Changes in Food Choice, Taste, Desire, and Enjoyment 1 Year after Sleeve Gastrectomy: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients’ Selection
2.2. Endpoints
2.3. Assessment of Frequency of Food Intake and of Changes in Taste Perception
2.4. Body Weight and BMI Assessment of the Study Population
2.5. Statistical Analysis
3. Results
3.1. Food Intake Frequency 12 Months after Sleeve Gastrectomy
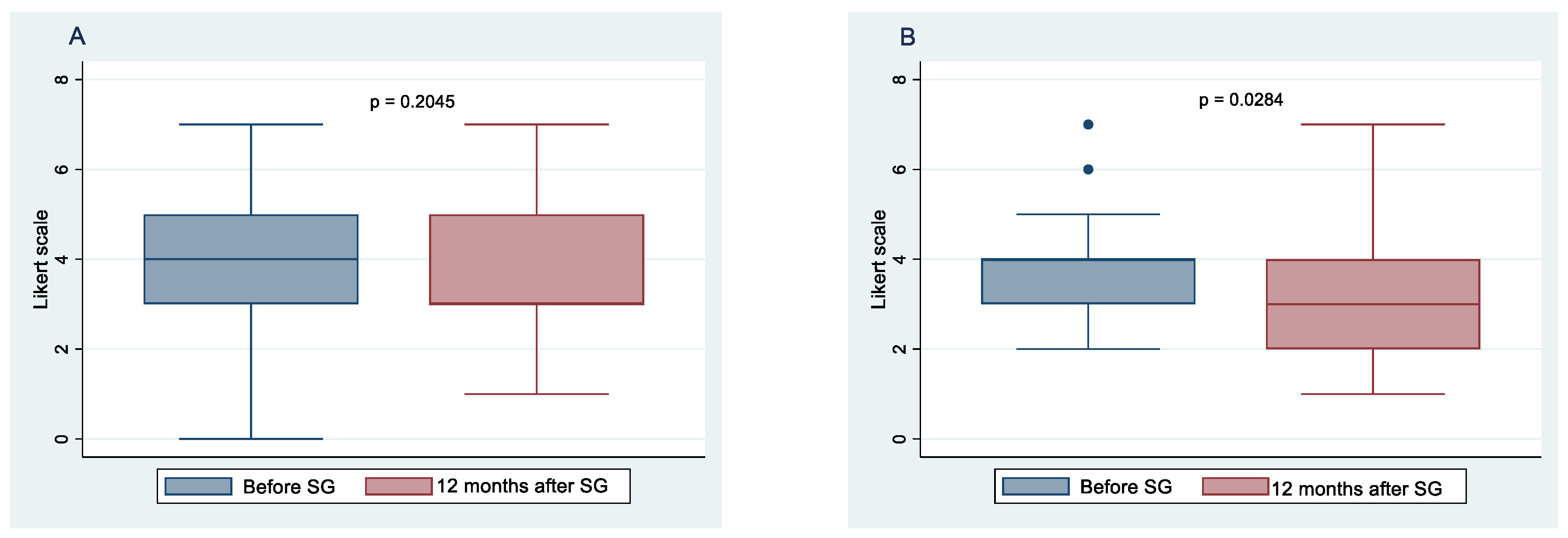
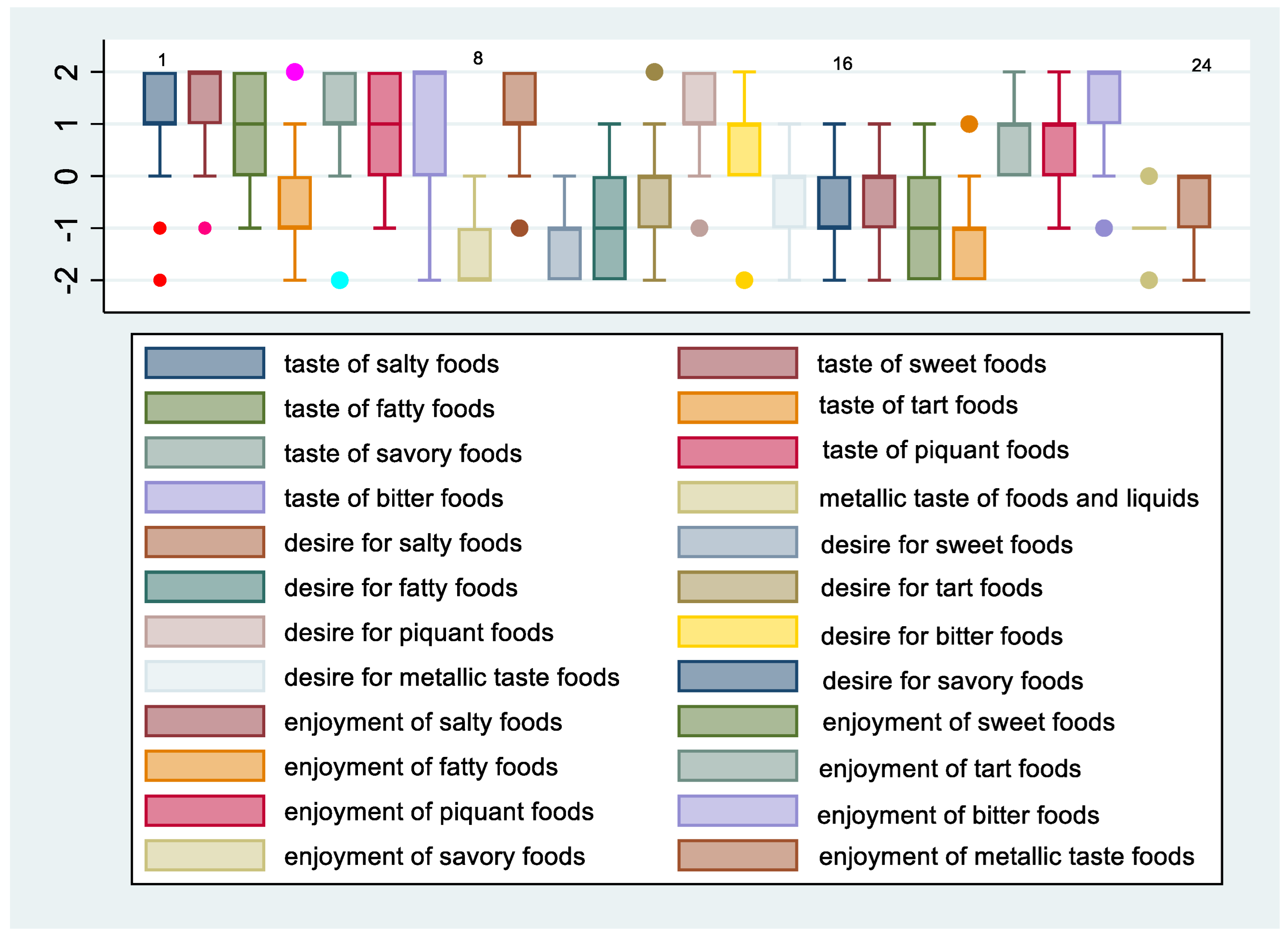
3.2. Changes in Taste Perception 12 Months after Sleeve Gastrectomy
3.3. Total Body Weight and BMI Changes 12 Months after SG
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Alsheikh, A.S.; Alabdulkader, S.; Johnson, B.; Goldstone, A.P.; Miras, A.D. Effect of Obesity Surgery on Taste. Nutrients 2022, 14, 866. [Google Scholar] [CrossRef] [PubMed]
- Boesveldt, S.; de Graaf, K. The Differential Role of Smell and Taste for Eating Behavior. Perception 2017, 46, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A. An evolutionary perspective on food and human taste. Curr. Biol. 2013, 23, R409–R418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrandies, W.; Zschieschang, R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Harnischfeger, F.; Dando, R. Obesity-induced taste dysfunction, and its implications for dietary intake. Int. J. Obes. 2021, 45, 1644–1655. [Google Scholar] [CrossRef]
- Rohde, K.; Schamarek, I.; Blüher, M. Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets? Diabetes Metab. J. 2020, 44, 509–528. [Google Scholar] [CrossRef]
- Kittrell, H.; Graber, W.; Mariani, E.; Czaja, K.; Hajnal, A.; Di Lorenzo, P.M. Taste and odor preferences following Roux-en-Y surgery in humans. PLoS ONE 2018, 13, e0199508. [Google Scholar] [CrossRef]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Archer, N.; Shaw, J.; Cochet-Broch, M.; Bunch, R.; Poelman, A.; Barendse, W.; Duesing, K. Obesity is associated with altered gene expression in human tastebuds. Int. J. Obes. 2019, 43, 1475–1484. [Google Scholar] [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [Google Scholar] [CrossRef] [Green Version]
- Wood, G.C.; Bailey-Davis, L.; Benotti, P.; Cook, A.; Dove, J.; Mowery, J.; Ramasamy, A.; Iyer, N.; Smolarz, B.G.; Kumar, N.; et al. Effects of sustained weight loss on outcomes associated with obesity comorbidities and healthcare resource utilization. PLoS ONE 2021, 16, e0258545. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Higa, K.; Himpens, J.; Buchwald, H.; Scopinaro, N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018, 28, 3783–3794. [Google Scholar] [CrossRef] [PubMed]
- Iannelli, A.; Treacy, P.; Sebastianelli, L.; Schiavo, L.; Martini, F. Perioperative complications of sleeve gastrectomy: Review of the literature. J. Minimal Access Surg. 2019, 15, 1–7. [Google Scholar]
- Brondel, L.; Quilliot, D.; Mouillot, T.; Khan, N.A.; Bastable, P.; Boggio, V.; Leloup, C.; Pénicaud, L. Taste of Fat and Obesity: Different Hypotheses and Our Point of View. Nutrients 2022, 14, 555. [Google Scholar] [CrossRef] [PubMed]
- Nance, K.; Acevedo, M.B.; Pepino, M.Y. Changes in taste function and ingestive behavior following bariatric surgery. Appetite 2020, 146, 104423. [Google Scholar] [CrossRef]
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; le Roux, C.W.; Silecchia, G. Food Intake and Changes in Eating Behavior after Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067. [Google Scholar] [CrossRef]
- Altun, H.; Hanci, D.; Altun, H.; Batman, B.; Serin, R.K.; Karip, A.B.; Akyuz, U. Improved Gustatory Sensitivity in Morbidly Obese Patients after Laparoscopic Sleeve Gastrectomy. Ann. Otol. Rhinol. Laryngol. 2016, 125, 536–540. [Google Scholar] [CrossRef]
- Leahey, T.M.; Bond, D.S.; Raynor, H.; Roye, D.; Vithiananthan, S.; Ryder, B.A.; Sax, H.C.; Wing, R.R. Effects of bariatric surgery on food cravings: Do food cravings and the consumption of craved foods “normalize” after surgery? Surg. Obes. Relat. Dis. 2012, 8, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Bueter, M.; Miras, A.D.H.; Chichger, W.; Fenske, M.A.; Ghatei, S.R.; Bloom, R.J.; Unwin, T.A.; Lutz, A.C.S.; le Roux, C.W. Alterations of sucrose preference after roux-en-Y gastric bypass. Physiol. Behav. 2011, 104, 709–721. [Google Scholar] [CrossRef] [Green Version]
- Pepino, M.Y.; Bradley, J.C.D.; Eagon, S.; Sullivan, N.A.A.; Klein, S. Changes in Taste Perception and Eating Behavior after Bariatric Surgery-Induced Weight Loss in Women. Obesity 2014, 22, E13–E20. [Google Scholar] [CrossRef]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and gustatory function after bariatric surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C.; Maedge, J.; Lam, L.; Blasche, G.; Shakeri-Leidenmühler, S.; Kundi, M.; Ludvik, B.; Langer, F.B.; Prager, G.; Schindler, K.; et al. Salt taste after bariatric surgery and weight loss in obese persons. PeerJ 2016, 4, e2086. [Google Scholar] [CrossRef] [Green Version]
- Nance, K.; Eagon, J.C.; Klein, S.; Pepino, M.Y. Effects of sleeve gastrectomy vs. roux-en-Y gastric bypass on eating behavior and sweet taste perception in subjects with obesity. Nutrients 2017, 10, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeen, G.N.; Miras, A.D.; Alqhatani, A.R.; Le Roux, C.W. Sugar detection threshold after laparoscopic sleeve gastrectomy in adolescents. Obes. Surg. 2018, 28, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Pintus, S.; Mastinu, M.; Fantola, G.; Moroni, R.; Pepino, M.Y.; Barbarossa, I.T. Changes of Taste, Smell and Eating Behavior in Patients Undergoing Bariatric Surgery: Associations with PROP Phenotypes and Polymorphisms in the Odorant-Binding Protein OBPIIa and CD36 Receptor Genes. Nutrients 2021, 13, 250. [Google Scholar] [CrossRef]
- Fried, M.; Yumuk, V.; Oppert, J.M.; Scopinaro, N.; Torres, A.; Weiner, R.; Yashkov, Y.; Frühbeck, G. International Federation for Surgery of Obesity and Metabolic Disorders-European Chapter (IFSO-EC), European Association for the Study of Obesity (EASO), & European Association for the Study of Obesity Obesity Management Task Force (EASO OMTF) Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes. Surg. 2014, 24, 42–55. [Google Scholar]
- Huybrechts, I.; Miglio, R.; Mistura, L.; Grioni, S.; Pozzebon, I.; Odorifero, C.; Borea, R.; Gitto, A.; Terrafino, M.; Scipioni, M.; et al. Relative Validity of an Italian EPIC Food Frequency Questionnaire for Dietary Factors in Children and Adolescents. A Rizzoli Orthopedic Institute Study. Nutrients 2021, 13, 1245. [Google Scholar] [CrossRef]
- Van Vuuren, M.; Strodl, E.; White, K.M.; Lockie, P.D. Taste, Enjoyment, and Desire of Flavors Change after Sleeve Gastrectomy-Short Term Results. Obes. Surg. 2017, 27, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, L.; Di Rosa, M.; Tramontano, S.; Rossetti, G.; Iannelli, A.; Pilone, V. Long-Term Results of the Mediterranean Diet after Sleeve Gastrectomy. Obes. Surg. 2020, 30, 3792–3802. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Iannelli, A.; Barbarisi, A. Fat mass, fat-free mass, and resting metabolic rate in weight-stable sleeve gastrectomy patients compared with weight-stable nonoperated patients. Surg. Obes. Relat. Dis. 2017, 13, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Quagliariello, V.; Iannelli, A.; Barbarisi, A. A Comparative Study Examining the Impact of a Protein-Enriched vs. Normal Protein Postoperative Diet on Body Composition and Resting Metabolic Rate in Obese Patients after Sleeve Gastrectomy. Obes. Surg. 2017, 27, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Scalera, G.; Barbarisi, A. Sleeve gastrectomy to treat concomitant polycystyc ovary syndrome, insulin and leptin resistance in a 27-years morbidly obese woman unresponsive to insulin-sensitizing drugs: A 3-year follow-up. Int. J. Surg. Case Rep. 2015, 17, 36–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Romano, M.; Pieretti, G.; Schneck, A.S.; Iannelli, A. Correcting micronutrient deficiencies before sleeve gastrectomy may be useful in preventing early postoperative micronutrient deficiencies. Int. J. Vitam. Nutr. Res. 2019, 89, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, R.; Batterham, R.L. Potential mechanisms underlying the effect of bariatric surgery on eating behaviour. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 3–11. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Zheng, H. Modulation of taste responsiveness and food preference by obesity and weight loss. Physiol. Behav. 2012, 107, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Miras, A.D.; Jackson, R.N.; Jackson, S.N.; Goldstone, A.P.; Olbers, T.; Hackenberg, T.; Spector, A.C.; le Roux, C.W. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am. J. Clin. Nutr. 2012, 96, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.A.; Moskowitz, H.R.; Campbell, R.G. Taste and olfaction in human obesity. Physiol. Behav. 1977, 19, 335–337. [Google Scholar] [CrossRef]
- Umabiki, M.; Tsuzaki, K.; Kotani, K.; Nagai, N.; Sano, Y.; Matsuoka, Y.; Kitaoka, K.; Okami, Y.; Sakane, N.; Higashi, A. The improvement of sweet taste sensitivity with decrease in serum leptin levels during weight loss in obese females. Tohoku J. Exp. Med. 2010, 220, 267–271. [Google Scholar] [CrossRef] [Green Version]
- Scruggs, D.M.; Buffington, C.; Cowan, G.S., Jr. Taste Acuity of the Morbidly Obese before and after Gastric Bypass Surgery. Obes. Surg. 1994, 4, 24–28. [Google Scholar] [CrossRef]
- Carreiro, A.L.; Dhillon, J.; Gordon, S.; Higgins, K.A.; Jacobs, A.G.; McArthur, B.M.; Redan, B.W.; Rivera, R.L.; Schmidt, L.R.; Mattes, R.D. The Macronutrients, Appetite, and Energy Intake. Annu. Rev. Nutr. 2016, 36, 73–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Jacobsen, S.H.; Clausen, T.R.; Worm, D.; Hartmann, B.; Rehfeld, J.F.; Damgaard, M.; et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 2013, 37, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nosso, G.; Griffo, E.; Cotugno, M.; Saldalamacchia, G.; Lupoli, R.; Pacini, G.; Riccardi, G.; Angrisani, L.; Capaldo, B. Comparative Effects of Roux-en-Y Gastric Bypass and Sleeve Gastrectomy on Glucose Homeostasis and Incretin Hormones in Obese Type 2 Diabetic Patients: A One-Year Prospective Study. Horm. Metab. Res. Horm. Stoffwechs. = Horm. Metab. 2016, 48, 312–317. [Google Scholar] [CrossRef] [Green Version]
- Goldstone, A.P.; Miras, A.D.; Scholtz, S.; Jackson, S.; Neff, K.J.; Pénicaud, L.; Geoghegan, J.; Chhina, N.; Durighel, G.; Bell, J.D.; et al. Link between Increased Satiety Gut Hormones and Reduced Food Reward after Gastric Bypass Surgery for Obesity. J. Clin. Endocrinol. Metab. 2016, 101, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Scholtz, S.; Miras, A.D.; Chhina, N.; Prechtl, C.G.; Sleeth, M.L.; Daud, N.M.; Ismail, N.A.; Durighel, G.; Ahmed, A.R.; Olbers, T.; et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014, 63, 891–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochner, C.N.; Laferrère, B.; Afifi, L.; Atalayer, D.; Geliebter, A.; Teixeira, J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci. Res. 2012, 74, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makaronidis, J.M.; Neilson, S.; Cheung, W.H.; Tymoszuk, U.; Pucci, A.; Finer, N.; Doyle, J.; Hashemi, M.; Elkalaawy, M.; Adamo, M.; et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite 2016, 107, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Makaronidis, J.M.; Batterham, R.L. Potential Mechanisms Mediating Sustained Weight Loss Following Roux-en-Y Gastric Bypass and Sleeve Gastrectomy. Endocrinol. Metab. Clin. N. Am. 2016, 45, 539–552. [Google Scholar] [CrossRef]
- Clemmensen, C.; Müller, T.D.; Woods, S.C.; Berthoud, H.R.; Seeley, R.J.; Tschöp, M.H. Gut-Brain Cross-Talk in Metabolic Control. Cell 2017, 168, 758–774. [Google Scholar] [CrossRef] [Green Version]
- van Bloemendaal, L.; IJzerman, R.G.; Ten Kulve, J.S.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, C.; Di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiPatrizio, N.V.; Astarita, G.; Schwartz, G.; Li, X.; Piomelli, D. Endocannabinoid signal in the gut controls dietary fat intake. Proc. Natl. Acad. Sci. USA 2011, 108, 12904–12908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteleone, P.; Piscitelli, F.; Scognamiglio, P.; Monteleone, A.M.; Canestrelli, B.; Di Marzo, V.; Maj, M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: A pilot study. J. Clin. Endocrinol. Metab. 2012, 97, E917–E924. [Google Scholar] [CrossRef]
- Mallipedhi, A.; Prior, S.L.; Dunseath, G.; Bracken, R.M.; Barry, J.; Caplin, S.; Eyre, N.; Morgan, J.; Baxter, J.N.; O’Sullivan, S.E.; et al. Changes in plasma levels of N-arachidonoyl ethanolamine and N-palmitoylethanolamine following bariatric surgery in morbidly obese females with impaired glucose homeostasis. J. Diabetes Res. 2015, 2015, 680867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahima, R.S.; Antwi, D.A. Brain regulation of appetite and satiety. Endocrinol. Metab. Clin. N. Am. 2008, 37, 811–823. [Google Scholar] [CrossRef] [Green Version]
- Burger, K.S.; Berner, L.A. A functional neuroimaging review of obesity, appetitive hormones and ingestive behavior. Physiol. Behav. 2014, 136, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Iannelli, A. The Role of the Nutritionist in a Multidisciplinary Bariatric Surgery Team. Obes. Surg. 2019, 29, 1028–1030. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavo, L.; Aliberti, S.M.; Calabrese, P.; Senatore, A.M.; Severino, L.; Sarno, G.; Iannelli, A.; Pilone, V. Changes in Food Choice, Taste, Desire, and Enjoyment 1 Year after Sleeve Gastrectomy: A Prospective Study. Nutrients 2022, 14, 2060. https://doi.org/10.3390/nu14102060
Schiavo L, Aliberti SM, Calabrese P, Senatore AM, Severino L, Sarno G, Iannelli A, Pilone V. Changes in Food Choice, Taste, Desire, and Enjoyment 1 Year after Sleeve Gastrectomy: A Prospective Study. Nutrients. 2022; 14(10):2060. https://doi.org/10.3390/nu14102060
Chicago/Turabian StyleSchiavo, Luigi, Silvana Mirella Aliberti, Pietro Calabrese, Anna Maria Senatore, Lucia Severino, Gerardo Sarno, Antonio Iannelli, and Vincenzo Pilone. 2022. "Changes in Food Choice, Taste, Desire, and Enjoyment 1 Year after Sleeve Gastrectomy: A Prospective Study" Nutrients 14, no. 10: 2060. https://doi.org/10.3390/nu14102060
APA StyleSchiavo, L., Aliberti, S. M., Calabrese, P., Senatore, A. M., Severino, L., Sarno, G., Iannelli, A., & Pilone, V. (2022). Changes in Food Choice, Taste, Desire, and Enjoyment 1 Year after Sleeve Gastrectomy: A Prospective Study. Nutrients, 14(10), 2060. https://doi.org/10.3390/nu14102060







