Impact of Seasonal Consumption of Local Tomatoes on the Metabolism and Absorption of (Poly)Phenols in Fischer Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Tomato Samples
2.3. Study Design
2.4. uHPLC-MSn Analyses of Tomato-Derived (Poly)Phenolic Metabolites in Rat Serum
2.5. Statistical Analysis
3. Results
3.1. Phenolic Metabolites in Serum after Tomato Administration
3.2. Effects of Photoperiod in (Poly)Phenolic Metabolites in Serum after Tomato Administration
3.3. Effects of the Geographical Origin of Tomatoes in Their (Poly)Phenolic Bioavailability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.Y.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Huélamo, M.; Tulipani, S.; Estruch, R.; Escribano, E.; Illán, M.; Corella, D.; Lamuela-Raventós, R.M. The tomato sauce making process affects the bioaccessibility and bioavailability of tomato phenolics: A pharmacokinetic study. Food Chem. 2015, 173, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Sanvicente, I.; Mallor, C.; Menal-Puey, S. Spanish traditional tomato. Effects of genotype, location and agronomic conditions on the nutritional quality and evaluation of consumer preferences. Food Chem. 2019, 270, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaličková, D.; Belović, M.; Ilić, N.; Kotur-Stevuljević, J.; Slanař, O.; Šobajić, S. Comparison of Polyphenol-Enriched Tomato Juice and Standard Tomato Juice for Cardiovascular Benefits in Subjects with Stage 1 Hypertension: A Randomized Controlled Study. Plant Foods Hum. Nutr. 2019, 74, 122–127. [Google Scholar] [CrossRef]
- Piao, X.-M.; Jang, E.-K.; Chung, J.-W.; Lee, G.-A.; Lee, H.-S.; Sung, J.-S.; Jeon, Y.-A.; Lee, J.-R.; Kim, Y.-G.; Lee, S.-Y. Variation in Antioxidant Activity and Polyphenol Content in Tomato Stems and Leaves. Plant Breed. Biotechnol. 2013, 1, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of flavonols in tomatoes and tomato-based products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- Slimestad, R.; Fossen, T.; Verheul, M.J. The flavonoids of tomatoes. J. Agric. Food Chem. 2008, 56, 2436–2441. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Sciarria, T.P.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and anti-inflammatory activities of the crude extracts of raw and fermented tomato pomace and their correlations with aglycate-polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Slimestad, R.; Verheul, M.; Slimestada, R.; Verheulb, M. Review of flavonoids and other phenolics from fruits of different tomato (lycopersicon esculentum mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Gonzali, S.; Perata, P. Anthocyanins from purple tomatoes as novel antioxidants to promote human health. Antioxidants 2020, 9, 1017. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Arranz, S.; Medina-Remón, A.; Casals-Ribes, I.; Lamuela-Raventós, R.M. Changes in phenolic content of tomato products during storage. J. Agric. Food Chem. 2011, 59, 9358–9365. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Di Lecce, G.; Valderas-Martínez, P.; Tulipani, S.; Jáuregui, O.; Escribano-Ferrer, E.; Estruch, R.; Illan, M.; Lamuela-Raventós, R.M. Bioavailability of tomato polyphenols is enhanced by processing and fat addition: Evidence from a randomized feeding trial. Mol. Nutr. Food Res. 2016, 60, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Exposure of Fischer 344 rats to distinct photoperiods influences the bioavailability of red grape polyphenols. J. Photochem. Photobiol. B Biol. 2019, 199, 111623. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Ho, C.-T.; Zhang, X.; Cao, J.; Wang, H.; Shao, X.; Pan, D.; Wu, Z. Oolong Tea Polyphenols Ameliorate Circadian Rhythm of Intestinal Microbiome and Liver Clock Genes in Mouse Model. J. Agric. Food Chem. 2019, 67, 11969–11976. [Google Scholar] [CrossRef]
- Cagampang, F.R.; Bruce, K.D. The role of the circadian clock system in nutrition and metabolism. Br. J. Nutr. 2012, 108, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Ruiz de Azua, M.J.; Cruz-Carrión, Á.; Muguerza, B.; Arola-Arnal, A.; Suarez, M. Seasonal consumption of cherries from different origins affects metabolic markers and gene expression of lipogenic enzymes in rat liver: A preliminary study. Nutrients 2021, 13, 3643. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Iglesias-Carres, L.; Quiñones, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Rat health status affects bioavailability, target tissue levels, and bioactivity of grape seed flavanols. Mol. Nutr. Food Res. 2017, 61, 1600342. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Carrión, Á.; Ruiz de Azua, M.J.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Suárez, M.; Arola-Arnal, A. Tomatoes consumed in-season prevent oxidative stress in Fischer 344 rats: Impact of geographical origin. Food Funct. 2021, 12, 8340–8350. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Carrión, Á.; Calani, L.; de Azua, M.J.R.; Mena, P.; Del Rio, D.; Suárez, M.; Arola-Arnal, A. (Poly)phenolic composition of tomatoes from different growing locations and their absorption in rats: A comparative study. Food Chem. 2022, 388, 132984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L. Voluntary oral administration of drugs in mice. Protoc. Exch. 2011, 2, 100330. [Google Scholar] [CrossRef]
- Ardid-Ruiz, A.; Ibars, M.; Mena, P.; Del Rio, D.; Muguerza, B.; Bladé, C.; Arola, L.; Aragonès, G.; Suárez, M. Potential involvement of peripheral leptin/STAT3 signaling in the effects of resveratrol and its metabolites on reducing body fat accumulation. Nutrients 2018, 10, 1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Caimari, A.; Arola, L. Cherry consumption out of season alters lipid and glucose homeostasis in normoweight and cafeteria-fed obese Fischer 344 rats. J. Nutr. Biochem. 2019, 63, 72–86. [Google Scholar] [CrossRef]
- Cruz-Carrión, Á.; de Azua, M.J.R.; Mulero, M.; Arola-Arnal, A.; Suárez, M. Oxidative Stress in Rats is Modulated by Seasonal Consumption of Sweet Cherries from Different Geographical Origins: Local vs. Non-Local. Nutrients 2020, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Gibert-Ramos, A.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode. Sci. Rep. 2018, 8, 13572. [Google Scholar] [CrossRef] [Green Version]
- Mena, P.; Del Rio, D. Gold Standards for Realistic (Poly)phenol Research. J. Agric. Food Chem. 2018, 66, 8221–8223. [Google Scholar] [CrossRef]
- Heideman, P.D.; John Sylvester, C. Reproductive Photoresponsiveness in Unmanipulated Male Fischer 344 Laboratory Rats1. Biol. Reprod. 1997, 57, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Oishi, K.; Yamamoto, S.; Oike, H.; Ohkura, N.; Taniguchi, M. Cinnamic acid shortens the period of the circadian clock in mice. Biochem. Biophys. Rep. 2017, 9, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Biological Timekeeping: Clocks, Rhythms and Behaviour; Springer (India) Private Ltd.: Delhi, India, 2017; ISBN 9788132236887. [Google Scholar]
- Bjørnerem, A.; Straume, B.; Øian, P.; Berntsen, G.K.R. Seasonal Variation of Estradiol, Follicle Stimulating Hormone, and Dehydroepiandrosterone Sulfate in Women and Men. J. Clin. Endocrinol. Metab. 2006, 91, 3798–3802. [Google Scholar] [CrossRef] [PubMed]
- Bourne, L.C.; Rice-Evans, C. Bioavailability of ferulic acid. Biochem. Biophys. Res. Commun. 1998, 253, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, S.; Bidanchi, R.M.; Murthy, M.K.; Gurusubramanian, G.; Roy, V.K. Influence of ferulic acid consumption in ameliorating the cadmium-induced liver and renal oxidative damage in rats. Environ. Sci. Pollut. Res. 2019, 26, 20631–20653. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and polyphenols: Roles and diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [Green Version]
- Russell, W.R.; Labat, A.; Scobbie, L.; Duncan, S.H. Availability of blueberry phenolics for microbial metabolism in the colon and the potential inflammatory implications. Mol. Nutr. Food Res. 2007, 51, 726–731. [Google Scholar] [CrossRef]
- Verzelloni, E.; Pellacani, C.; Tagliazucchi, D.; Tagliaferri, S.; Calani, L.; Costa, L.G.; Brighenti, F.; Borges, G.; Crozier, A.; Conte, A.; et al. Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol. Nutr. Food Res. 2011, 55, S35–S43. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Trombley, J.D.; Loegel, T.N.; Danielson, N.D.; Hagerman, A.E. Capillary electrophoresis methods for the determination of covalent polyphenol-protein complexes. Anal. Bioanal. Chem. 2011, 401, 1527–1533. [Google Scholar] [CrossRef]
- Van Der Burg-Koorevaar, M.C.D.; Miret, S.; Duchateau, G.S.M.J.E. Effect of milk and brewing method on black tea catechin bioaccessibility. J. Agric. Food Chem. 2011, 59, 7752–7758. [Google Scholar] [CrossRef] [PubMed]

| Compound | LT | NLT |
|---|---|---|
| Flavonoids | ||
| Kaempferol-O-rutinoside a | 3.51 ± 0.48 | 9.27 ± 1.25 * |
| Kaempferol-O-rutinoside-O-pentoside a | 4.81 ± 0.58 | 11.22 ± 2.02 * |
| Luteolin-O-hexoside-C-hexoside a | 34.76 ± 3.6 | 21.2 ± 1.02 * |
| Naringenin | 1.64 ± 0.27 | 2.18 ± 0.47 |
| Naringenin chalcone b | 1.12 ± 0.20 | 1.43 ± 0.11 |
| Phloretin 3′,5′- di-C-β-glucopyranoside c | 107.97 ± 11.5 | 51.19 ± 4.19 * |
| Quercetin-O-dihexoside a | 5.87 ± 0.76 | 5.20 ± 0.34 |
| QHRP-O-hexoside a | 1.79 ± 0.43 | 1.24 ± 0.56 |
| QHRP-coumaric acid a | 21.16 ± 1.13 | 10.13 ± 1.57 * |
| QHRP-ferulic acid a | 10.98 ± 0.4 | 6.86 ± 0.12 * |
| QHRP-sinapic acid a | 4.24 ± 0.16 | 3.66 ± 0.37 |
| QHRP-syringic acid a | 6.55 ± 0.81 | 8.28 ± 1.54 |
| Quercetin-O-rutinoside-O-hexoside a | 2.52 ± 0.27 | 0.87 ± 0.13 * |
| Quercetin-O-rutinoside-O-pentoside a | 81.03 ± 2.80 | 64.25 ± 5.82 * |
| Rutin | 111.33 ± 1.11 | 86.38 ± 11.77 * |
| Total, flavonoids | 399.32 ± 16.15 | 283.37 ± 30.38 * |
| Caffeic and dihydrocaffeic acid derivatives | ||
| Caffeic acid derivative I d | 33.24 ± 1.12 | 65.55 ± 17.50 * |
| Caffeic acid derivative II d | 28.10 ± 2.15 | 14.81 ± 3.34 * |
| Caffeic acid derivative III d | 13.95 ± 0.68 | 24.97 ± 6.96 |
| Caffeic acid derivative IV d | 4.19 ± 0.10 | 5.37 ± 0.47 * |
| Caffeic acid derivative V d | 4.79 ± 0.41 | 4.91 ± 0.98 |
| Caffeoylmalic acid d | 62.93 ± 3.55 | 66.80 ± 9.10 |
| Dihydrocaffeic acid derivative f | 38.14 ± 2.12 | 32.09 ± 5.61 |
| Total, caffeic and dihydrocaffeic acid derivatives | 185.34 ± 10.14 | 214.49 ± 43.97 |
| Free phenolic acids | ||
| Caffeic acid | 27.30 ± 3.03 | 40.92 ± 1.25 * |
| Dihydrocaffeic acid | n.d. | 8.72 ± 2.73 * |
| p-Coumaric acid | 15.57 ± 1.14 | 16.19 ± 2.02 |
| Salicylic acid | 29.04 ± 3.22 | 54.39 ± 12.34 * |
| Total, free phenolic acids | 71.90 ± 7.39 | 120.22 ± 18.34 * |
| Hydroxybenzoic acid derivatives | ||
| Dihydroxybenzoic acid-O-pentoside g | 24.44 ± 1.46 | 18.30 ± 1.64 * |
| Hydroxybenzoic acid-O-hexoside g | 40.36 ± 1.28 | 33.59 ± 3.08 * |
| Syringic acid-O-hexoside g | 37.53 ± 0.93 | 37.53 ± 5.59 |
| Total, hydroxybenzoic acid derivatives | 102.34 ± 3.66 | 89.42 ± 10.30 |
| Hydroxycinnamic acid derivatives | ||
| Caffeic acid-O-hexoside I g | 169.42 ± 5.07 | 235.81 ± 8.06 * |
| Caffeic acid-O-hexoside II g | 67.52 ± 4.03 | 60.86 ± 5.82 |
| Caffeic acid-O-hexoside III g | 194.60 ± 4.23 | 159.57 ± 19.72 * |
| Coumaric acid derivative | 14.08 ± 1.85 | 15.00 ± 1.15 |
| Coumaric acid-O-hexoside I g | 87.86 ± 2.09 | 132.38 ± 1.72 * |
| Coumaric acid-O-hexoside II and III g | 161.79 ± 7.83 | 113.07 ± 0.57 * |
| Dicaffeoyl-O-hexoside g | 72.09 ± 1.97 | 109.38 ± 14.05 * |
| Ferulic acid-O-hexoside g | 72.85 ± 2.58 | 24.30 ± 2.35 * |
| Sinapic acid-O-hexoside g | 27.05 ± 2.16 | 31.30 ± 3.01 |
| Total, hydroxycinnamic acid derivatives | 867.26 ± 31.81 | 881.67 ± 56.44 |
| Hydroxycinnamoylquinic acids | ||
| 3-O-Caffeoylquinic acid | 30.09 ± 4.38 | 44.24 ± 4.39 * |
| 4-O-Caffeoylquinic acid | 223.02 ± 7.43 | 247.29 ± 7.59 * |
| 5-O-Caffeoylquinic acid | 200.14 ± 33.63 | 280.73 ± 9.04 |
| Caffeoylquinic acid-O-hexoside I j | 39.64 ± 2.68 | 37.24 ± 5.62 |
| Caffeoylquinic acid-O-hexoside II j | 52.89 ± 5.94 | 49.50 ± 5.53 |
| Coumaroylquinic acid j | 93.38 ± 9.33 | 96.80 ± 4.70 |
| Dicaffeoylquinic acid I i | 88.27 ± 3.34 | 103.29 ± 8.83 |
| Dicaffeoylquinic acid II h | 39.97 ± 1.91 | 68.08 ± 1.60 * |
| Dicaffeoylquinic acid III i | 75.77 ± 2.30 | 155.79 ± 0.01 * |
| Dicaffeoylquinic acid-O-hexoside h | 44.75 ± 2.45 | 37.09 ± 1.56 * |
| Feruloylquinic acid j | 31.76 ± 1.34 | 31.81 ± 2.03 |
| Tricaffeoylquinic acid h | 177.11 ± 3.55 | 390.57 ± 61.65 * |
| Tricaffeoylquinic acid-O-hexoside i | 53.05 ± 4.62 | 17.93 ± 0.69 * |
| Total, hydroxycinnamoylquinic acids | 1149.83 ± 82.90 | 1560.35 ± 113.23 * |
| Phenylpropanoic acid-glycosides | ||
| Dihydrocaffeic acid-O-hexoside I g | 63.44 ± 3.84 | 74.47 ± 4.54 |
| Dihydrocaffeic acid-O-hexoside II g | 144.74 ± 4.37 | 160.71 ± 14.94 |
| Dihydrocaffeoyl-caffeoyl-O-hexoside g | 79.63 ± 4.81 | 95.00 ± 18.17 |
| Dihydroferulic acid-O-hexoside g | 152.76 ± 9.93 | 94.48 ± 3.04 * |
| Hydroxyphenylpropionic acid-O-hexoside g | 145.74 ± 6.55 | 171.36 ± 0.72 * |
| Total, phenylpropanoic acid-glycosides | 586.30 ± 29.49 | 596.02 ± 41.42 |
| Total, (Poly)phenolic compounds | 3362.30 ±189.92 | 3745.54 ± 315.02 |
| Metabolite | Serum Concentration (nM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Winter-DH | Spring/Autumn-DH | Summer-DH | 2wA | |||||
| LT | NLT | LT | NLT | LT | NLT | |||
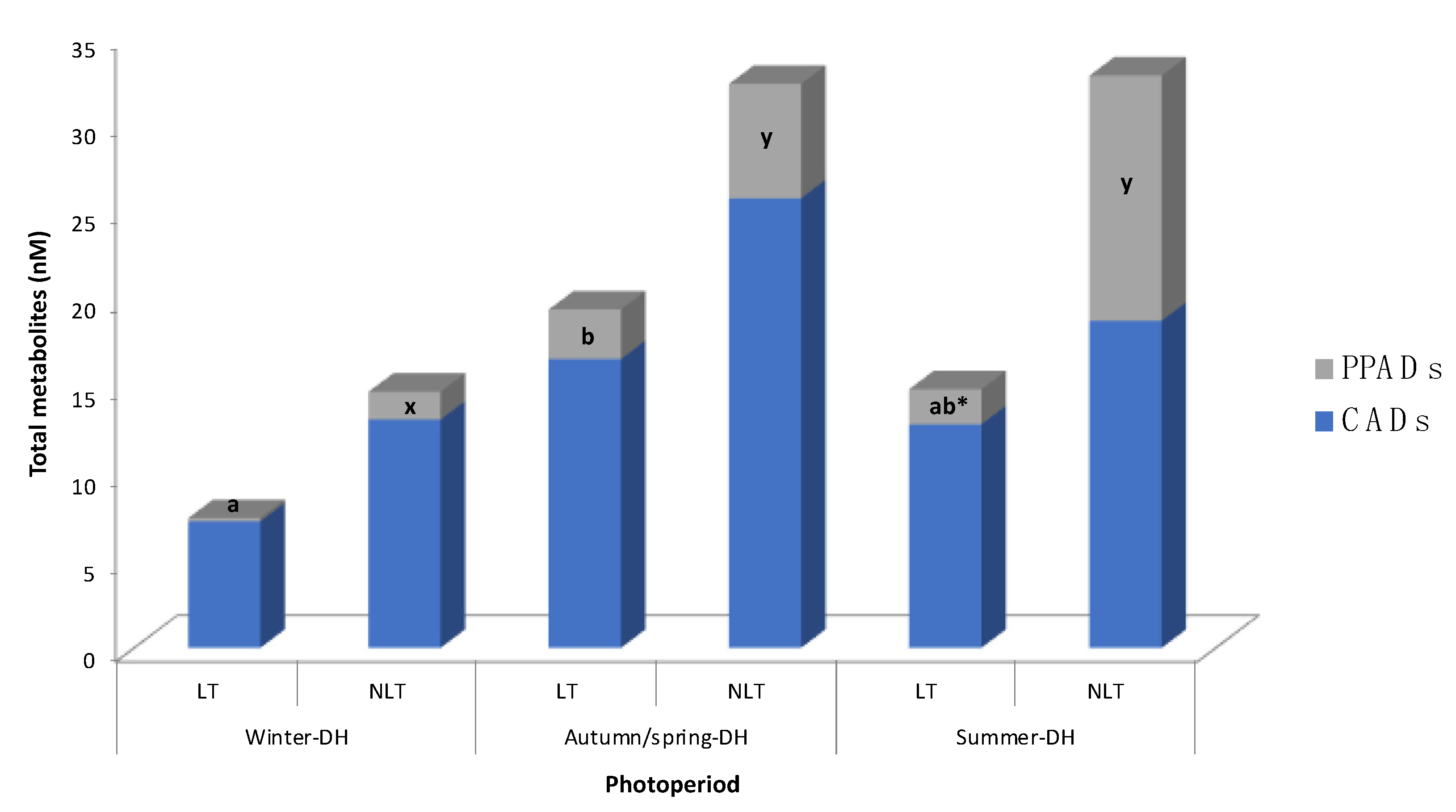
| CADs | 3′-methoxycinnamic acid-4′-sulfate | n.d. a,* | 3.8 ± 3.8 x | 11.3 ± 5.6 b | 18.5 ± 9.1 x | 8.8 ± 4.9 b | 13.1 ± 6.1 x | p |
| 4′-hydroxycinnamic acid-3′-glucuronide | n.q. | n.q. | n.q. | n.q. | n.q. | n.q. | n.s. | |
| 4′-methoxycinnamic acid-3′-sulfate | n.d. | n.q. | n.q. | n.q. | n.q. | n.q. | n.s. | |
| Hydroxycinnamic acid sulfate I | n.q. | n.q. | n.q. | n.q. | n.q. | n.q. | n.s. | |
| Hydroxycinnamic acid sulfate II | 7.2 ± 3.7 a | 9.2 ± 2.0 x | 5.2 ± 1.3 a | 7.2 ± 1.3 x | 4.0 ± 1.3 a | 5.6 ± 2.1 x | n.s. | |
| PPADs | 3-(3′-methoxyphenyl)propanoic acid-4′-sulfate | n.d. a,* | 1.4 ± 0.9 x | 0.8 ± 0.3 b* | 2.6 ± 1.2 x,y | 0.5 ± 0.5 b,* | 9.4 ± 3.7 y | P, T |
| 3-(4′-hydroxyphenyl)propanoic acid-3′-sulfate | n.q. a | n.q. x | 2.0 ± 0.8 b | 3.9 ± 1.3 y | 1.5 ± 0.8 b | 4.7 ± 2.0 y | P | |
| Total metabolites | 7.4 ± 3.7 | 14.7 ± 6.1 | 19.3 ± 6.8 | 32.3 ± 10.8 | 14.8 ± 6.6 | 32.7 ± 11.9 | n.s. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Carrión, Á.; Calani, L.; Ruiz de Azua, M.J.; Mena, P.; Del Rio, D.; Arola-Arnal, A.; Suárez, M. Impact of Seasonal Consumption of Local Tomatoes on the Metabolism and Absorption of (Poly)Phenols in Fischer Rats. Nutrients 2022, 14, 2047. https://doi.org/10.3390/nu14102047
Cruz-Carrión Á, Calani L, Ruiz de Azua MJ, Mena P, Del Rio D, Arola-Arnal A, Suárez M. Impact of Seasonal Consumption of Local Tomatoes on the Metabolism and Absorption of (Poly)Phenols in Fischer Rats. Nutrients. 2022; 14(10):2047. https://doi.org/10.3390/nu14102047
Chicago/Turabian StyleCruz-Carrión, Álvaro, Luca Calani, Ma. Josefina Ruiz de Azua, Pedro Mena, Daniele Del Rio, Anna Arola-Arnal, and Manuel Suárez. 2022. "Impact of Seasonal Consumption of Local Tomatoes on the Metabolism and Absorption of (Poly)Phenols in Fischer Rats" Nutrients 14, no. 10: 2047. https://doi.org/10.3390/nu14102047
APA StyleCruz-Carrión, Á., Calani, L., Ruiz de Azua, M. J., Mena, P., Del Rio, D., Arola-Arnal, A., & Suárez, M. (2022). Impact of Seasonal Consumption of Local Tomatoes on the Metabolism and Absorption of (Poly)Phenols in Fischer Rats. Nutrients, 14(10), 2047. https://doi.org/10.3390/nu14102047