697. Sci. Rep. 2020, 10, 15919. [Google Scholar] [CrossRef]
- Roura, E.; Koopmans, S.J.; Lallès, J.P.; Le Huerou-Luron, I.; de Jager, N.; Schuurman, T.; Val-Laillet, D. Critical review evaluating the pig as a model for human nutritional physiology. Nutr. Res. Rev. 2016, 29, 60–90. [Google Scholar] [CrossRef]
- Burrin, D.; Sangild, P.T.; Stoll, B.; Thymann, T.; Buddington, R.; Marini, J.; Olutoye, O.; Shulman, R.J. Translational advances in pediatric nutrition and gastroenterology: New insights from pig models. Annu. Rev. Anim. Biosci. 2020, 15, 321–354. [Google Scholar] [CrossRef] [Green Version]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.; Dilger, R.; Donovan, S.M.; Li, Q.; Gross, G.; Brink, L.; Fleming, S. Developing a reference framework for typical development in the young pig. Curr. Dev. Nutr. 2021, 5, 546. [Google Scholar] [CrossRef]
- Fleming, S.A.; Mudd, A.T.; Hauser, J.; Yan, J.; Metairon, S.; Steiner, P.; Donovan, S.M.; Dilger, R.N. Dietary oligofructose alone or in combination with 2’-Fucosyllactose differentially improves recognition memory and hippocampal mRNA expression. Nutrients 2020, 12, 2131. [Google Scholar] [CrossRef] [PubMed]
- Hartke, J.L.; Monaco, M.H.; Wheeler, M.B.; Donovan, S.M. Effect of a short-term fast on intestinal disaccharidase activity and villus morphology of piglets suckling insulin-like growth factor-I transgenic sows. J. Anim. Sci. 2005, 83, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.A.; Jahoor, F.; Burrin, D.G.; Reeds, P.J. Brush-border disaccharidase synthesis in infant pigs measured in vivo with [2H3]. Am. J. Physiol. 1994, 267, G1128–G1134. [Google Scholar] [CrossRef]
- Wang, M.; Radlowski, E.C.; Monaco, M.H.; Fahey, G.C., Jr.; Gaskins, H.R.; Donovan, S.M. Mode of delivery and early nutrition modulate microbial colonization and fermentation products in neonatal piglets. J. Nutr. 2013, 143, 795803. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Bauer, L.L.; Chen, X.; Wang, M.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Fahey, G.C., Jr.; Donovan, S.M. Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J. Nutr. 2012, 142, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Kok, R.G.; de Waal, A.; Schut, F.; Welling, G.W.; Weenk, G.; Hellingwerf, K.J. Specific detection and analysis of a probiotic Bifidobacterium strain in infant feces. Appl. Environ. Microbiol. 1996, 62, 3668–3672. [Google Scholar] [CrossRef] [Green Version]
- Matsuki, T.; Watanabe, K.; Tanaka, R.; Fukuda, M.; Oyaizu, H. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 1999, 65, 4506–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome composition in pediatric populations from birth to adolescence: Impact of diet and prebiotic and probiotic interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borewicz, K.; Suarez-Diez, M.; Hechler, C.; Beijers, R.; de Weerth, C.; Arts, I.; Penders, J.; Thijs, C.; Nauta, A.; Lindner, C.; et al. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci. Rep. 2019, 21, 2434. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.P.; Martin, J.C.; Duncan, S.H.; Flint, H.J. Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro. FEMS Microbiol. Ecol. 2014, 87, 30–40. [Google Scholar] [CrossRef] [Green Version]
- Heinritz, S.; Mosenthin, R.; Weiss, E. Use of pigs as a potential model for research into dietary modulation of the human gut microbiota. Nutr. Res. Rev. 2013, 26, 191–209. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C., Jr.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- Monaco, M.H.; Wang, M.; Pan, X.; Li, Q.; Richards, J.D.; Chichlowski, M.; Berg, B.M.; Dilger, R.N.; Donovan, S.M. Evaluation of Sialyllactose supplementation of a prebiotic-containing formula on growth, intestinal development, and bacterial colonization in the neonatal piglet. Curr. Dev. Nutr. 2018, 2, nzy067. [Google Scholar] [CrossRef]
- Wang, M.; Monaco, M.H.; Hauser, J.; Yan, J.; Dilger, R.N.; Donovan, S.M. Bovine milk oligosaccharides and human milk oligosaccharides modulate the gut microbiota composition and volatile fatty acid concentrations in a preclinical neonatal model. Microorganisms 2021, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Cilieborg, M.S.; Sangild, P.T.; Jensen, M.L.; Østergaard, M.V.; Christensen, L.; Rasmussen, S.O.; Mørbak, A.L.; Jørgensen, C.B.; Bering, S.B. α1,2-Fucosyllactose does not improve intestinal function or prevent Escherichia coli F18 diarrhea in newborn pigs. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanlon, P.R.; Thorsrud, B.A. A 3-week pre-clinical study of 2’-fucosyllactose in farm piglets. Food Chem. Toxicol. 2014, 74, 343–348. [Google Scholar] [CrossRef]
- Reznikov, E.A.; Comstock, S.S.; Hoeflinger, J.L.; Wang, M.; Miller, M.J.; Donovan, S.M. Dietary bovine lactoferrin reduces Staphylococcus aureus in the tissues and modulates the immune response in piglets systemically infected with S. aureus. Curr. Dev. Nutr. 2017, 2, nzy001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azagra-Boronat, I.; Massot-Cladera, M.; Mayneris-Perxachs, J.; Knipping, K.; Van’t Land, B.; Tims, S.; Stahl, B.; Garssen, J.; Franch, À.; Castell, M.; et al. Immunomodulatory and prebiotic effects of 2’-fucosyllactose in suckling rats. Front. Immunol. 2019, 31, 1773. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Berger, B.; Carnielli, V.P.; Ksiazyk, J.; Lagström, H.; Sanchez Luna, M.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; et al. Human milk oligosaccharides: 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT) in infant formula. Nutrients 2018, 10, 1161. [Google Scholar] [CrossRef] [Green Version]
- Marriage, B.J.; Buck, R.H.; Goehring, K.C.; Oliver, J.S.; Williams, J.A. Infants fed a lower calorie formula with 2’FL showed growth and 2’FL uptake like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 649–658. [Google Scholar] [CrossRef] [Green Version]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of infant formula with human milk oligosaccharides on growth and morbidity: A randomized multicenter trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human milk oligosaccharide composition is associated with excessive weight gain during exclusive breastfeeding—An explorative study. Front. Pediatr. 2019, 18, 297. [Google Scholar] [CrossRef] [Green Version]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Ryoo, J.H.; Bode, L.; Goran, M.I. Human milk oligosaccharides and Hispanic infant weight gain in the first 6 months. Obesity 2020, 28, 1519–1525. [Google Scholar] [CrossRef]
- Dror, T.; Dickstein, Y.; Dubourg, G.; Paul, M. Microbiota manipulation for weight change. Microb. Pathog. 2017, 106, 146–161. [Google Scholar] [CrossRef]
- Sattler, V.A.; Bayer, K.; Schatzmayr, G.; Haslberger, A.G.; Klose, V. Impact of a probiotic, inulin, or their combination on the piglets’ microbiota at different intestinal locations. Benef. Microbes 2015, 6, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Musilova, S.; Modrackova, N.; Hermanova, P.; Hudcovic, T.; Svejstil, R.; Rada, V.; Tejnecky, V.; Bunesova, V. Assessment of the synbiotic properites of human milk oligosaccharides and Bifidobacterium longum subsp. infantis in vitro and in humanised mice. Benef. Microbes 2017, 8, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Donovan, S.M. Human microbiota-associated swine: Current progress and future opportunities. ILAR J. 2015, 56, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Breastfeeding Report Card-United States. 2020. Available online: https://www.cdc.gov/breastfeeding/data/reportcard.htm (accessed on 7 April 2021).
- Almeida, C.C.; Mendonça Pereira, B.F.; Leandro, K.C.; Costa, M.P.; Spisso, B.F.; Conte-Junior, C.A. Bioactive compounds in infant formula and their effects on infant nutrition and health: A systematic literature review. Int. J. Food Sci. 2021, 2021, 8850080. [Google Scholar] [CrossRef]
- Kosmerl, E.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Jiménez-Flores, R.; García-Cano, I. Improving human health with milk fat globule membrane, lactic acid bacteria, and bifidobacteria. Microorganisms 2021, 9, 341. [Google Scholar] [CrossRef]
- Chichlowski, M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. The influence of milk oligosaccharides on microbiota of infants: Opportunities for formulas. Ann. Rev. Food Sci. Technol. 2011, 2, 331–351. [Google Scholar] [CrossRef] [Green Version]
| Dietary Treatment 2 | ||||
|---|---|---|---|---|
| CON | FL | CON | FL | |
| Dietary Form | Powder | Powder | Liquid | Liquid |
| Units | g/100 g DM | g/100 g DM | g/L | g/L |
| Dry matter | 97.22 | 97.21 | 194.44 | 194.42 |
| Organic matter | 90.75 | 90.84 | 176.45 | 176.611 |
| Lactose | 40.05 | 39.16 | 77.88 | 76.13 |
| 2’-fucosyllactose | ND | 0.63 | ND | 1.22 |
| Ash | 9.25 | 9.16 | 17.99 | 17.81 |
| Phosphorus | 0.73 | 0.72 | 1.43 | 1.40 |
| Calcium | 0.95 | 0.92 | 1.85 | 1.79 |
| Acid-hydrolyzed fat | 26.29 | 26.32 | 51.12 | 51.17 |
| Crude protein | 23.71 | 23.95 | 46.10 | 46.56 |
| Total amino acids | 23.56 | 24.72 | 45.81 | 48.06 |
| Essential amino acids | ||||
| Arginine | 0.66 | 0.68 | 1.28 | 1.32 |
| Histidine | 0.47 | 0.48 | 0.91 | 0.93 |
| Isoleucine | 1.42 | 1.44 | 2.76 | 2.80 |
| Leucine | 2.45 | 2.49 | 4.76 | 4.84 |
| Lysine | 2.21 | 3.10 | 4.30 | 6.03 |
| Methionine | 0.42 | 0.45 | 0.82 | 0.87 |
| Phenylalanine | 0.89 | 0.91 | 1.73 | 1.77 |
| Threonine | 1.48 | 1.50 | 2.88 | 2.92 |
| Tryptophan | 0.43 | 0.44 | 0.84 | 0.86 |
| Valine | 1.37 | 1.39 | 2.66 | 2.70 |
| Non-essential amino acids | ||||
| Alanine | 1.11 | 1.12 | 2.16 | 2.18 |
| Aspartic acid 3 | 2.39 | 2.42 | 4.65 | 4.70 |
| Cyst(e)ine 4 | 0.50 | 0.51 | 0.97 | 0.99 |
| Glutamic acid 5 | 3.92 | 3.94 | 7.62 | 7.66 |
| Glycine | 0.50 | 0.50 | 0.97 | 0.97 |
| Proline | 1.41 | 1.40 | 2.74 | 2.72 |
| Serine | 1.07 | 1.10 | 2.08 | 2.14 |
| Tyrosine | 0.68 | 0.70 | 1.32 | 1.36 |
| p-Value | |||||||
|---|---|---|---|---|---|---|---|
| CON | BI | FL | FLBI | Diet | Probiotic | Interaction | |
| Villus length (μm) | 529 ± 25.6 | 555 ± 34.4 | 605 ± 39.4 | 542 ± 37.5 | 0.9371 | 0.3502 | 0.5789 |
| Villus width (μm) | 127 ± 5.5 | 115 ± 4.9 | 127 ± 4.5 | 124 ± 3.4 | 0.3234 | 0.1300 | 0.2957 |
| Villus area (μm2) | 65.5 ± 3.7 | 64.8 ± 6.6 | 76 ± 4.9 | 67.4 ± 5.3 | 0.2564 | 0.4062 | 0.4613 |
| Crypt width (μm) | 38.4 ± 0.8 | 41.6 ± 1.4 | 42.3 ± 1.1 | 41.3 ± 1.7 | 0.2285 | 0.5432 | 0.0709 |
| Crypt depth (μm) | 168 ± 7.0 | 145 ± 6.0 | 184 ± 7.5 | 159 ± 4.3 | 0.0402 | 0.0006 | 0.6804 |
| Crypt volume (μm3) | 5853 ± 320 | 5728 ± 362 | 7080 ± 443 | 5961 ± 366 | 0.0962 | 0.0659 | 0.0686 |
| Surface area (μm2) | 205 ± 28 | 204 ± 27.3 | 238 ± 15.2 | 212 ± 17.2 | 0.2499 | 0.4324 | 0.4893 |
| Villus-to-crypt ratio | 3.6 ± 0.3 | 3.6 ± 0.3 | 3.2 ± 0.2 | 3.5 ± 0.2 | 0.3483 | 0.5480 | 0.5369 |
| p-Value | |||||||
|---|---|---|---|---|---|---|---|
| CON | BI | FL | FLBI | Diet | Probiotic | Interaction | |
| Jejunum | 25.5 ± 0.5 | 22.3 ± 3.5 | 23.3 ± 2.4 | 20.6 ± 3.9 | 0.6302 | 0.3396 | 0.6227 |
| Ileum | 14.1 ± 1.7 | 13.9 ± 2.0 | 21.6 ± 3.6 | 25.0 ± 4.6 | 0.0116 | 0.7903 | 0.5158 |
| Presence | Abundance (No Imputations) * | |||
|---|---|---|---|---|
| Positive/Total of Animal (%) | Log10 Copies/g Feces | |||
| Ascending Colon Contents | Rectal Contents | Ascending Colon Contents | Rectal Contents | |
| CON | 0/10 (0) a | 0/12 (0) a | BLD | BLD |
| BI | 6/12 (50) b | 8/13 (62) b | 7.58 ± 0.34 | 8.03 ± 0.13 |
| FL | 0/15 (0) a | 0/15 (0) a | BLD | BLD |
| FLBI | 9/12 (75) b | 8/12 (67) b | 7.46 ± 0.11 | 7.40 ± 0.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniels, V.C.; Monaco, M.H.; Wang, M.; Hirvonen, J.; Jensen, H.M.; Ouwehand, A.C.; Mukherjea, R.; Dilger, R.N.; Donovan, S.M. Evaluation of 2’-Fucosyllactose and Bifidobacterium longum Subspecies infantis on Growth, Organ Weights, and Intestinal Development of Piglets. Nutrients 2022, 14, 199. https://doi.org/10.3390/nu14010199
Daniels VC, Monaco MH, Wang M, Hirvonen J, Jensen HM, Ouwehand AC, Mukherjea R, Dilger RN, Donovan SM. Evaluation of 2’-Fucosyllactose and Bifidobacterium longum Subspecies infantis on Growth, Organ Weights, and Intestinal Development of Piglets. Nutrients. 2022; 14(1):199. https://doi.org/10.3390/nu14010199
Chicago/Turabian StyleDaniels, Victoria C., Marcia H. Monaco, Mei Wang, Johanna Hirvonen, Henrik Max Jensen, Arthur C. Ouwehand, Ratna Mukherjea, Ryan N. Dilger, and Sharon M. Donovan. 2022. "Evaluation of 2’-Fucosyllactose and Bifidobacterium longum Subspecies infantis on Growth, Organ Weights, and Intestinal Development of Piglets" Nutrients 14, no. 1: 199. https://doi.org/10.3390/nu14010199







