Probiotic Supplementation for Promotion of Growth in Children: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
3. Results
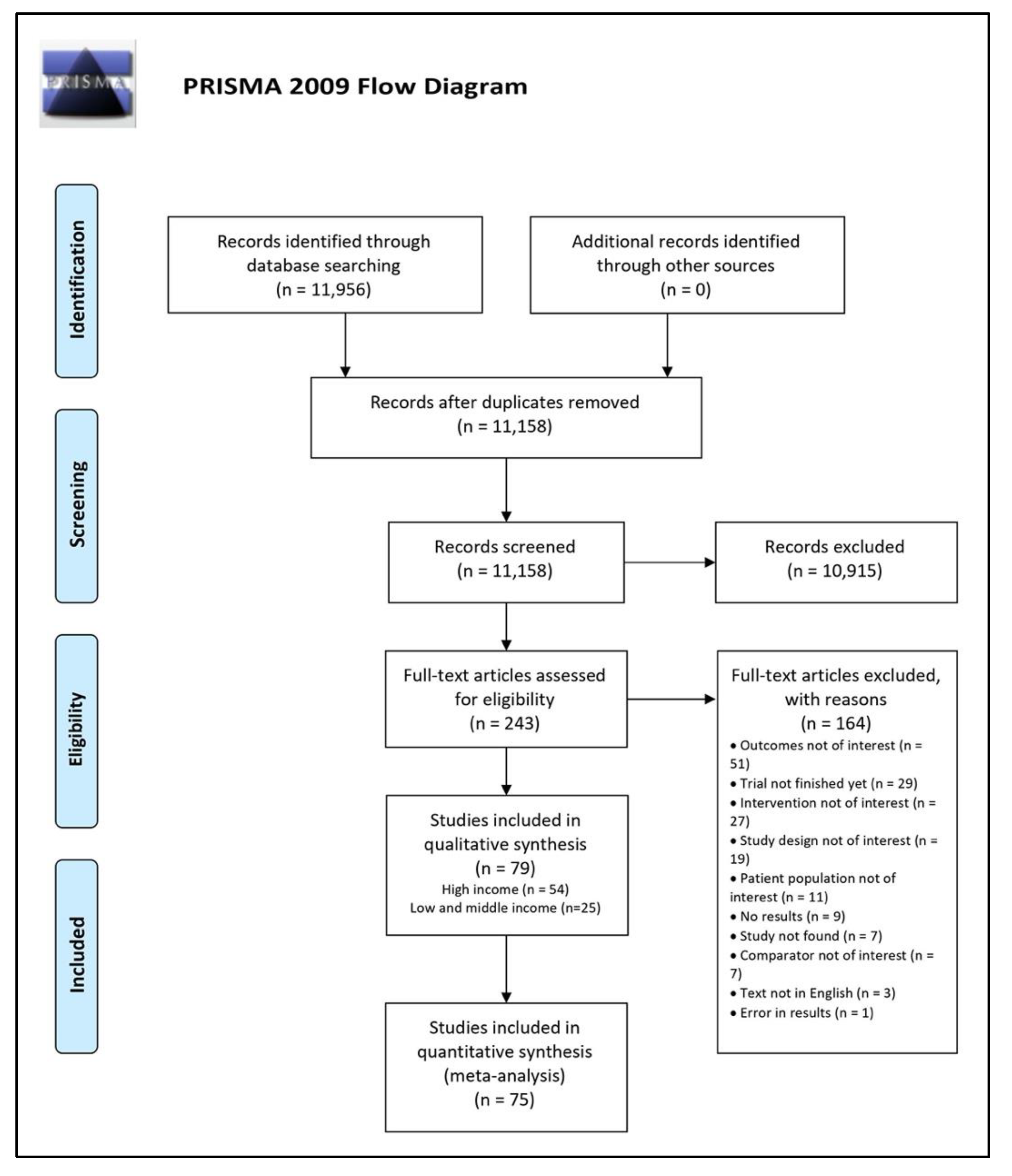
3.1. Literature Search
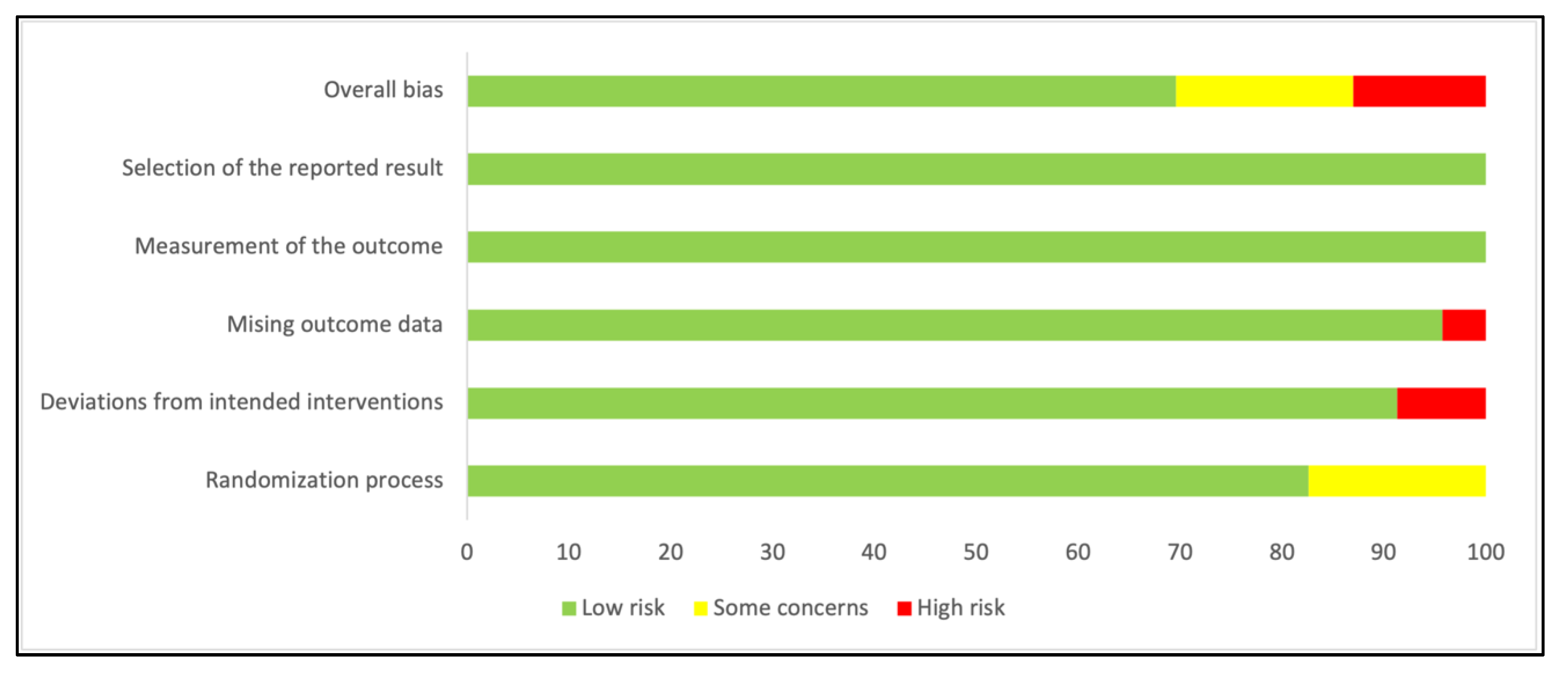
3.2. Characteristics of Included Studies
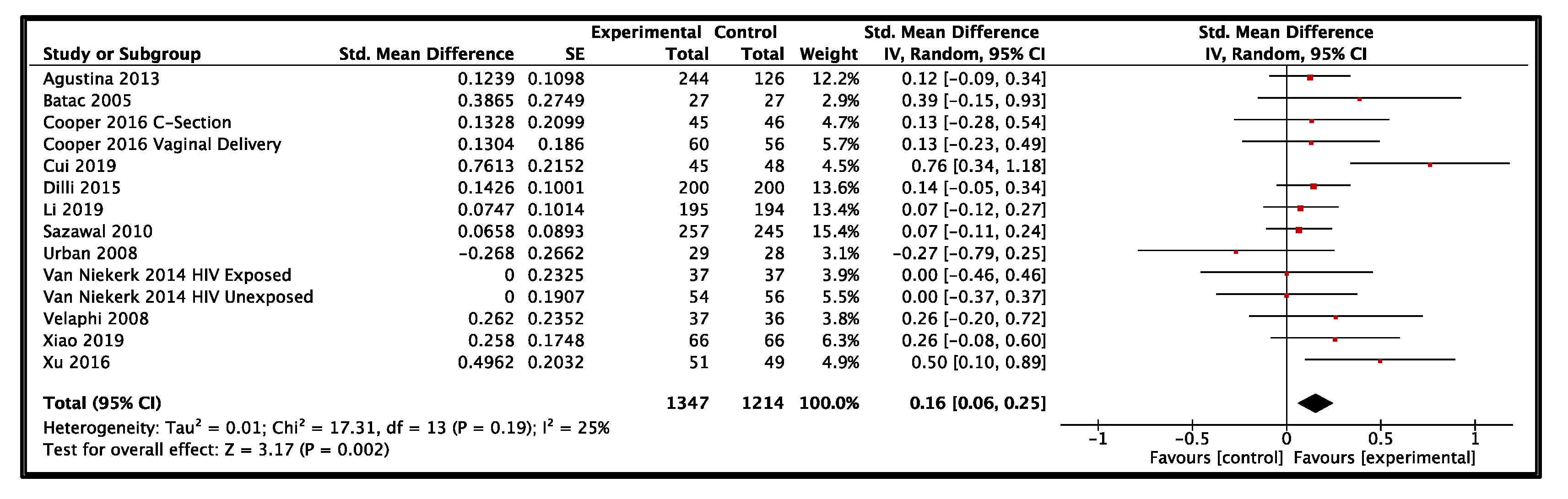
3.3. LMIC Results
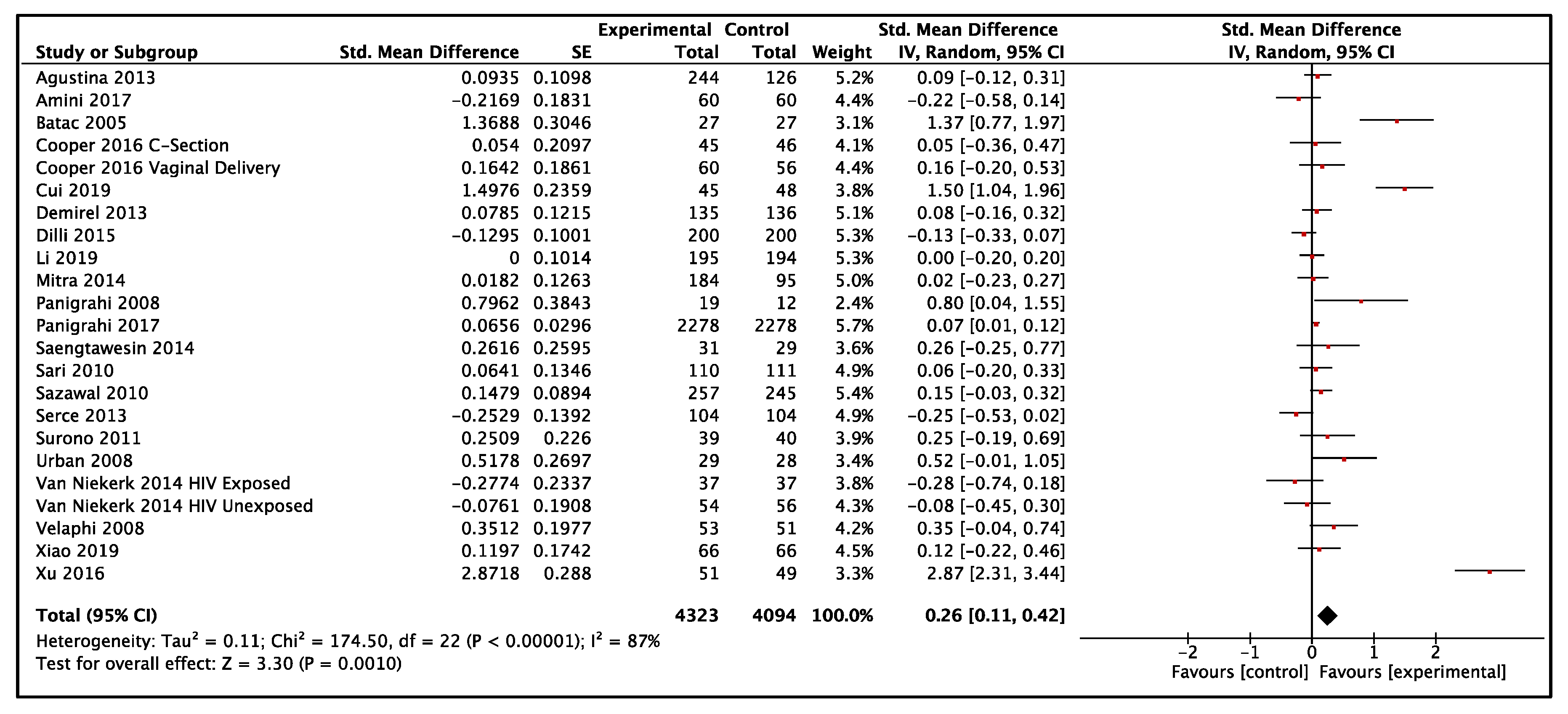
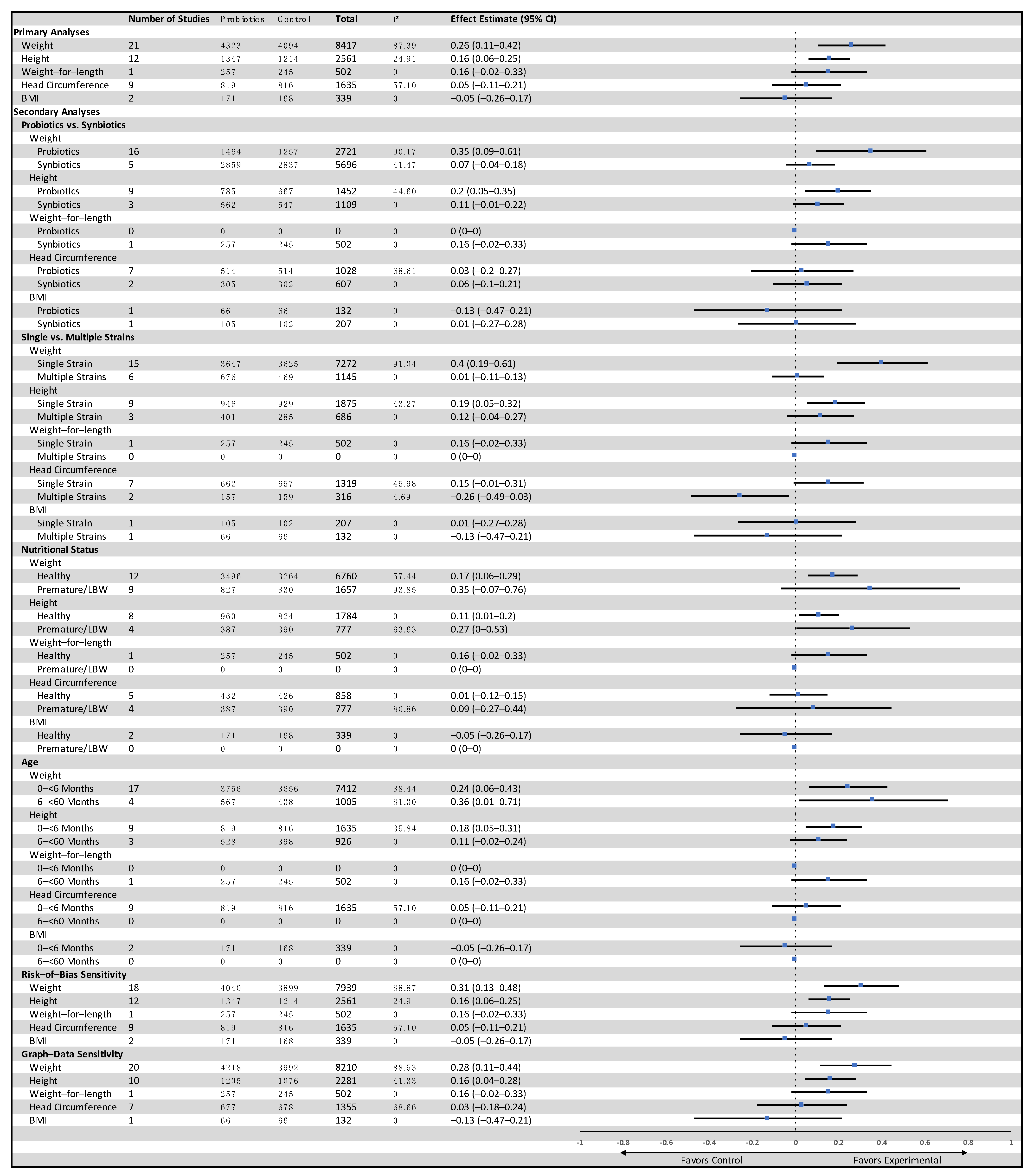
3.3.1. Weight-for-Age
3.3.2. Height-for-Age
3.3.3. Other Outcomes
3.4. HIC Results
3.4.1. Weight-for-Age
3.4.2. Height-for-Age
3.4.3. Other Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rackaityte, E.; Lynch, S.V. The human microbiome in the 21(st) century. Nat. Commun. 2020, 11, 5256. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, 6275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.Y.; Mostafa, I.; Hibberd, M.C.; Das, S.; Mahfuz, M.; Naila, N.N.; Islam, M.M.; Huq, S.; Alam, M.A.; Zaman, M.U.; et al. A Microbiota-Directed Food Intervention for Undernourished Children. N. Engl. J. Med. 2021, 384, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, J.L.; Venkatesh, S.; Chang, H.W.; Hibberd, M.C.; Kung, V.L.; Cheng, J.; Chen, R.Y.; Subramanian, S.; Cowardin, C.A.; Meier, M.F.; et al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science 2019, 365, 6449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The Human Microbiome and Child Growth—First 1000 Days and Beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Probiotics Market Size, Share & Trends Analysis Report by Product (Food & Beverages, Dietary Supplements), By Ingredient (Bacteria, Yeast), By End Use, By Distribution Channel, And Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com (accessed on 6 August 2021).
- Depoorter, L.; Vandenplas, Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients 2021, 13, 2176. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.B. Prebiotics: Preferential substrates for specific germs? Am. J. Clin. Nutr. 2001, 73, 406S–409S. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.D.; Gibson, G.R. Probiotics, prebiotics, and synbiotics: Approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999, 69, 1052S–1057S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Million, M.; Angelakis, E.; Paul, M.; Armougom, F.; Leibovici, L.; Raoult, D. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb. Pathog. 2012, 53, 100–108. [Google Scholar] [CrossRef]
- Onubi, O.J.; Poobalan, A.S.; Dineen, B.; Marais, D.; McNeill, G. Effects of probiotics on child growth: A systematic review. J. Health Popul. Nutr. 2015, 34, 8. [Google Scholar] [CrossRef] [Green Version]
- Steenhout, P.G.; Rochat, F.; Hager, C. The effect of Bifidobacterium lactis on the growth of infants: A pooled analysis of randomized controlled studies. Ann. Nutr. Metab. 2009, 55, 334–340. [Google Scholar] [CrossRef]
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 6 November 2020).
- Serce, O.; Gursoy, T.; Karatekin, G.; Ovali, F. Effects of prebiotic and probiotic combination on necrotizing enterocolitis and sepsis prophylaxis in very low birth weight infants. J. Perinat. Med. 2013, 41. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 15; StataCorp LLC.: College Station, TX, USA, 2017. [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Higgins, J.P.T., Green, S., Eds.; 2011. Available online: www.handbook.cochrane.org (accessed on 6 November 2020).
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse-Berkeveld, M.; Alles, M.; Franke-Beckmann, E.; Helm, K.; Knecht, R.; Kollges, R.; Sandner, B.; Knol, J.; Ben Amor, K.; Bufe, A. Infant formula containing galacto-and fructo-oligosaccharides and Bifidobacterium breve M-16V supports adequate growth and tolerance in healthy infants in a randomised, controlled, double-blind, prospective, multicentre study. J. Nutr. Sci. 2016, 5, e42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustina, R.; Bovee-Oudenhoven, I.M.; Lukito, W.; Fahmida, U.; van de Rest, O.; Zimmermann, M.B.; Firmansyah, A.; Wulanti, R.; Albers, R.; van den Heuvel, E.G.; et al. Probiotics Lactobacillus reuteri DSM 17938 and Lactobacillus casei CRL 431 modestly increase growth, but not iron and zinc status, among Indonesian children aged 1-6 years. J. Nutr. 2013, 143, 1184–1193. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, B.; Hellmuth, C.; Haiden, N.; Olbertz, D.; Hamelmann, E.; Vusurovic, M.; Fleddermann, M.; Roehle, R.; Knoll, A.; Koletzko, B.; et al. Hydrolyzed Formula with Reduced Protein Content Supports Adequate Growth: A Randomized Controlled Noninferiority Trial. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 822–830. [Google Scholar] [CrossRef]
- Al-Hosni, M.; Duenas, M.; Hawk, M.; Stewart, L.A.; Borghese, R.A.; Cahoon, M.; Atwood, L.; Howard, D.; Ferrelli, K.; Soll, R. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 2012, 32, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.J.; Jordan, S.; Storey, M.; Thornton, C.A.; Gravenor, M.; Garaiova, I.; Plummer, S.F.; Wang, D.; Morgan, G. Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J. Nutr. 2010, 140, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Aloisio, I.; Prodam, F.; Giglione, E.; Bozzi Cionci, N.; Solito, A.; Bellone, S.; Baffoni, L.; Mogna, L.; Pane, M.; Bona, G.; et al. Three-Month Feeding Integration with Bifidobacterium Strains Prevents Gastrointestinal Symptoms in Healthy Newborns. Front. Nutr. 2018, 5, 39. [Google Scholar] [CrossRef] [Green Version]
- Amini, E.; Dalili, H.; Niknafs, N.; Shariat, M.; Nakhostin, M.; Jedari-Attari, S. The effect of probiotics in prevention of necrotising enterocolitis in preterm neonates in comparison with control group. Iran. J. Pediatrics 2017, 27. [Google Scholar] [CrossRef] [Green Version]
- Batac, M.C.R.; Guno, M.J.V.; Caparas-de Castro, C.; Gutierrez-Santos, K.; Tondoc, A. Effects of a probiotic formula on measles, mumps and rubella IgG production and on anthropometric measurements of infants aged 11-15 months in a tertiary hospital. St. Tomas J. Med. 2005, 52, 124–130. [Google Scholar]
- Bazanella, M.; Maier, T.V.; Clavel, T.; Lagkouvardos, I.; Lucio, M.; Maldonado-Gòmez, M.X.; Autran, C.; Walter, J.; Bode, L.; Schmitt-Kopplin, P.; et al. Randomized controlled trial on the impact of early-life intervention with bifidobacteria on the healthy infant fecal microbiota and metabolome. Am. J. Clin. Nutr. 2017, 106, 1274–1286. [Google Scholar] [CrossRef] [Green Version]
- Bin-Nun, A.; Bromiker, R.; Wilschanski, M.; Kaplan, M.; Rudensky, B.; Caplan, M.; Hammerman, C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J. Pediatr. 2005, 147, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Cekola, P.L.; Czerkies, L.A.; Storm, H.M.; Wang, M.H.; Roberts, J.; Saavedra, J.M. Growth and Tolerance of Term Infants Fed Formula With Probiotic Lactobacillus re.e.euteri. Clin. Pediatr. 2015, 54, 1175–1184. [Google Scholar] [CrossRef]
- Chouraqui, J.P.; Grathwohl, D.; Labaune, J.M.; Hascoet, J.M.; de Montgolfier, I.; Leclaire, M.; Giarre, M.; Steenhout, P. Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am. J. Clin. Nutr. 2008, 87, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Chrzanowska-Liszewska, D.; Seliga-Siwecka, J.; Kornacka, M.K. The effect of Lactobacillus rhamnosus GG supplemented enteral feeding on the microbiotic flora of preterm infants-double blinded randomized control trial. Early Hum. Dev. 2012, 88, 57–60. [Google Scholar] [CrossRef]
- Cooper, P.; Bolton, K.D.; Velaphi, S.; de Groot, N.; Emady-Azar, S.; Pecquet, S.; Steenhout, P. Early Benefits of a Starter Formula Enriched in Prebiotics and Probiotics on the Gut Microbiota of Healthy Infants Born to HIV+ Mothers: A Randomized Double-Blind Controlled Trial. Clin. Med. Insights Pediatr. 2016, 10, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Costalos, C.; Skouteri, V.; Gounaris, A.; Sevastiadou, S.; Triandafilidou, A.; Ekonomidou, C.; Kontaxaki, F.; Petrochilou, V. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum. Dev. 2003, 74, 89–96. [Google Scholar] [CrossRef]
- Costeloe, K.; Hardy, P.; Juszczak, E.; Wilks, M.; Millar, M.R. Bifidobacterium breve BBG-001 in very preterm infants: A randomised controlled phase 3 trial. Lancet 2016, 387, 649–660. [Google Scholar] [CrossRef]
- Cui, X.; Shi, Y.; Gao, S.; Xue, X.; Fu, J. Efffects of Lactobacillus reuteri DSM 17938 in preterm infants: A double-blinded randomized controlled study. Ital. J. Pediatr. 2019, 45, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, J.W.; Wickens, K.; Black, P.N.; Stanley, T.V.; Mitchell, E.A.; Fitzharris, P.; Tannock, G.W.; Purdie, G.; Crane, J. Safety aspects of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis subsp. lactis HN019 in human infants aged 0–2 years. Int. Dairy J. 2009, 19, 149–154. [Google Scholar] [CrossRef]
- Demirel, G.; Erdeve, O.; Celik, I.H.; Dilmen, U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: A randomized, controlled study. Acta Paediatr 2013, 102, e560–e565. [Google Scholar] [CrossRef] [PubMed]
- Dilli, D.; Aydin, B.; Fettah, N.D.; Ozyazici, E.; Beken, S.; Zenciroglu, A.; Okumus, N.; Ozyurt, B.M.; Ipek, M.S.; Akdag, A.; et al. The propre-save study: Effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr 2015, 166, 545–551. [Google Scholar] [CrossRef]
- Escribano, J.; Ferre, N.; Gispert-Llaurado, M.; Luque, V.; Rubio-Torrents, C.; Zaragoza-Jordana, M.; Polanco, I.; Codoner, F.M.; Chenoll, E.; Morera, M.; et al. Bifidobacterium longum subsp infantis CECT7210-supplemented formula reduces diarrhea in healthy infants: A randomized controlled trial. Pediatr. Res. 2018, 83, 1120–1128. [Google Scholar] [CrossRef] [PubMed]
- Gil-Campos, M.; Lopez, M.A.; Rodriguez-Benitez, M.V.; Romero, J.; Roncero, I.; Linares, M.D.; Maldonado, J.; Lopez-Huertas, E.; Berwind, R.; Ritzenthaler, K.L.; et al. Lactobacillus fermentum CECT 5716 is safe and well tolerated in infants of 1-6 months of age: A randomized controlled trial. Pharmacol. Res. 2012, 65, 231–238. [Google Scholar] [CrossRef]
- Guney Varal, I.; Koksal, N.; Ozkan, H.; Bagci, O.; Dogan, P. Potential use of multi-strain synbiotics for improving postnatal head circumference. Pak. J. Med. Sci. 2018, 34, 1502–1506. [Google Scholar] [CrossRef]
- Harvey, B.M.; Langford, J.E.; Harthoorn, L.F.; Gillman, S.A.; Green, T.D.; Schwartz, R.H.; Burks, A.W. Effects on growth and tolerance and hypoallergenicity of an amino acid-based formula with synbiotics. Pediatr. Res. 2014, 75, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hays, S.; Jacquot, A.; Gauthier, H.; Kempf, C.; Beissel, A.; Pidoux, O.; Jumas-Bilak, E.; Decullier, E.; Lachambre, E.; Beck, L.; et al. Probiotics and growth in preterm infants: A randomized controlled trial, PREMAPRO study. Clin. Nutr. 2015, 35, 802–811. [Google Scholar] [CrossRef] [Green Version]
- Hojsak, I.; Snovak, N.; Abdovic, S.; Szajewska, H.; Misak, Z.; Kolacek, S. Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2010, 29, 312–316. [Google Scholar] [CrossRef]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Cavallo, L.; Francavilla, R. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 2008, 152, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Tobin, J.M.; Opie, G.F.; Donath, S.; Tabrizi, S.N.; Pirotta, M.; Morley, C.J.; Garland, S.M.; ProPrems Study, G. Probiotic effects on late-onset sepsis in v.very preterm infants: A randomized controlled trial. Pediatrics 2013, 132, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kankaanpaa, P.E.; Yang, B.; Kallio, H.P.; Isolauri, E.; Salminen, S.J. Influence of probiotic supplemented infant formula on composition of plasma lipids in atopic infants. J. Nutr. Biochem. 2002, 13, 364–369. [Google Scholar] [CrossRef]
- Kukkonen, K.; Savilahti, E.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Poussa, T.; Tuure, T.; Kuitunen, M. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: Randomized, double-blind, placebo-controlled trial. Pediatrics 2008, 122, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Peng, Y.; Li, Z.; Christensen, B.; Heckmann, A.B.; Stenlund, H.; Lönnerdal, B.; Hernell, O. Feeding Infants Formula with Probiotics or Milk Fat Globule Membrane: A Double-Blind, Randomized Controlled Trial. Front. Pediatrics 2019, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Hsu, C.H.; Chen, H.L.; Chung, M.Y.; Hsu, J.F.; Lien, R.I.; Tsao, L.Y.; Chen, C.H.; Su, B.H. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: A multicenter, randomized, controlled trial. Pediatrics 2008, 122, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Luoto, R.; Kalliomaki, M.; Laitinen, K.; Isolauri, E. The impact of perinatal probiotic intervention on the development of overweight and obesity: Follow-up study from birth to 10 years. Int. J. Obes. 2010, 34, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, J.; Canabate, F.; Sempere, L.; Vela, F.; Sanchez, A.R.; Narbona, E.; Lopez-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, J.; Gil-Campos, M.; Maldonado-Lobon, J.A.; Benavides, M.R.; Flores-Rojas, K.; Jaldo, R.; Jimenez Del Barco, I.; Bolivar, V.; Valero, A.D.; Prados, E.; et al. Evaluation of the safety, tolerance and efficacy of 1-year consumption of infant formula supplemented with Lactobacillus fermentum CECT5716 Lc40 or Bifidobacterium breve CECT7263: A randomized controlled trial. BMC Pediatr. 2019, 19, 361. [Google Scholar] [CrossRef]
- Maldonado, J.; Lara-Villoslada, F.; Sierra, S.; Sempere, L.; Gomez, M.; Rodriguez, J.M.; Boza, J.; Xaus, J.; Olivares, M. Safety and tolerance of the human milk probiotic strain Lactobacillus salivarius CECT5713 in 6-month-old children. Nutrition 2010, 26, 1082–1087. [Google Scholar] [CrossRef]
- Manzano, S.; De Andres, J.; Castro, I.; Rodriguez, J.M.; Jimenez, E.; Espinosa-Martos, I. Safety and tolerance of three probiotic strains in healthy infants: A multi-centre randomized, double-blind, placebo-controlled trial. Benef. Microbes 2017, 8, 569–578. [Google Scholar] [CrossRef]
- Millar, M.R.; Bacon, C.; Smith, S.L.; Walker, V.; Hall, M.A. Enteral feeding of premature infants with Lactobacillus GG. Arch. Dis. Child. 1993, 69, 483–487. [Google Scholar] [CrossRef]
- Mitra, M.; Adarsh, E.; Narang, A.; Agrawal, R.; Vaidya, U.; Ganguly, S. Safety and tolerance of infant formulas containing probiotics in India: A multicenter randomized controlled trial. J. Matern.-Fetal Neonatal Med. 2014, 27, 346. [Google Scholar] [CrossRef]
- Mohan, R.; Koebnick, C.; Schildt, J.; Mueller, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 2008, 64, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Chandel, D.S.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [Google Scholar] [CrossRef]
- Panigrahi, P.; Parida, S.; Pradhan, L.; Mohapatra, S.S.; Misra, P.R.; Johnson, J.A.; Chaudhry, R.; Taylor, S.; Hansen, N.I.; Gewolb, I.H. Long-term colonization of a Lactobacillus plantarum synbiotic preparation in the neonatal gut. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 45–53. [Google Scholar] [CrossRef]
- Papagaroufalis, K.; Fotiou, A.; Egli, D.; Tran, L.A.; Steenhout, P. A Randomized Double Blind Controlled Safety Trial Evaluating d-Lactic Acid Production in Healthy Infants Fed a Lactobacillus reuteri-containing Formula. Nutr. Metab. Insights 2014, 7, 19–27. [Google Scholar] [CrossRef]
- Puccio, G.; Cajozzo, C.; Meli, F.; Rochat, F.; Grathwohl, D.; Steenhout, P. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum.m.m BL999 and prebiotics. Nutrition 2007, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Radke, M.; Picaud, J.C.; Loui, A.; Cambonie, G.; Faas, D.; Lafeber, H.N.; de Groot, N.; Pecquet, S.S.; Steenhout, P.G.; Hascoet, J.M. Starter formula enriched in prebiotics and probiotics ensures normal growth of infants and promotes gut health: A randomized clinical trial. Pediatr. Res. 2017, 81, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouge, C.; Piloquet, H.; Butel, M.J.; Berger, B.; Rochat, F.; Ferraris, L.; Des Robert, C.; Legrand, A.; de la Cochetiere, M.F.; N’Guyen, J.M.; et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2009, 89, 1828–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roze, J.C.; Barbarot, S.; Butel, M.J.; Kapel, N.; Waligora-Dupriet, A.J.; De Montgolfier, I.; Leblanc, M.; Godon, N.; Soulaines, P.; Darmaun, D.; et al. An alpha-lactalbumin-enriched and symbiotic-supplemented v. a standard infant formula: A multicentre, double-blind, randomised trial. Br. J. Nutr. 2012, 107, 1616–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, J.M.; Abi-Hanna, A.; Moore, N.; Yolken, R.H. Long-term consumption of infant formulas containing live probiotic bacteria: Tolerance and safety. Am. J. Clin. Nutr. 2004, 79, 261–267. [Google Scholar] [CrossRef]
- Saengtawesin, V.; Tangpolkaiwalsak, R.; Kanjanapattankul, W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thail. 2014, 97, S20–S25. [Google Scholar]
- Sari, F.N.; Dizdar, E.A.; Oguz, S.; Erdeve, O.; Uras, N.; Dilmen, U. Oral probiotics: Lactobacillus sporogenes in prevention of necrotizing enterocolitis in very low birth weight infants: A randomized, controlled trial. Early Hum. Dev. 2010, 86, S87. [Google Scholar] [CrossRef]
- Sazawal, S.; Dhingra, U.; Hiremath, G.; Sarkar, A.; Dhingra, P.; Dutta, A.; Menon, V.P.; Black, R.E. Effects of Bifidobacterium lactis HN019 and prebiotic oligosaccharide added to milk on iron status, anemia, and growth among children 1 to 4 years old. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 341–346. [Google Scholar] [CrossRef]
- Scalabrin, D.M.; Johnston, W.H.; Hoffman, D.R.; P’Pool, V.L.; Harris, C.L.; Mitmesser, S.H. Growth and tolerance of healthy term infants receiving hydrolyzed infant formulas supplemented with Lactobacillus rhamnosus GG: Randomized, double-blind, controlled trial. Clin. Pediatr. 2009, 48, 734–744. [Google Scholar] [CrossRef]
- Serce, O.; Benzer, D.; Gursoy, T.; Karatekin, G.; Ovali, F. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: A randomised controlled trial. Early Hum. Dev. 2013, 89, 1033–1036. [Google Scholar] [CrossRef]
- Simeoni, U.; Berger, B.; Junick, J.; Blaut, M.; Pecquet, S.; Rezzonico, E.; Grathwohl, D.; Sprenger, N.; Brussow, H.; Study, T.; et al. Gut microbiota analysis reveals a ma.arked shift to bifidobacteria by a starter infant formula containing a synbiotic of bovine milk-derived oligosaccharides and Bifidobacterium animalis subsp. lactis CNCM I-3446. Environ. Microbiol. 2016, 18, 2185–2195. [Google Scholar] [CrossRef] [Green Version]
- Smilowitz, J.T.; Moya, J.; Breck, M.A.; Cook, C.; Fineberg, A.; Angkustsiri, K.; Underwood, M.A. Safety and tolerability of Bifidobacterium longum subspecies infantis EVC001 supplementation in healthy term breastfed infants: A phase I clinical trial. BMC Pediatr. 2017, 17, 133. [Google Scholar] [CrossRef]
- Stratiki, Z.; Costalos, C.; Sevastiadou, S.; Kastanidou, O.; Skouroliakou, M.; Giakoumatou, A.; Petrohilou, V. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum. Dev. 2007, 83, 575–579. [Google Scholar] [CrossRef]
- Sur, D.; Manna, B.; Niyogi, S.K.; Ramamurthy, T.; Palit, A.; Nomoto, K.; Takahashi, T.; Shima, T.; Tsuji, H.; Kurakawa, T.; et al. Role of probiotic in preventing acute diarrhoea in children: A community-based, randomized, double-blind placebo-controlled field trial in an urban slum. Epidemiol. Infect. 2011, 139, 919–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surono, I.S.; Koestomo, F.P.; Novitasari, N.; Zakaria, F.R.; Yulianasari; Koesnandar. Novel probiotic Enterococcus faecium IS-27526 supplementation increased total salivary sIgA level and bodyweight of pre-school children: A pilot study. Anaerobe 2011, 17, 496–500. [Google Scholar] [CrossRef]
- Szajewska, H.; Ruszczynski, M.; Szymanski, H.; Sadowska-Krawczenko, I.; Piwowarczyk, A.; Rasmussen, P.B.; Kristensen, M.B.; West, C.E.; Hernell, O. Effects of infant formula supplemented with prebiotics compared with synbiotics on growth up to the age of 12 mo: A randomized controlled trial. Pediatr. Res. 2017, 81, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.L.; Dunstan, J.A.; Prescott, S.L. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: A randomized controlled trial. J. Allergy Clin. Immunol. 2007, 119, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Totsu, S.; Yamasaki, C.; Terahara, M.; Uchiyama, A.; Kusuda, S. Bifidobacterium and enteral feeding in preterm infants: Cluster-randomized trial. Pediatr. Int. 2014, 56, 714–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.A.; Marcobal, A.; Tancredi, D.J.; Bevins, C.L.; Sherman, M.P. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: Impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Urban, M.F.; Bolton, K.D.; Mokhachane, M.; Mphahlele, R.M.; Bomela, H.N.; Monaheng, L.; Beckh-Arnold, E.; Cooper, P.A. Growth of infants born to HIV-infected women when fed a biologically acidified starter formula with and without probiotics. South Afr. J. Clin. Nutr. 2008, 21, 28–32. [Google Scholar] [CrossRef]
- Van Niekerk, E.; Kirsten, G.F.; Nel, D.G.; Blaauw, R. Probiotics, feeding tolerance, and growth: A comparison between HIV-exposed and unexposed very low birth weight infants. Nutrition 2014, 30, 645–653. [Google Scholar] [CrossRef]
- Velaphi, S.C.; Cooper, P.A.; Bolton, K.D.; Mokhachane, M.; Mphahlele, R.M.; Beckh-Arnold, E.; Monaheng, L.; Haschke-Becher, E. Growth and metabolism of infants born to women infected with human immunodeficiency virus and fed acidified whey-adapted starter formulas. Nutrition 2008, 24, 203–211. [Google Scholar] [CrossRef]
- Vendt, N.; Grunberg, H.; Tuure, T.; Malminiemi, O.; Wuolijoki, E.; Tillmann, V.; Sepp, E.; Korpela, R. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: Double-blind, randomized trial. J. Hum. Nutr. Diet. 2006, 19, 51–58. [Google Scholar] [CrossRef]
- Vlieger, A.M.; Robroch, A.; van Buuren, S.; Kiers, J.; Rijkers, G.; Benninga, M.A.; te Biesebeke, R. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: A randomised controlled trial. Br. J. Nutr. 2009, 102, 869–875. [Google Scholar] [CrossRef] [Green Version]
- Weizman, Z.; Alsheikh, A. Safety and tolerance of a probiotic formula in early infancy comparing two probiotic agents: A pilot study. J. Am. Coll Nutr. 2006, 25, 415–419. [Google Scholar] [CrossRef]
- Weizman, Z.; Asli, G.; Alsheikh, A. Effect of a probiotic infant formula on infections in child care centers: Comparison of two probiotic agents. Pediatrics 2005, 115, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Wejryd, E.; Marchini, G.; Frimmel, V.; Jonsson, B.; Abrahamsson, T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. 2019, 108, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics maintain intestinal secretory immunoglobulin A levels in healthy formula-fed infants: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.; Wang, Y.; Fu, J.; Sun, M.; Mao, Z.; Vandenplas, Y. A double-blinded randomized trial on growth and feeding tolerance with Saccharomyces boulardii CNCM I-745 in formula-fed preterm infants. J. Pediatr. (Rio J.) 2016, 92, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, N.N.; Wang, D.; Grathwohl, D.; Lan, P.N.; Kim, H.V.; Goyer, A.; Benyacoub, J. Effect of a Growing-up Milk Containing Synbiotics on Immune Function and Growth in Children: A Cluster Randomized, Multicenter, Double-blind, Placebo Controlled Study. Clin. Med. Insights Pediatr. 2013, 7, 49–56. [Google Scholar] [CrossRef]
- Ziegler, E.E.; Jeter, J.M.; Drulis, J.M.; Nelson, S.E.; Haschke, F.; Steenhout, P.; Brown, C.; Maire, J.C.; Hager, C. Formula with reduced content of improved, partially hydrolyzed protein and probiotics: Infant growth and health. Mon. Fur Kinderheilkd. 2003, 151, S65–S71. [Google Scholar] [CrossRef]
- Jalali, S.Z.; Shiri, M.R.; Shirazi, M.G. Effect of probiotics on full intestinal feeding in premature infants: A double blind, clinical trial. Iran. J. Pediatrics 2020, 30, e100139. [Google Scholar] [CrossRef] [Green Version]
- Meli, F.; Puccio, G.; Cajozzo, C.; Ricottone, G.L.; Pecquet, S.; Sprenger, N.; Steenhout, P. Growth and safety evaluation of infant formulae containing oligosaccharides derived from bovine milk: A randomized, double-blind, noninferiority trial. BMC Pediatr. 2014, 14, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshiro, T.; Nagata, S.; Wang, C.; Takahashi, T.; Tsuji, H.; Asahara, T.; Nomoto, K.; Takei, H.; Nittono, H.; Yamashiro, Y. Bifidobacterium Supplementation of Colostrum and Breast Milk Enhances Weight Gain and Metabolic Responses Associated with Microbiota Establishment in Very-Preterm Infants. Biomed. Hub. 2019, 4, 502935. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Kotch, J.B.; Jensen, E.T.; Savage, E.; Weber, D.J. Randomized, double-blind, placebo-controlled study of synbiotic yogurt effect on the health of children. J. Pediatr. 2015, 166, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.V.; Barratt, M.J.; Charbonneau, M.R.; Ahmed, T.; Gordon, J.I. Childhood undernutrition, the gut microbiota, and microbiota-directed therapeutics. Science 2016, 352, 1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef] [PubMed]
| Certainty Assessment | № of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Probiotics | Control | Relative(95% CI) | Absolute (95% CI) | |
| Weight-for-age | |||||||||||
| 21 | RCT | not serious a | very serious b | not serious c | not serious d | none | 4323 | 4094 | - | SMD 0.26 higher (0.11 higher to 0.42 higher) | ⨁⨁◯◯ LOW |
| Height-for-age | |||||||||||
| 12 | RCT | not serious e | serious f | not serious c | not serious | none | 1347 | 1214 | - | SMD 0.16 higher (0.06 higher to 0.25 higher) | ⨁⨁⨁◯ MODERATE |
| Head Circumference | |||||||||||
| 9 | RCT | not serious e | serious g | not serious c | serious h | none | 819 | 816 | - | SMD 0.05 higher (0.11 lower to 0.21 higher) | ⨁⨁◯◯ LOW |
| BMI | |||||||||||
| 2 | RCT | not serious e | serious i | not serious | serious j | none | 171 | 168 | - | SMD 0.05 lower (0.26 lower to 0.17 higher) | ⨁⨁◯◯ LOW |
| Sepsis | |||||||||||
| 9 | RCT | not serious k | serious l | not serious | not serious | none | 312/3026 (10.3%) | 441/3024 (14.6%) | RR 0.74 (0.64 to 0.87) | 38 fewer per 1000 (from 53 fewer to 19 fewer) | ⨁⨁⨁◯ MODERATE |
| Certainty Assessment | № of Patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | Probiotics | Control | Relative (95% CI) | Absolute (95% CI) | |
| Weight-for-age | |||||||||||
| 51 | RCT | not serious a | serious b | not serious c | not serious d | none | 5759 | 5073 | - | SMD 0.01 higher (0.04 lower to 0.05 higher) | ⨁⨁⨁◯ MODERATE |
| Height-for-age | |||||||||||
| 32 | RCT | not serious a | serious e | not serious c | not serious d | none | 3350 | 2768 | - | SMD 0.01 lower (0.06 lower to 0.04 higher) | ⨁⨁⨁◯ MODERATE |
| Head Circumference | |||||||||||
| 28 | RCT | not serious f | serious g | not serious c | not serious d | none | 2655 | 2117 | - | SMD 0.04 lower (0.2 lower to 0.11 higher) | ⨁⨁⨁◯ MODERATE |
| BMI | |||||||||||
| 5 | RCT | not serious | serious e | not serious c | serious h | none | 415 | 305 | - | SMD 0.09 higher (0.06 lower to 0.25 higher) | ⨁⨁◯◯ LOW |
| Sepsis | |||||||||||
| 12 | RCT | not serious i | serious j | not serious c | serious k | none | 275/1778 (15.5%) | 278/1749 (15.9%) | RR 1.03 (0.84 to 1.26) | 5 more per 1000 (from 25 fewer to 41 more) | ⨁⨁◯◯ LOW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catania, J.; Pandit, N.G.; Ehrlich, J.M.; Zaman, M.; Stone, E.; Franceschi, C.; Smith, A.; Tanner-Smith, E.; Zackular, J.P.; Bhutta, Z.A.; et al. Probiotic Supplementation for Promotion of Growth in Children: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 83. https://doi.org/10.3390/nu14010083
Catania J, Pandit NG, Ehrlich JM, Zaman M, Stone E, Franceschi C, Smith A, Tanner-Smith E, Zackular JP, Bhutta ZA, et al. Probiotic Supplementation for Promotion of Growth in Children: A Systematic Review and Meta-Analysis. Nutrients. 2022; 14(1):83. https://doi.org/10.3390/nu14010083
Chicago/Turabian StyleCatania, Joseph, Natasha G. Pandit, Julie M. Ehrlich, Muizz Zaman, Elizabeth Stone, Courtney Franceschi, Abigail Smith, Emily Tanner-Smith, Joseph P. Zackular, Zulfiqar A. Bhutta, and et al. 2022. "Probiotic Supplementation for Promotion of Growth in Children: A Systematic Review and Meta-Analysis" Nutrients 14, no. 1: 83. https://doi.org/10.3390/nu14010083
APA StyleCatania, J., Pandit, N. G., Ehrlich, J. M., Zaman, M., Stone, E., Franceschi, C., Smith, A., Tanner-Smith, E., Zackular, J. P., Bhutta, Z. A., & Imdad, A. (2022). Probiotic Supplementation for Promotion of Growth in Children: A Systematic Review and Meta-Analysis. Nutrients, 14(1), 83. https://doi.org/10.3390/nu14010083







