Diagnostic Accuracy of IgA Anti-Transglutaminase and IgG Anti-Deamidated Gliadin for Diagnosis of Celiac Disease in Children under Two Years of Age: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.3. Information Sources and Search
2.4. Study Selection
2.5. Data Collection Process
2.6. Definitions for Data Extraction
2.7. Risk of Bias and Applicability
2.8. Diagnostic Accuracy Measures
2.9. Meta-Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
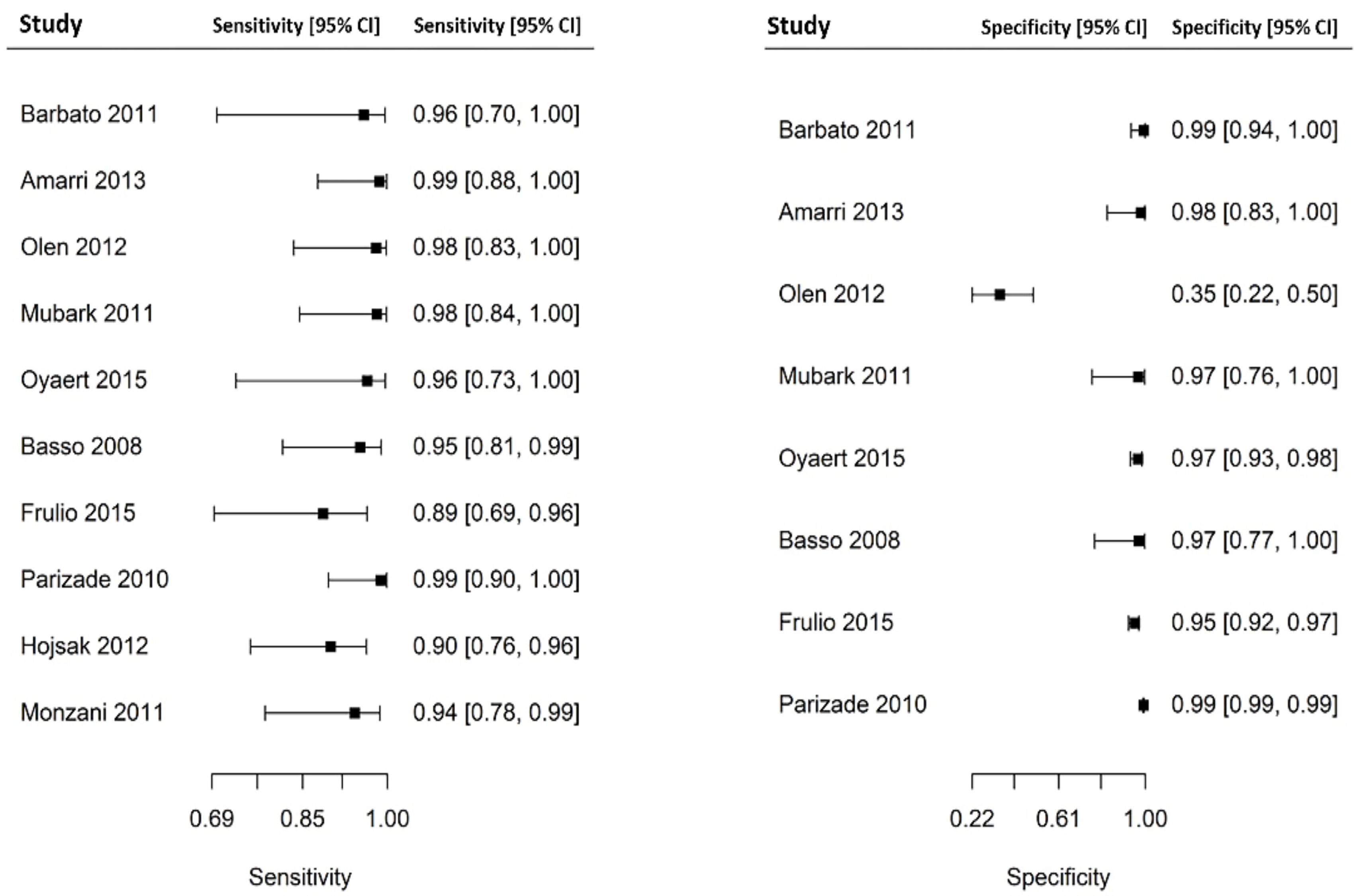
3.3. Results of Individual Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fasano, A.; Catassi, C. Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Kelly, C.P.; Calderwood, A.H.; Murray, J.A. ACG clinical guidelines: Diagnosis and management of celiac disease. Am. J. Gastroenterol. 2013, 108, 656–676. [Google Scholar] [CrossRef] [Green Version]
- Bürgin-Wolff, A.; Gaze, H.; Hadziselimovic, F.; Huber, H.; Lentze, M.J.; Nussle, D.; Reymond-Berthet, C. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch. Dis. Child. 1991, 66, 941–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgin-Wolff, A.; Hadziselimovic, F. Screening test for coeliac disease. Lancet 1997, 349, 1843–1844. [Google Scholar] [CrossRef]
- Lagerqvist, C.; Dahlbom, I.; Hansson, T.; Jidell, E.; Juto, P.; Olcén, P.; Stenlund, H.; Hernell, O.; Ivarsson, A. Antigliadin immunoglobulin A best in finding celiac disease in children younger than 18 months of age. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 428. [Google Scholar] [CrossRef] [PubMed]
- Agarth, D. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin. Gastroenterol. Hepatol. 2007, 5, 1276–1281. [Google Scholar] [CrossRef] [PubMed]
- Salameh, J.P.; Bossuyt, P.M.; McGrath, T.A.; Thombs, B.D.; Hyde, C.J.; Macaskill, P.; Deeks, J.J.; Leeflang, M.; Korevaar, D.A.; Whiting, P.; et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 2020, 370, m2632. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; PRISMA-S Group. PRISMA-S: An extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Fleiss, J.L. The statistical basis of meta-analysis. Stat. Methods Med. Res. 1993, 2, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Parizade, M.; Shainberg, B. Positive deamidated gliadin peptide antibodies and negative tissue transglutaminase IgA antibodies in a pediatric population: To biopsy or not to biopsy. Clin. Vaccine Immunol. 2010, 17, 884–886. [Google Scholar] [CrossRef] [Green Version]
- Barbato, M.; Maiella, G.; Di Camillo, C.; Guida, S.; Valitutti, F.; Lastrucci, G.; Mainiero, F.; Cucchiara, S. The anti-deamidated gliadin peptide antibodies unmask celiac disease in small children with chronic diarrhoea. Dig. Liver Dis. 2011, 43, 465–469. [Google Scholar] [CrossRef]
- Monzani, A.; Rapa, A.; Fonio, P.; Tognato, E.; Panigati, L.; Oderda, G. Use of deamidated gliadin peptide antibodies to monitor diet compliance in childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Amarri, S.; Alvisi, P.; De Giorgio, R.; Gelli, M.C.; Cicola, R.; Tovoli, F.; Sassatelli, R.; Caio, G.; Volta, U. Antibodies to deamidated gliadin peptides: An accurate predictor of coeliac disease in infancy. J. Clin. Immunol. 2013, 33, 1027–1030. [Google Scholar] [CrossRef]
- Oyaert, M.; Vermeersch, P.; De Hertogh, G.; Hiele, M.; Vandeputte, N.; Hoffman, I.; Bossuyt, X. Combining antibody tests and taking into account antibody levels improves serologic diagnosis of celiac disease. Clin. Chem. Lab. Med. 2015, 53, 1537–1546. [Google Scholar] [CrossRef]
- Basso, D.; Guariso, G.; Fogar, P.; Meneghel, A.; Zambon, C.-F.; Navaglia, F.; Greco, E.; Schiavon, S.; Rugge, M.; Plebani, M. Antibodies against synthetic deamidated gliadin peptides for celiac disease diagnosis and follow-up in children. Clin. Chem. 2009, 55, 150–157. [Google Scholar] [CrossRef]
- Mubarak, A.; Gmelig-Meyling, F.H.J.; Wolters, V.M.; Ten Kate, F.J.W.; Houwen, R.H.J. Immunoglobulin G antibodies against deamidated-gliadin-peptides outperform anti-endomysium and tissue transglutaminase antibodies in children <two years age. APMIS 2011, 119, 894–900. [Google Scholar] [CrossRef]
- Olen, O.; Gudjónsdóttir, A.H.; Browaldh, L.; Hessami, M.; Elvin, K.; Liedberg, A.-S.; Neovius, M.; Grahnquist, L. Antibodies against deamidated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Frulio, G.; Polimeno, A.; Palmieri, D.; Fumi, M.; Auricchio, R.; Piccolo, E.; Giarrusso, P.C. Evaluating diagnostic accuracy of anti-tissue Transglutaminase IgA antibodies as first screening for Celiac Disease in very young children. Clin. Chim. Acta 2015, 446, 237–240. [Google Scholar] [CrossRef]
- Hojsak, I.; Mozer-Glassberg, Y.; Gilboa, N.S.; Weinberger, R.; Hartman, C.; Shamir, R. Celiac disease screening assays for children younger than 3 years of age: The performance of three serological tests. Dig. Dis. Sci. 2012, 57, 127–132. [Google Scholar] [CrossRef]
- Catassi, G.N.; Vallorani, M.; Cerioni, F.; Lionetti, E.; Catassi, C. A negative fallout of COVID-19 lockdown in Italy: Life-threatening delay in the diagnosis of celiac disease. Dig. Liver Dis. 2020, 52, 1092–1093. [Google Scholar] [CrossRef]
- Arigliani, M.; Morassutti, F.R.; Fabris, M.; Melli, P.; Tonutti, E.; Cogo, P. Coeliac disease in infants: Antibodies to deamidated gliadin peptide come first! Ital. J. Pediatr. 2017, 43, 70. [Google Scholar] [CrossRef] [Green Version]
- Pacitto, A.; Paglino, A.; Di Genova, L.; Leonardi, A.; Farinelli, E.; Principi, N.; Di Cara, G.; Esposito, S. Celiac disease presenting with peripheral neuropathy in children: A case report. Int. J. Environ. Res. Public Health 2017, 14, 785. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.; Li, M.; Emery, L.; Taki, I.; Barriga, K.; Tiberti, C.; Eisenbarth, G.S.; Rewers, M.J.; Hoffenberg, E.J. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Lammi, A.; Arikoski, P.; Hakulinen, A.; Schwab, U.; Uusitupa, M.; Heinonen, S.; Savilahti, E.; Kinnunen, T.; Ilonen, J. Development of gliadin-specific immune responses in children with HLA-associated genetic risk for celiac disease. Scand. J. Gastroenterol. 2016, 51, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Mallon, D.; Hajjat, T.M. Serologic Evaluation of Celiac Disease for Patients Younger Than two years of Age. J. Pediatr. 2020, 224, 16–17. [Google Scholar] [CrossRef]
- Walker-Smith, J.A.; Guandalini, S.; Schmitz, J. Revised criteria for diagnosis of coeliac disease. Arch. Dis. Child. 1990, 65, 909–911. [Google Scholar]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]




| Publication | Study Type and Location | Total No. Included | Gender (%Female) | Age | Population | Target Condition | Reference Test | Index Tests | TP | TN | TP DGP IgG | TP TTG IgA | TN DGP IgG | TN TTG IgA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parizade 2010 [15] | Prospective controlled, Israel | 5002 | NR | <2 yr | 200 symptomatic children; 4802 healthy children | CD | Intestinal biopsy | DGP IgG and TTG IgA | 41 | 4961 | 41/41 | 35/41 | 4912/4961 | 4961/4961 |
| Barbato 2011 [16] | Prospective controlled, Italy | 80 | 43 | 4–24 mo | 40 children with classic symptoms of CD and with DGP IgG positive and TTG IgA negative; 40 healthy children | CD | Intestinal biopsy | DGP IgG and TTG IgA | 11 | 69 | 11/11 | 0/11 | 69/69 | 69/69 |
| Monzani 2011 [17] | Prospective, controlled, Italy | 130 | NR | <2 yr | 24 children with classic symptoms of CD; 106 healthy children | CD | Intestinal biopsy | DGP IgG and TTG IgA | 24 | 106 | 24/24 | 24/24 | NR | NR |
| Amarri 2013 [18] | Prospective controlled, Italy | 57 | 62 | <2 yr | 34 children with classic symptoms of CD; 23 healthy children | CD | Intestinal biopsy | DGP IgG and TTG IgA | 34 | 23 | 34/34 | 33/34 | 23/23 | 23/23 |
| Oyaert 2015 [19] | Prospective controlled, Belgium | 210 | 44 | <2 yr | 13 symptomatic children; 197 healthy children | CD | Intestinal biopsy | DGP IgG and TTG IgA | 13 | 197 | 13/13 | 12/13 | 191/197 | 196/197 |
| Basso 2008 [20] | Retrospective, Italy | 46 | NR | <2 yr | 46 symptomatic children undergoing intestinal biopsy | CD | Intestinal biopsy | DGP IgG and TTG IgA | 30 | 16 | 29/30 | 29/30 | 16/16 | 16/16 |
| Mubarak 2011 [21] | Retrospective, The Netherlands | 41 | NR | <2 yr | 41 symptomatic children undergoing intestinal biopsy | CD | Intestinal biopsy | DGP IgG and TTG IgA | 26 | 15 | 26/26 | 25/26 | 15/15 | 15/15 |
| Olen 2012 [22] | Retrospective, Sweden | 71 | NR | <2 yr | 71 symptomatic children undergoing intestinal biopsy | CD | Intestinal biopsy | 65 children tested for DGP IgG and TTG IgA; 2 tested for TTG IgA only; 4 tested for DGP IgG only) | 26 (24 tested for both index tests; 2 tested only for TTG IgA). | 45 (41 tested for both index tests; 4 tested only for DGP IgG) | 24/24 | 24/26 | 14/45 | 40/41 |
| Frulio 2015 [23] | Retrospective, Italy | 348 | 49 | 6–24 yr | 348 symptomatic children undergoing intestinal biopsy | CD | Intestinal biopsy | DGP IgG and TTG IgA | 21 | 327 | 19/21 | 21/21 | 312/327 | 327/327 |
| Hojsak 2012 [24] | Retrospective, Israel | 31 | 46 | <2 yr | 31 symptomatic children undergoing intestinal biopsy | CD | Intestinal biopsy | DGP IgG and TTG IgA | 31 | NR | 31/31 | 30/30 | NR | NR |
| Catassi 2020 [25] | Case report, Italy | 1 | 100 | 18 mo | 1 child presenting with CD crisis | CD | Intestinal biopsy | DGP IgG and TTG IgA | 1 | NA | 1/1 | 0 | NA | NA |
| Arigliani 2017 [26] | Case report, Italy | 1 | 0 | 8 mo | 1 child presenting with failure to thrive, constipation, and developmental delay | CD | Intestinal biopsy | DGP IgG and TTG IgA | 1 | NA | 1/1 | 0 | NA | NA |
| Pacitto 2017 [27] | Case report, Italy | 1 | 0 | 23 mo | 1 child presenting with malabsorption syndrome and peripheral neuropathy | CD | Intestinal biopsy | DGP IgG and TTG IgA | 1 | NA | 1/1 | 1/1 | NA | NA |
| Liu 2015 [28] | Longitudinal cohort, USA | 1243 | NR | 0.5–17 yr | 1243 newborn at genetic risk of CD screened with both index tests | CD | Intestinal biopsy | DGP IgG and TTG IgA | 50 | 1193 | 50/50 | 50/50 | NR | NR |
| Lammi 2016 [29] | Longitudinal cohort, Finland | 291 | NR | 6–48 mo | 291 newborn at genetic risk of CD screened with both index tests | CD | Intestinal biopsy | DGP IgG and TTG IgA | 9 | 282 | 9/9 | 9/9 | NR | NR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catassi, G.N.; Pulvirenti, A.; Monachesi, C.; Catassi, C.; Lionetti, E. Diagnostic Accuracy of IgA Anti-Transglutaminase and IgG Anti-Deamidated Gliadin for Diagnosis of Celiac Disease in Children under Two Years of Age: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 7. https://doi.org/10.3390/nu14010007
Catassi GN, Pulvirenti A, Monachesi C, Catassi C, Lionetti E. Diagnostic Accuracy of IgA Anti-Transglutaminase and IgG Anti-Deamidated Gliadin for Diagnosis of Celiac Disease in Children under Two Years of Age: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(1):7. https://doi.org/10.3390/nu14010007
Chicago/Turabian StyleCatassi, Giulia N., Alfredo Pulvirenti, Chiara Monachesi, Carlo Catassi, and Elena Lionetti. 2022. "Diagnostic Accuracy of IgA Anti-Transglutaminase and IgG Anti-Deamidated Gliadin for Diagnosis of Celiac Disease in Children under Two Years of Age: A Systematic Review and Meta-Analysis" Nutrients 14, no. 1: 7. https://doi.org/10.3390/nu14010007
APA StyleCatassi, G. N., Pulvirenti, A., Monachesi, C., Catassi, C., & Lionetti, E. (2022). Diagnostic Accuracy of IgA Anti-Transglutaminase and IgG Anti-Deamidated Gliadin for Diagnosis of Celiac Disease in Children under Two Years of Age: A Systematic Review and Meta-Analysis. Nutrients, 14(1), 7. https://doi.org/10.3390/nu14010007







