Dietary Alpha-Ketoglutarate Partially Abolishes Adverse Changes in the Small Intestine after Gastric Bypass Surgery in a Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Breeding and Experimental Design
2.2. Histology Preparation and Histomorphometric Analysis
2.3. Immunohistochemical Analysis
2.4. Statistical Analysis
3. Results
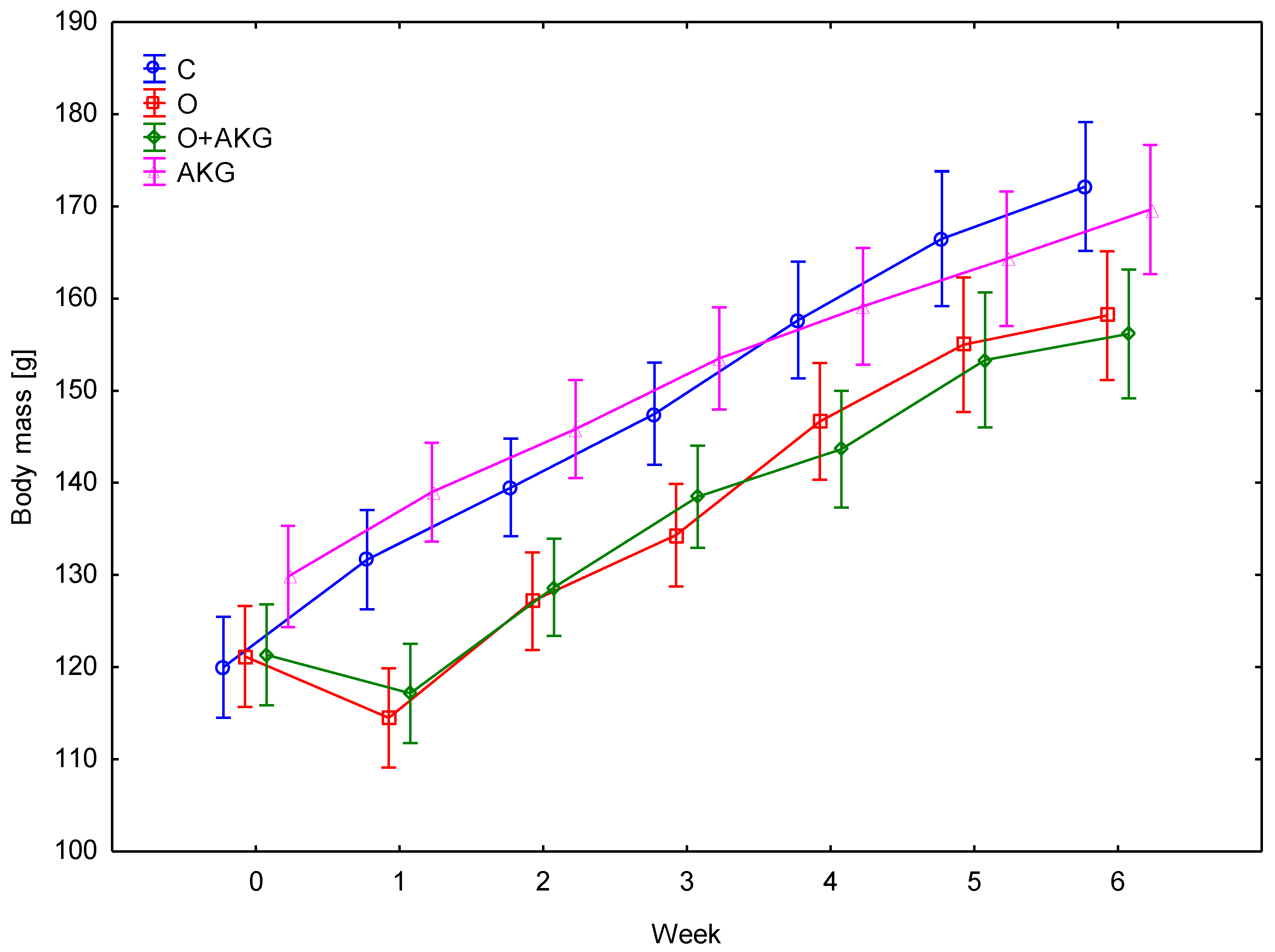
3.1. Body Weight
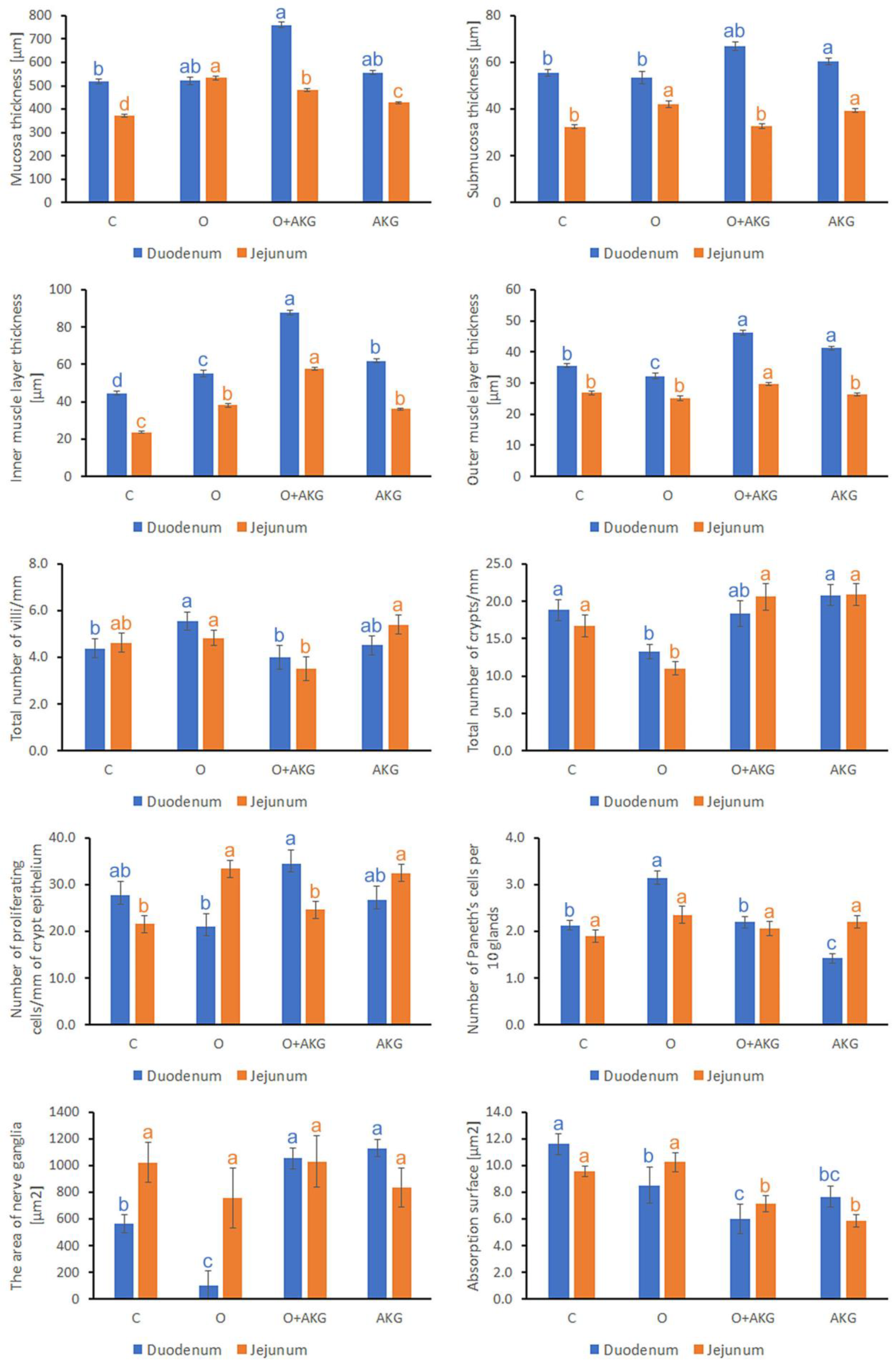
3.2. Histomorphometry of the Small Intestine
3.3. Biochemical Analysis of Gastrin and CCK
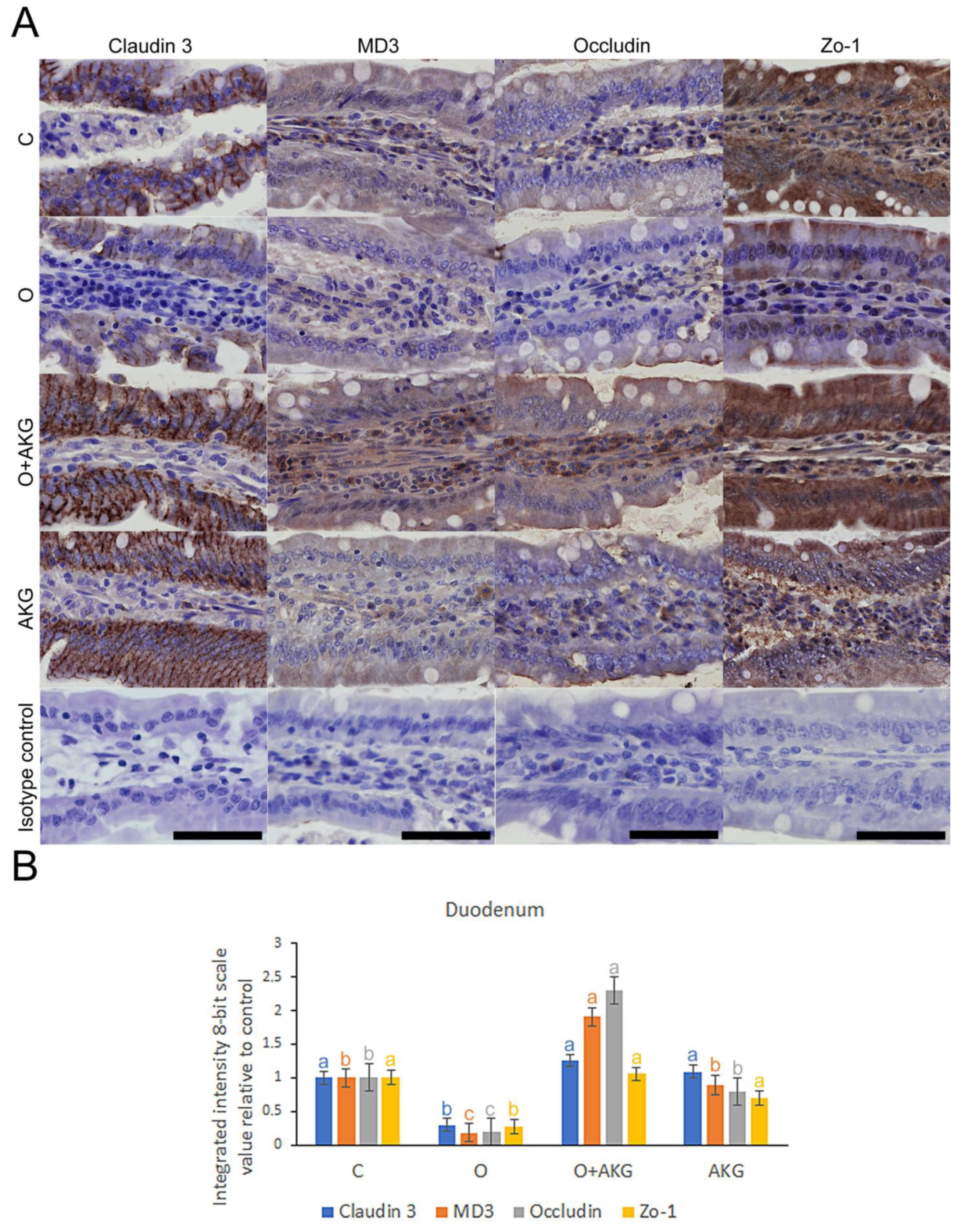
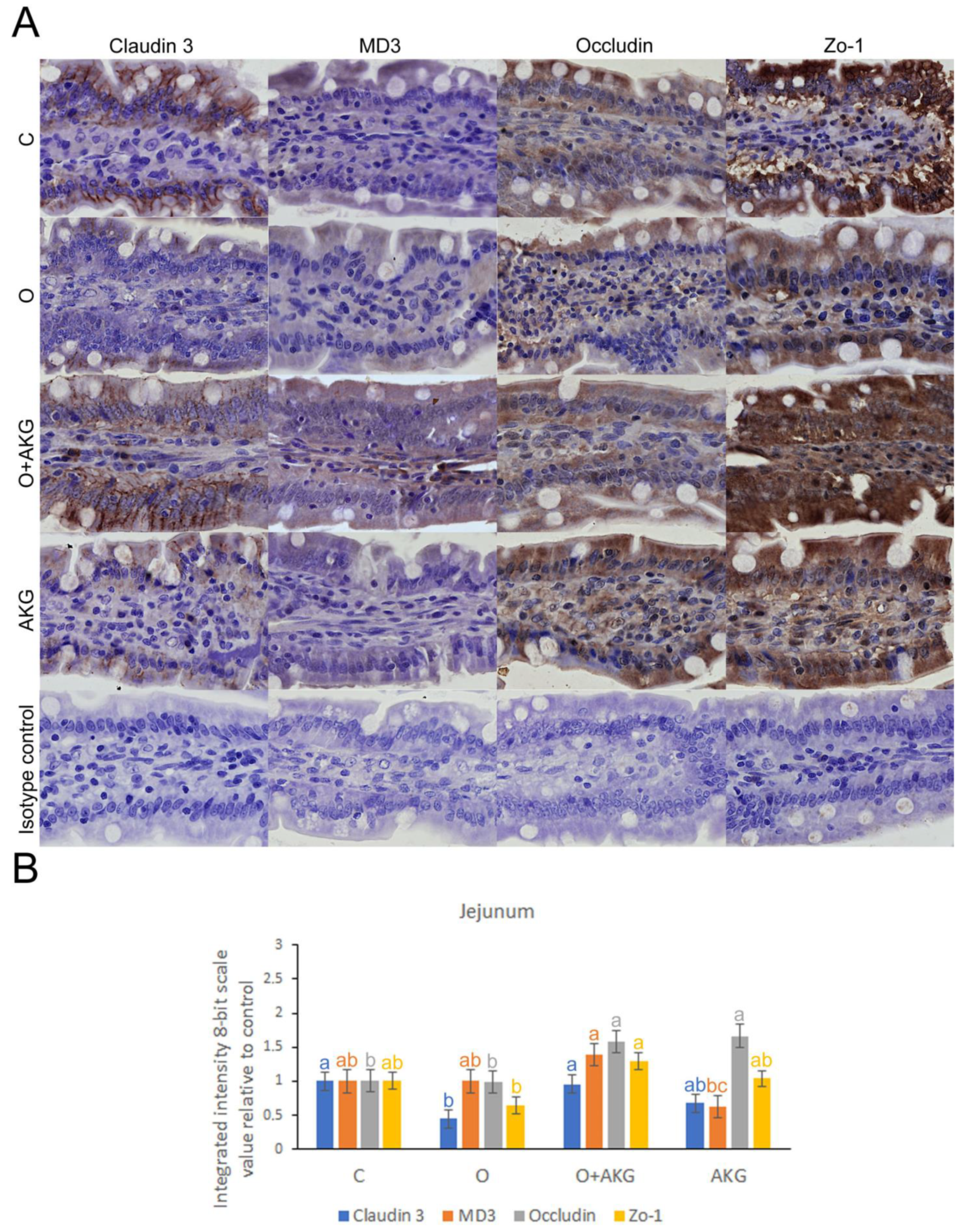
3.4. Immunohistochemical Analysis of Selected Proteins of the Small Intestinal Barrier
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reilly, J.J.; Methven, E.; McDowell, Z.C.; Hacking, B.; Alexander, D.; Stewart, L.; Kelnar, C.J.H. Health consequences of obesity. Arch. Dis. Child. 2003, 88, 748–752. [Google Scholar] [CrossRef]
- Bray, G.A. Medical consequences of obesity. Proc. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [Green Version]
- Birks, S.; Peeters, A.; Backholer, K.; O’Brien, P.; Brown, W. A systematic review of the impact of weight loss on cancer incidence and mortality. Obes. Rev. 2012, 13, 868–891. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Li, Z.; Zhang, N.; Yu, H.-T.; Wang, S.-R.; Liu, J.-R. Effects of gastrointestinal motility on obesity. Nutr. Metab. 2014, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.Y. Obesity-Related Digestive Diseases and Their Pathophysiology. Gut Liver 2017, 11, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Balan, H.; Costache, C.; Angelescu, G.; Popescu, L. Bariatric surgery—A real hope or just fake news? Arch. Balk. Med. Union 2017, 52, A39. [Google Scholar]
- Seo, G.H.; Kang, H.Y.; Choe, E.K. Osteoporosis and fracture after gastrectomy for stomach cancer. Medicine 2018, 97, e0532. [Google Scholar] [CrossRef]
- Kapoor, V.K. Complications of pancreato-duodenectomy. Rozhl. V Chir. Mesic. Ceskoslovenske Chir. Spol. 2016, 95, 53–59. [Google Scholar]
- Sah, B.; Zhu, Z.; Wang, X.; Yang, Q.; Chen, M.; Xiang, M.; Chen, J.; Yan, M. Post-operative complications of gastric cancer surgery: Female gender at high risk. Eur. J. Cancer Care 2009, 18, 202–208. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New Concepts in Nutraceuticals as Alternative for Pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Ling, K.H.; El-Nezami, H.; Wang, M.F. Influence of functional food components on gut health. Crit. Rev. Food Sci. Nutr. 2018, 59, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Harrison, A.P.; Pierzynowski, S.G. Biological effects of 2-oxoglutarate with particular emphasis on the regulation of protein, mineral and lipid absorption/metabolism, muscle performance, kidney function, bone formation and cancerogenesis, all viewed from a healthy ageing perspective state of the art—Review article. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 91–106. [Google Scholar]
- Xiao, D.; Zeng, L.; Yao, K.; Kong, X.; Wu, G.; Yin, Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 2016, 48, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.; Hanson, R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junghans, P.; Derno, M.; Pierzynowski, S.; Hennig, U.; Rudolph, P.E.; Souffrant, W.B. Intraduodenal infusion of α-ketoglutarate decreases whole body energy expenditure in growing pigs. Clin. Nutr. 2006, 25, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowski, S.; Pierzynowska, K. Alpha-ketoglutarate, a key molecule involved in nitrogen circulation in both animals and plants, in the context of human gut microbiota and protein metabolism. Adv. Med. Sci. 2022, 67, 142–147. [Google Scholar] [CrossRef]
- Pierzynowski, S.G.; Sjodin, A. Perspectives of glutamine and its derivatives as feed additives for farm animals. J. Anim. Feed Sci. 1998, 7, 79–91. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Bin, P.; Ren, W.; Gao, W.; Liu, G.; Yin, J.; Duan, J.; Li, Y.; Yao, K.; Huang, R.; et al. Alpha-ketoglutarate (AKG) lowers body weight and affects intestinal innate immunity through influencing intestinal microbiota. Oncotarget 2017, 8, 38184–38192. [Google Scholar] [CrossRef]
- Rzeski, W.; Walczak, K.; Juszczak, M.; Langner, E.; PoŻarowski, P.; Kandefer-Szerszeń, M.; Pierzynowski, S.G. Alpha-ketoglutarate (AKG) inhibits proliferation of colon adenocarcinoma cells in normoxic conditions. Scand. J. Gastroenterol. 2012, 47, 565–571. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Wu, X.; Yin, Y.; Wu, G. Dietary α-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids 2010, 39, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Buddington, R.K.; Pajor, A.; Buddington, K.K.; Pierzynowski, S. Absorption of α-ketoglutarate by the gastrointestinal tract of pigs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 138, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Śliwa, E.; Dobrowolski, P.; Siwicki, A.K.; Pierzynowski, S.G. Changes of a non-specific defence mechanism in blood serum of piglets induced by prenatal and postnatal administration of α-ketoglutarate. Bull. Vet. Inst. Pulawy 2007, 51, 297–301. [Google Scholar]
- Śliwa, E.; Dobrowolski, P.; Tatara, M.R.; Pierzynowski, S.G. Alpha-ketoglutarate partially protects newborns from metabolic changes evoked by chronic maternal exposure to glucocorticoids. J. Pre-Clin. Clin. Res. 2007, 1, 55–59. [Google Scholar]
- Filip, R.; Pierzynowski, S.G. The absorption, tissue distribution and excretion of enteraly administered α-ketoglutarate in rats. J. Anim. Physiol. Anim. Nutr. 2008, 92, 182–189. [Google Scholar] [CrossRef]
- Grzesiak, P.; Słupecka-Ziemilska, M.; Woliński, J. The biological role of a-ketoglutaric acid in physiological processes and its therapeutic potential. Dev. Period Med. 2016, 20, 61–67. [Google Scholar]
- Dobrowolski, P.; Tomaszewska, E.; Radzki, R.P.; Bienko, M.; Wydrych, J.; Zdybel, A.; Pierzynowski, S.G. Can 2-oxoglutarate prevent changes in bone evoked by omeprazole? Nutrition 2013, 29, 556–561. [Google Scholar] [CrossRef]
- Dobrowolski, P.; Tomaszewska, E.; Kurlak, P.; Pierzynowski, S.G. Dietary 2-oxoglutarate mitigates gastrectomy-evoked structural changes in cartilage of female rats. Exp. Biol. Med. 2016, 241, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, P.J.; Piersiak, T.; Surve, V.V.; Kruszewska, D.; Gawron, A.; Pacuska, P.; Håkanson, R.; Pierzynowski, S.G. Dietary α-ketoglutarate reduces gastrectomy-evoked loss of calvaria and trabecular bone in female rats. Scand. J. Gastroenterol. 2008, 43, 551–558. [Google Scholar] [CrossRef]
- Kim, S.S.; Christopher, L.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-7020-6887-4. [Google Scholar]
- Rich, L.; Whittaker, P. Collagen and Picrosirius Red Staining: A Polarized Light Assessment of Fibrillar Hue and Spatial Distribution. Braz. J. Morphol. Sci. 2005, 22, 97–104. [Google Scholar]
- Dobrowolski, P.; Huet, P.; Karlsson, P.; Eriksson, S.; Tomaszewska, E.; Gawron, A.; Pierzynowski, S.G. Potato fiber protects the small intestinal wall against the toxic influence of acrylamide. Nutrition 2012, 28, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kisielinski, K.; Willis, S.; Prescher, A.; Klosterhalfen, B.; Schumpelick, V. A simple new method to calculate small intestine absorptive surface in the rat. Clin. Exp. Med. 2002, 2, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.; Świątkiewicz, S.; Arczewka-włosek, A.; Muszyński, S.; Szymań, S. Modern hybrid rye as an alternative energy source for broiler chickens ameliorates absorption surface of initial segments of intestines regardless of xylanase supplementation. Animals 2021, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Seeley, R.J.; Chambers, A.P.; Sandoval, D.A. The Role of Gut Adaptation in the Potent Effects of Multiple Bariatric Surgeries on Obesity and Diabetes. Cell Metab. 2015, 21, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Blachier, F.; Mariotti, F.; Huneau, J.F.; Tomé, D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Amino Acids 2006, 33, 547–562. [Google Scholar] [CrossRef]
- Eklou-Lawson, M.; Bernard, F.; Neveux, N.; Chaumontet, C.; Bos, C.; Davila-Gay, A.-M.; Tomé, D.; Cynober, L.; Blachier, F. Colonic luminal ammonia and portal blood l-glutamine and l-arginine concentrations: A possible link between colon mucosa and liver ureagenesis. Amino Acids 2008, 37, 751–760. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Ding, B.; Liu, Y.; Zhu, H.; Liu, J.; Li, Y.; Kang, P.; Yin, Y.; Wu, G. Alpha-Ketoglutarate and intestinal function. Front. Biosci. 2011, 16, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Bergen, W.G.; Wu, G. Intestinal Nitrogen Recycling and Utilization in Health and Disease. J. Nutr. 2009, 139, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Dokladny, K.; Zuhl, M.N.; Moseley, P.L. Intestinal epithelial barrier function and tight junction proteins with heat and exercise. J. Appl. Physiol. 2016, 120, 692–701. [Google Scholar] [CrossRef]
- He, L.; Zhou, X.; Huang, N.; Li, H.; Cui, Z.; Tian, J.; Jiang, Q.; Liu, S.; Wu, J.; Li, T.; et al. Administration of alpha-ketoglutarate improves epithelial restitution under stress injury in early-weaning piglets. Oncotarget 2017, 8, 91965–91978. [Google Scholar] [CrossRef] [Green Version]
- Chou, H.-C.; Chen, C.-M. Neonatal hyperoxia disrupts the intestinal barrier and impairs intestinal function in rats. Exp. Mol. Pathol. 2017, 102, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steed, E.; Elbediwy, A.; Vacca, B.; Dupasquier, S.; Hemkemeyer, S.A.; Suddason, T.; Costa, A.C.; Beaudry, J.-B.; Zihni, C.; Gallagher, E.; et al. MarvelD3 couples tight junctions to the MEKK1–JNK pathway to regulate cell behavior and survival. J. Cell Biol. 2014, 204, 821–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, Q.; Xu, H.; Tao, L.; Lu, J.; Cai, L.; Wang, C. Somatostatin regulates tight junction proteins expression in colitis mice. Int. J. Clin. Exp. Pathol. 2014, 7, 2153–2162. [Google Scholar]
- Wang, B.; Wu, Z.; Ji, Y.; Sun, K.; Dai, Z.; Wu, G. L-Glutamine Enhances Tight Junction Integrity by Activating CaMK Kinase 2–AMP-Activated Protein Kinase Signaling in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, M.; Zhao, Y.; Chen, J.; Li, B.; Cai, W. JNK inhibitor SP600125 protects against lipopolysaccharide-induced acute lung injury via upregulation of claudin-4. Exp. Ther. Med. 2014, 8, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Zdzisin, B. Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch. Immunol. Ther. Exp. 2016, 65, 20–33. [Google Scholar]
- Bayliak, M.M.; Lylyk, M.P.; Shmihel, H.V.; Sorochynska, O.M.; Semchyshyn, O.I.; Storey, J.M.; Storey, K.B.; Lushchak, V.I. Dietary alpha-ketoglutarate promotes higher protein and lower triacylglyceride levels and induces oxidative stress in larvae and young adults but not in middle-aged Drosophila melanogaster. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 204, 28–39. [Google Scholar] [CrossRef]
- He, L.; Xu, Z.; Yao, K.; Wu, G.; Yin, Y.; Nyachoti, C.; Kim, S. The Physiological Basis and Nutritional Function of Alpha-ketoglutarate. Curr. Protein Pept. Sci. 2015, 16, 576–581. [Google Scholar] [CrossRef]
- SSzefel, J.; Kruszewski, W.J.; Buczek, T. Enteral feeding and its impact on the gut immune system and intestinal mucosal barrier. Gastroenterol. Rev. 2015, 10, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Lu, Y.; Srikant, C.B.; Gao, Z.-H.; Liu, J.-L. Intestinal adaptation and Reg gene expression induced by antidiabetic duodenal-jejunal bypass surgery in Zucker fatty rats. Am. J. Physiol. Liver Physiol. 2013, 304, G635–G645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- le Roux, C.W.; Borg, C.; Wallis, K.; Vincent, R.P.; Bueter, M.; Goodlad, R.; Ghatei, M.A.; Patel, A.; Bloom, S.R.; Aylwin, S.J.B. Gut Hypertrophy after Gastric Bypass Is Associated With Increased Glucagon-Like Peptide 2 and Intestinal Crypt Cell Proliferation. Ann. Surg. 2010, 252, 50–56. [Google Scholar] [CrossRef] [PubMed]
- TTaqi, E.; Wallace, L.E.; de Heuvel, E.; Chelikani, P.; Zheng, H.; Berthoud, H.-R.; Holst, J.J.; Sigalet, D.L. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J. Pediatr. Surg. 2010, 45, 987–995. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, L.A.; Bucher, B.T.; Erwin, C.R.; Wakeman, D.; White, F.V.; Warner, B.W. Intestinal adaptation after small bowel resection in human infants. J. Pediatr. Surg. 2011, 46, 1045–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, W.M.; Starling, E.H. The movements and innervation of the small intestine. J. Physiol. 1899, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef]
- Ahlman, H.; Nilsson, O. The gut as the largest endocrine organ in the body. Ann. Oncol. 2001, 12 (Suppl. S2), S63–S68. [Google Scholar] [CrossRef]
- Edkins, J.S. On the chemical mechanism of gastric secretion. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1905, 76, 376. [Google Scholar] [CrossRef] [Green Version]
- Grossman, M.I. Gastrin, cholecystokinin, and secretin act on one receptor. Lancet 1970, 295, 1088–1089. [Google Scholar] [CrossRef]
- Ahlman, H.; Dahlström, A. Vagal mechanisms controlling serotonin release from the gastrointestinal tract and pyloric motor function. J. Auton. Nerv. Syst. 1983, 9, 119–140. [Google Scholar] [CrossRef]
- Chaudhri, O.; Small, C.; Bloom, S. Gastrointestinal hormones regulating appetite. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1187–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducroc, R.; Guilmeau, S.; Akasbi, K.; Devaud, H.; Buyse, M.; Bado, A. Luminal Leptin Induces Rapid Inhibition of Active Intestinal Absorption of Glucose Mediated by Sodium-Glucose Cotransporter 1. Diabetes 2005, 54, 348–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewski, M.L.; Arterburn, D.E.; Van Scoyoc, L.; Smith, V.A.; Yancy, W.S.; Weidenbacher, H.J.; Livingston, E.H.; Olsen, M.K. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016, 151, 1046–1055. [Google Scholar] [CrossRef]
| Parameter | C | O | O + AKG | AKG |
|---|---|---|---|---|
| Duodenum | ||||
| Villi width/µm | 119.8 ± 43.4 c | 121.6 ± 33.8 bc | 313.3 ± 100.6 a | 200.9 ± 69.0 b |
| Villi length/µm | 623.8 ± 153.3 a | 433.6 ± 51.6 c | 572.5 ± 89.8 ab | 521.3 ± 79.8 b |
| Crypt depth/µm | 97.3 ± 52.9 c | 160.6 ± 28.8 bc | 205.4 ± 94.0 a | 152.2 ± 38.7 b |
| Crypt width/µm | 44.4 ± 6.8 b | 41.7 ± 7.4 bc | 48.9 ± 9.0 a | 40.9 ± 7.1 c |
| The height of the villi epithelium [µm] | 32.3 ± 0.3 b | 32.2 ± 0.5 b | 36.0 ± 0.4 a | 32.4 ± 0.3 b |
| Number of enterocytes per 100 μm of villi epithelium | 18.6 ± 4.0 a | 17.8 ± 4.0a | 17.8 ± 3.8 a | 15.0 ± 3.7 b |
| Number of apoptotic cells/mm2 of tissue | 2.4 ± 0.2 a | 1.0 ± 0.2 bc | 0.5 ± 0.1c | 1.3 ± 0.2 b |
| Jejunum | ||||
| Villi width [µm] | 79.3 ± 32.0 b | 101.6 ± 23.4 b | 228.2 ± 26.9 a | 206.8 ± 32.6 a |
| Villi length [µm] | 439.5 ± 70.0 b | 486.5 ± 74.7 ab | 515.9 ± 113.7 a | 395.4 ± 52.9 c |
| Crypt depth [µm] | 66.3 ± 22.8 c | 163.7 ± 38.4 a | 161.0 ± 40.1 a | 128.4 ± 28.7 b |
| Crypt width [µm] | 43.6 ± 8.9 a | 40.4 ± 7.8 ab | 42.7 ± 7.7 a | 38.0 ± 7.1 b |
| The height of the villi epithelium [µm] | 30.8 ± 0.3 b | 30.4 ± 0.6 b | 33.8 ± 0.4 a | 30.7 ± 0.4 b |
| Number of enterocytes per 100 μm of villi epithelium | 24.3 ± 5.4 a | 21.6 ± 3.9 ab | 18.2 ± 3.2 bc | 15.7 ± 4.3 c |
| Number of apoptotic cells/mm2 of tissue | 0.7 ± 0.2 b | 0.7 ± 0.1 b | 0.6 ± 0.1 b | 1.8 ± 0.2 a |
| Parameter | C | O | O + AKG | AKG |
|---|---|---|---|---|
| Cholecystokinin | 6.3 ± 1.9 a | 3.6 ± 1.4 ab | 2.7 ± 1.1 b | 5.6 ± 1.3 ab |
| Gastrin | 61.2 ± 16.1 a | 9.3 ± 1.5 b | 14.0 ± 2.9 b | 57.8 ± 17.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaniak, P.; Tomaszewska, E.; Muszyński, S.; Marszałek-Grabska, M.; Pierzynowski, S.G.; Dobrowolski, P. Dietary Alpha-Ketoglutarate Partially Abolishes Adverse Changes in the Small Intestine after Gastric Bypass Surgery in a Rat Model. Nutrients 2022, 14, 2062. https://doi.org/10.3390/nu14102062
Iwaniak P, Tomaszewska E, Muszyński S, Marszałek-Grabska M, Pierzynowski SG, Dobrowolski P. Dietary Alpha-Ketoglutarate Partially Abolishes Adverse Changes in the Small Intestine after Gastric Bypass Surgery in a Rat Model. Nutrients. 2022; 14(10):2062. https://doi.org/10.3390/nu14102062
Chicago/Turabian StyleIwaniak, Paulina, Ewa Tomaszewska, Siemowit Muszyński, Marta Marszałek-Grabska, Stefan Grzegorz Pierzynowski, and Piotr Dobrowolski. 2022. "Dietary Alpha-Ketoglutarate Partially Abolishes Adverse Changes in the Small Intestine after Gastric Bypass Surgery in a Rat Model" Nutrients 14, no. 10: 2062. https://doi.org/10.3390/nu14102062
APA StyleIwaniak, P., Tomaszewska, E., Muszyński, S., Marszałek-Grabska, M., Pierzynowski, S. G., & Dobrowolski, P. (2022). Dietary Alpha-Ketoglutarate Partially Abolishes Adverse Changes in the Small Intestine after Gastric Bypass Surgery in a Rat Model. Nutrients, 14(10), 2062. https://doi.org/10.3390/nu14102062









