Inner Shell of the Chestnut (Castanea crenatta) Suppresses Inflammatory Responses in Ovalbumin-Induced Allergic Asthma Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Chestnut Inner Shell Extract
2.2. HPLC Analysis
2.3. Animals
2.4. Experimental Procedure
2.5. Airway Hyperresponsiveness (AHR)
2.6. Analysis of Immunoglobulin E (IgE) OVA-Specific IgE in Serum
2.7. Analysis of Bronchoalveolar Lavage Fluid (BALF)
2.8. Histopathological Analysis of the Lung Tissue
2.9. Western Blot Analysis
2.10. Analysis of MMP-9 Level in Lung Tissue
2.11. Statistical Analysis
3. Results
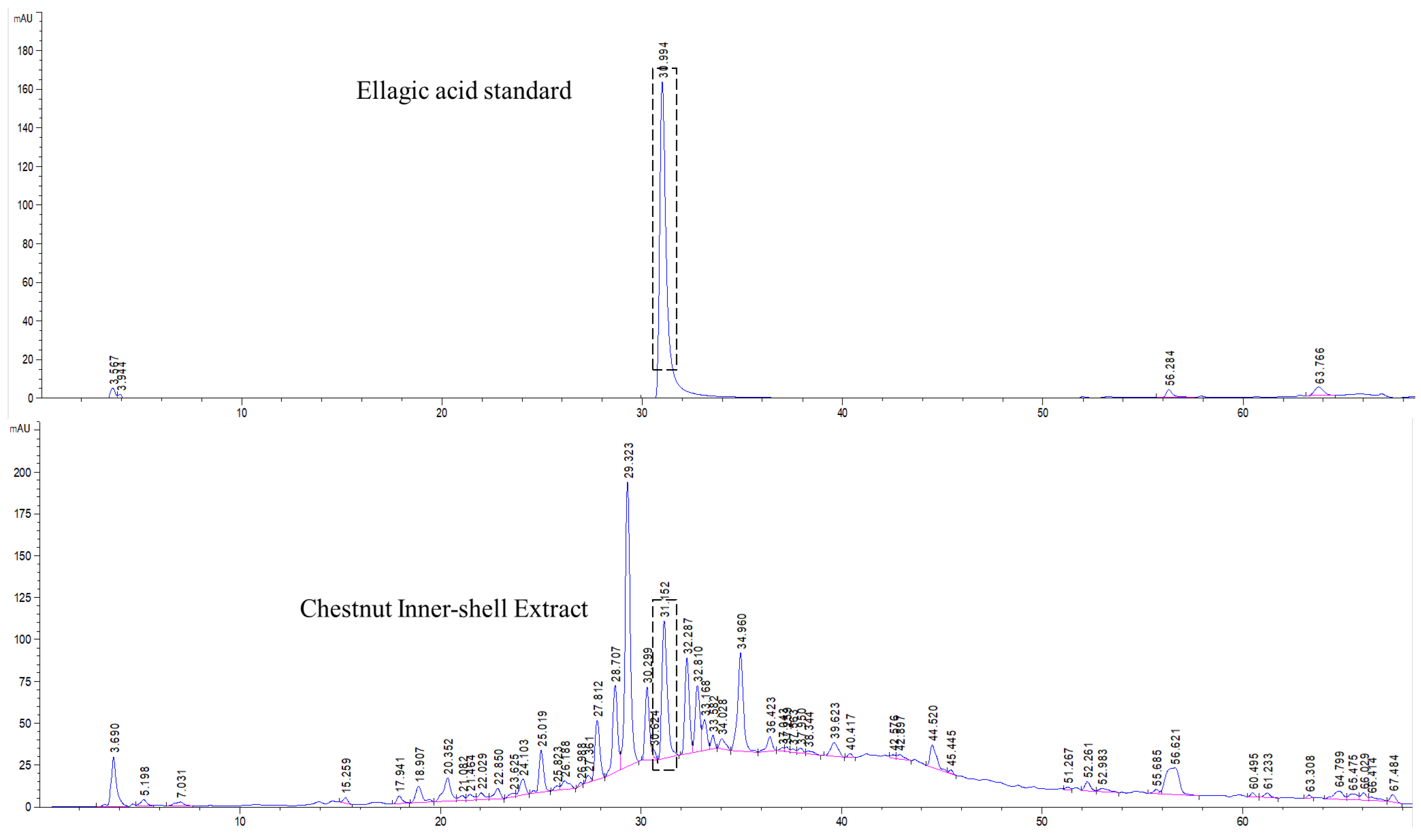
3.1. Standardization of CIE
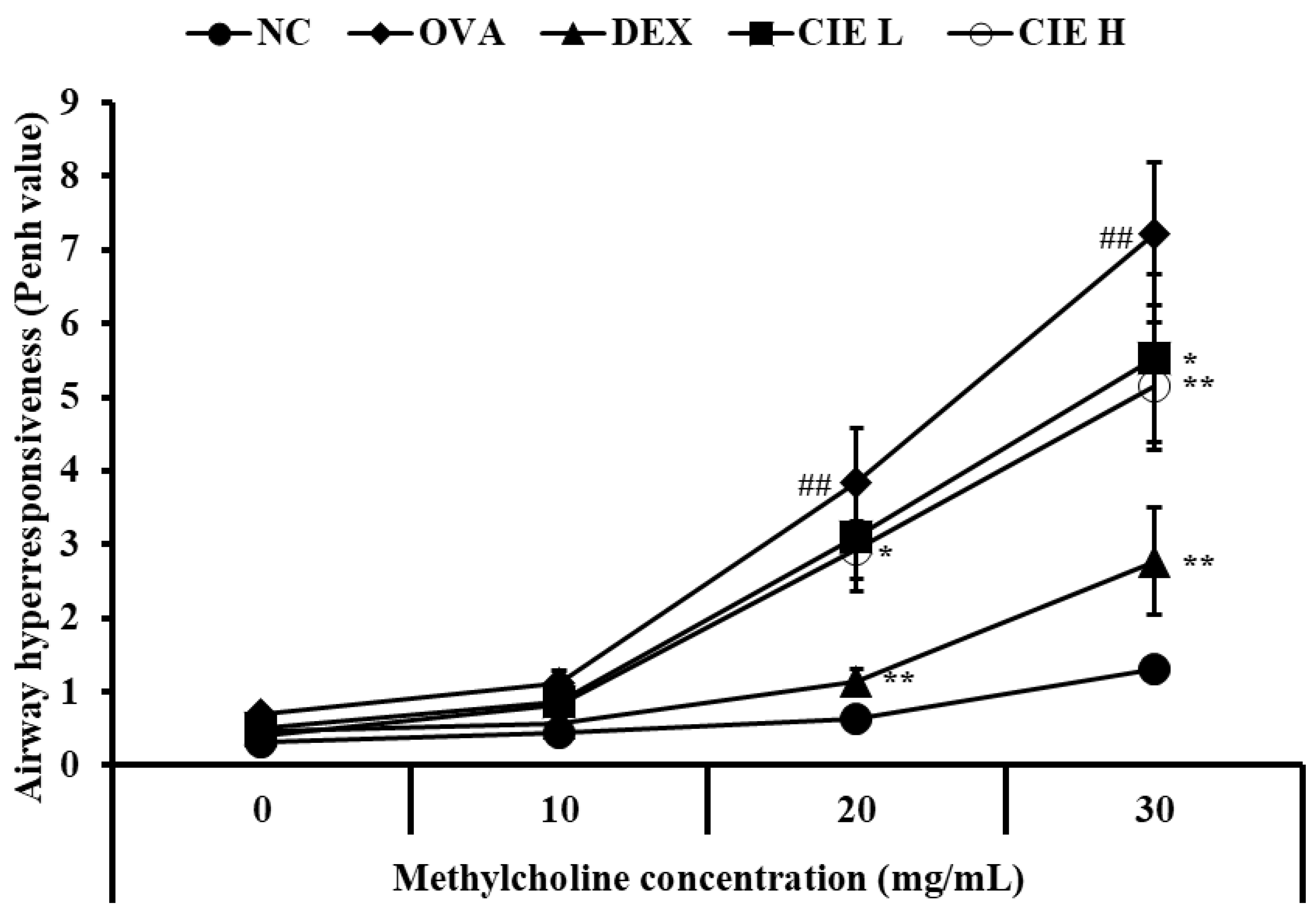
3.2. Effects of CIE on AHR in Asthmatic Mice
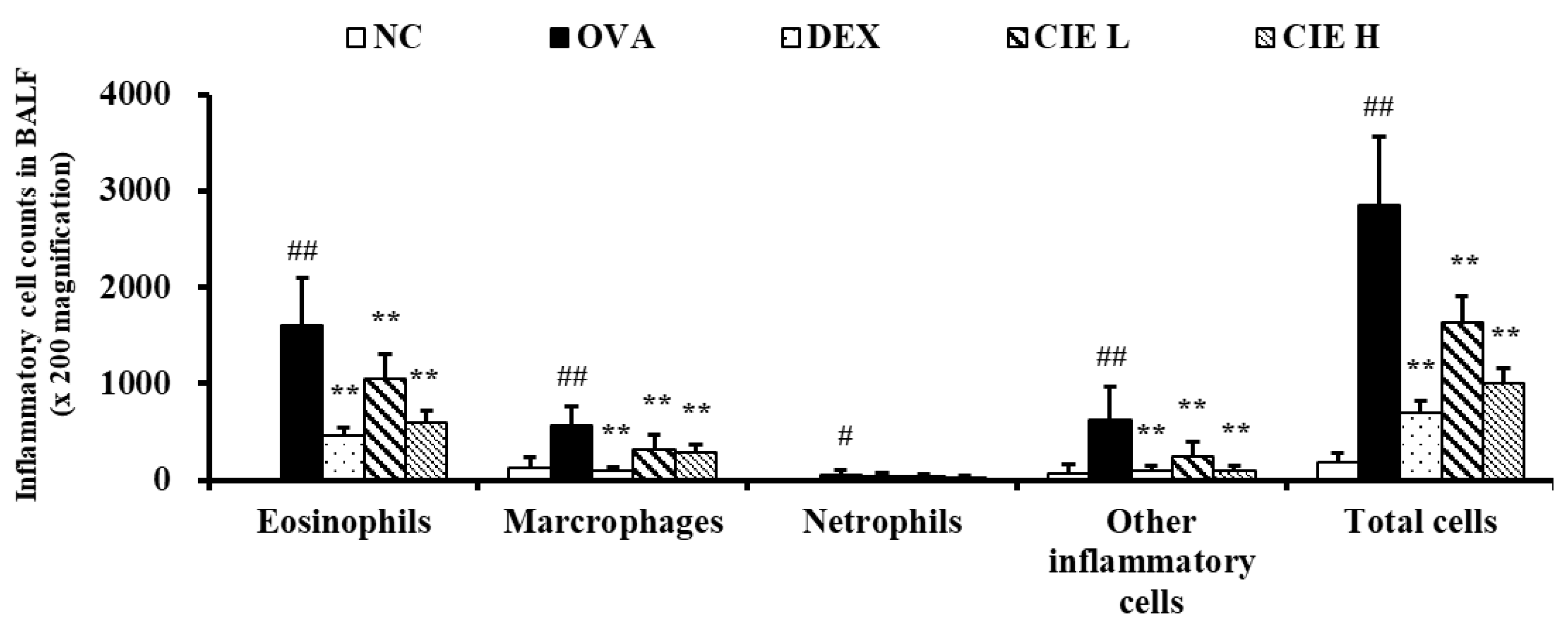
3.3. Effects of CIE on Inflammatory Cell Counts in BALF from Asthmatic Mice
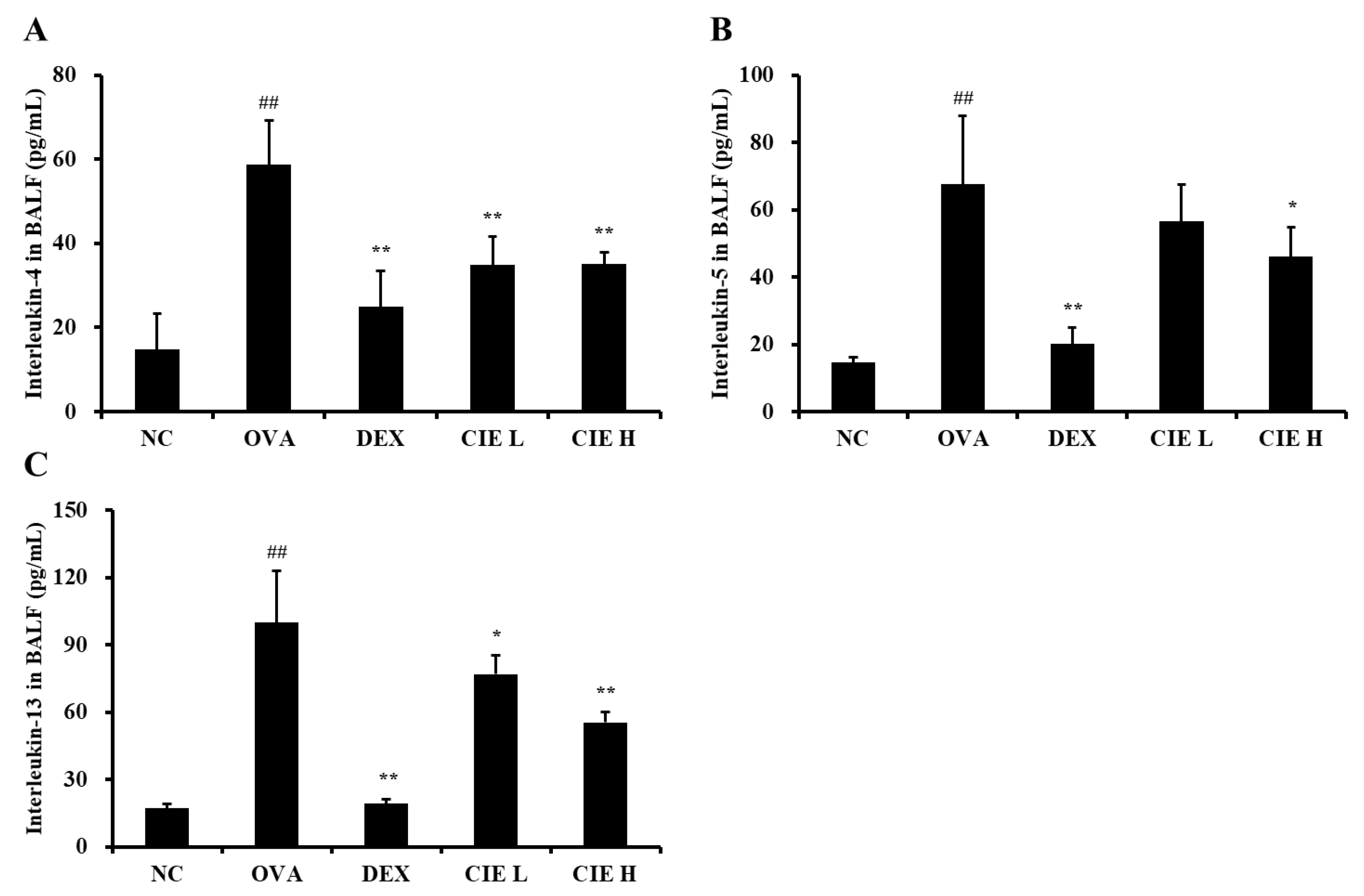
3.4. Effects of CIE on the Production of Type 2 Cytokines in BALF and Levels of IgE in Serum from OVA-Induced Asthmatic Mice
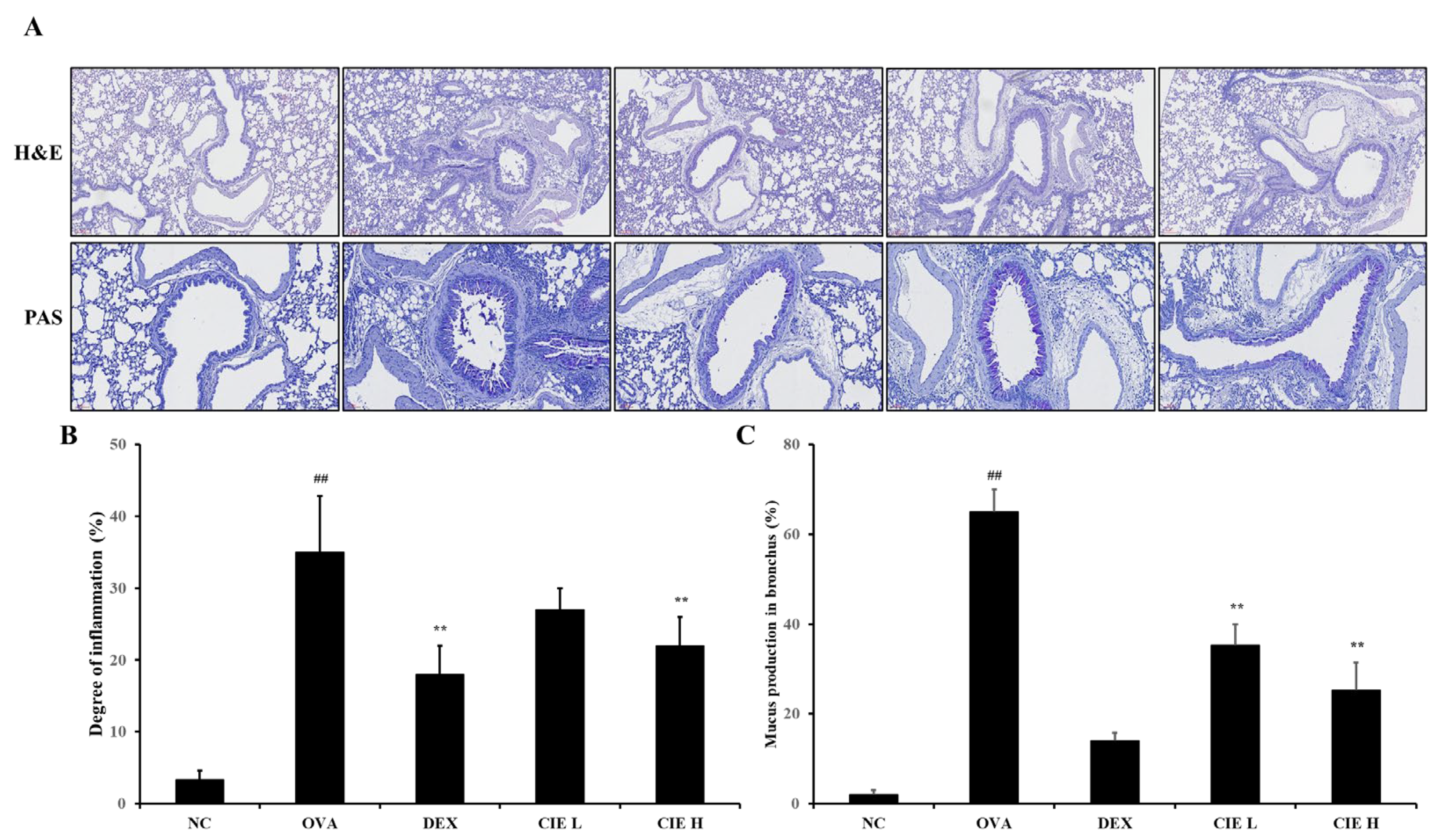
3.5. Effects of CIE on Airway Inflammation and Mucus Production in Lung Tissue from OVA-Induced Asthmatic Mice
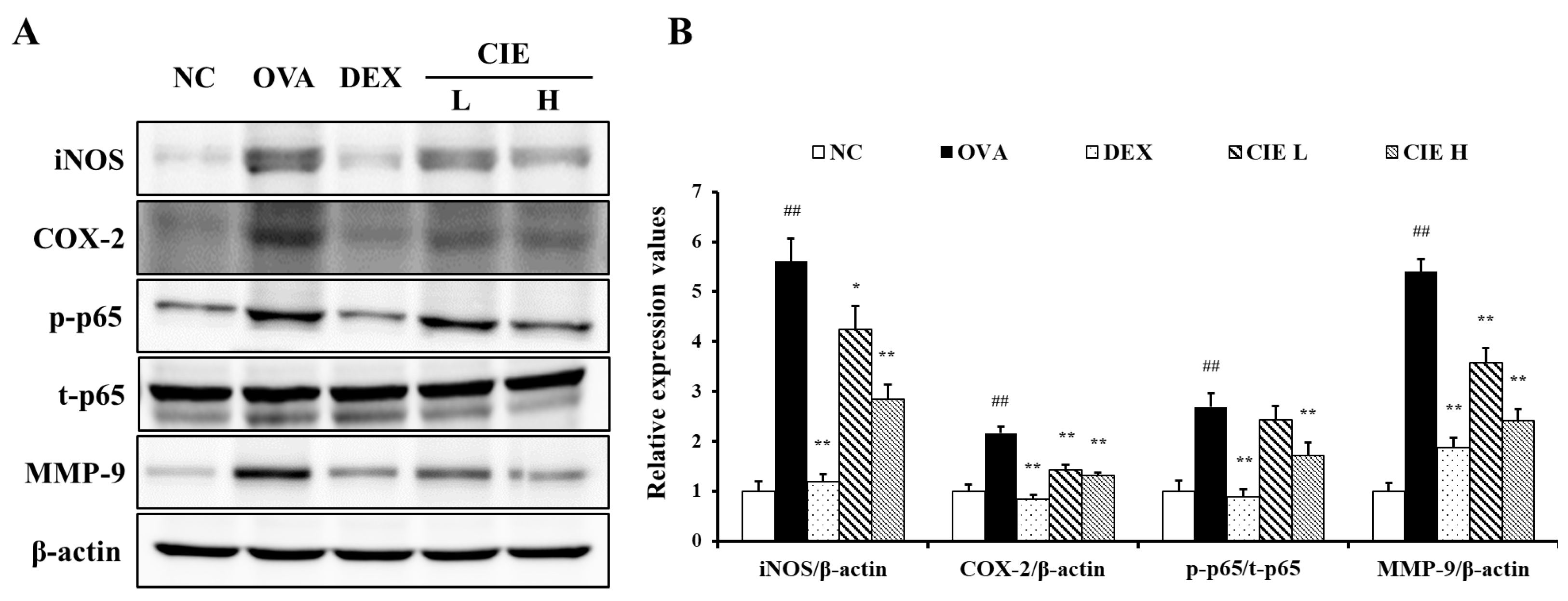
3.6. Effects of CIE on the Expression of iNOS, COX-2, NF-kB, and MMP-9 in Lung Tissue from OVA-Induced Asthmatic Mice
3.7. Effects of CIE on the Activity and Expression of MMP-9 in Lung Tissue from OVA-Induced Asthmatic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fuentes, N.; McCullough, M.; Panettieri, R.A., Jr.; Druey, K.M. RGS proteins, GRKs, and beta-arrestins modulate G protein-mediated signaling pathways in asthma. Pharmacol. Ther. 2021, 223, 107818. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Amin, F.; Sadeeqa, S. Prevalence of asthma and its management: A review. J. Pak. Med. Assoc. 2018, 68, 1823–1827. [Google Scholar] [PubMed]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; Menzies-Gow, A.N.; Price, D.B.; Bourdin, A.; Sweet, S.; Martin, A.L.; Alacqua, M.; Tran, T.N. Systematic Literature Review of Systemic Corticosteroid Use for Asthma Management. Am. J. Respir. Crit. Care Med. 2020, 201, 276–293. [Google Scholar] [CrossRef]
- Lloyd, C.M.; Hessel, E.M. Functions of T cells in asthma: More than just TH2 cells. Nat. Rev. Immunol. 2010, 10, 838–848. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Inam, A.; Shahzad, M.; Shabbir, A.; Shahid, H.; Shahid, K.; Javeed, A. Carica papaya ameliorates allergic asthma via down regulation of IL-4, IL-5, eotaxin, TNF-α, NF-ĸB, and iNOS levels. Phytomedicine 2021, 32, 1–7. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.H.; Kim, S.H.; Ahn, T.; Kim, S.W.; Kwak, Y.S.; Cho, I.H.; Nah, S.Y.; Cho, S.S.; Park, K.M.; et al. Korean Red Ginseng affects ovalbumin-induced asthma by modulating IL-12, IL-4, and IL-6 levels and the NF-κB/COX-2 and PGE2 pathways. J. Ginseng Res. 2021, 45, 482–489. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Dash, D.; Singh, R. Intranasal curcumin inhibits pulmonary fibrosis by modulating matrix metalloproteinase-9 (MMP-9) in ovalbumin-induced chronic asthma. Inflammation 2017, 40, 248–258. [Google Scholar] [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive phenolic components and potential health effects of chestnut shell: A review. J. Food Biochem. 2021, 45, e13696. [Google Scholar] [CrossRef]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’Acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-Sustainable Recovery of Phenolic and Antioxidant Compounds from Industrial Chestnut Shells Using Ultrasound-Assisted Extraction: Optimization and Evaluation of Biological Activities In Vitro. Antioxidant 2022, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, J.R.; Fang, G.T.; Kim, Y.H.; Yang, K.J.; Hwang, J.H.; Lee, H.S.; Oh, W.K.; Song, K.S.; Lee, C.H. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4- and high-fat diet-treated mice. Food Chem. Toxicol. 2010, 48, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Cerulli, A.; Napolitano, A.; Masullo, M.; Hošek, J.; Pizza, C.; Piacente, S. Chestnut shells (Italian cultivar "Marrone di Roccadaspide" PGI): Antioxidant activity and chemical investigation with in depth LC-HRMS/MS n rationalization of tannins. Food Res. Int. 2020, 129, 108787. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.S.; Lee, N.K.; Na, D.S.; Yu, H.H.; Paik, H.D. Comparative analysis of the antioxidant and anticancer activities of chestnut inner shell extracts prepared with various solvents. J. Sci. Food Agric. 2016, 96, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Jo, S.L.; Heo, J.H.; Kim, T.W.; Lee, K.P.; Hong, E.J. The aqueous fraction of Castanea crenata inner shell extract reduces obesity and intramuscular lipid accumulation via induction of mitochondrial respiration and fatty acid oxidation in muscle. Phytomedicine 2022, 98, 153974. [Google Scholar] [CrossRef]
- Kim, R.N.; Jo, S.H.; Choi, Y.J.; Kim, T.W.; Park, J.E. Chestnut inner shell extract inhibits viral entry of porcine epidemic diarrhea virus and other coronaviruses in vitro. Phytomedicine 2022. under review. [Google Scholar]
- Ko, J.W.; Kwon, H.J.; Seo, C.S.; Choi, S.J.; Shin, N.R.; Kim, S.H.; Kim, Y.H.; Kim, J.C.; Kim, M.S.; Shin, I.S. 4-Hydroxycinnamic acid suppresses airway inflammation and mucus hypersecretion in allergic asthma induced by ovalbumin challenge. Phytother. Res. 2019, 34, 624–633. [Google Scholar] [CrossRef]
- Lim, J.O.; Lee, S.J.; Kim, W.I.; Pak, S.W.; Moon, C.; Shin, I.S.; Heo, J.D.; Ko, J.W.; Kim, J.C. Titanium Dioxide Nanoparticles Exacerbate Allergic Airway Inflammation via TXNIP Upregulation in a Mouse Model of Asthma. Int. J. Mol. Sci. 2021, 22, 9924. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, J.H.; Kim, C.Y.; Jeong, J.S.; Lim, J.O.; Kim, J.C.; Ko, J.W.; Kim, T.W. Diallyl disulfide prevents 1,3-dichloro-2-propanol-induced hepatotoxicity through mitogen-activated protein kinases signaling. Food Chem. Toxicol. 2022, 160, 112814. [Google Scholar] [CrossRef]
- Cloutier, M.M.; Dixon, A.E.; Krishnan, J.A.; Lemanske, R.F.; Pace, W.; Schatz, M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program. JAMA 2020, 324, 2301–2317. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The cytokines of asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Al-Nour, M.Y.; Ibrahim, M.M.; Elsaman, T. Ellagic acid, Kaempferol, and Quercetin from Acacia nilotica: Promising combined drug with multiple mechanisms of action. Curr. Pharmacol. Rep. 2019, 5, 255–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, E.; Fu, Y.; Wei, Z.; Yang, Z. Inhibition of allergic airway inflammation through the blockage of NF-κB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014, 5, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Finotto, S. Resolution of allergic asthma. Semin. Immunopathol. 2019, 41, 665–674. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The regulatory function of eosinophils. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Dong, J.; Ji, L.; Jiang, P.; Leung, T.F.; Liu, D.; Ng, L.G.; Tsang, M.S.; Jiao, D.; Lam, C.W.; et al. Anti-allergic inflammatory activity of interleukin-37 is mediated by novel signaling cascades in human eosinophils. Front. Immunol. 2018, 9, 1445. [Google Scholar] [CrossRef]
- Mahmutovic Persson, I.; Akbarshahi, H.; Menzel, M.; Brandelius, A.; Uller, L. Increased expression of upstream TH2-cytokines in a mouse model of viral-induced asthma exacerbation. J. Transl. Med. 2016, 14, 52. [Google Scholar] [CrossRef] [Green Version]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Luan, B.; Xu, Q.R.; Wang, X.F. Effect of TLR7 gene expression mediating NF-κB signaling pathway on the pathogenesis of bronchial asthma in mice and the intervention role of IFN-γ. Eur. Rev. Med. Pharmacol. Sci. 2021, 5, 866–879. [Google Scholar]
- Medeiros, M.L.; de Oliveira, M.G.; Tavares, E.G.; Mello, G.C.; Anhê, G.F.; Mónica, F.Z.; Antunes, E. Long-term methylglyoxal intake aggravates murine Th2-mediated airway eosinophil infiltration. Int. Immunopharmacol. 2020, 81, 106254. [Google Scholar] [CrossRef]
- Bignon, E.; Rizza, S.; Filomeni, G.; Papaleo, E. Use of computational biochemistry for elucidating molecular mechanisms of nitric oxide synthase. Comput. Struct. Biotechnol. J. 2019, 17, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Comer, B.S.; Camoretti-Mercado, B.; Kogut, P.C.; Halayko, A.J.; Solway, J.; Gerthoffer, W.T. Cyclooxygenase-2 and microRNA-155 expression are elevated in asthmatic airway smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2015, 52, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chibana, K.; Trudeau, J.B.; Mustovich, A.T.; Hu, H.; Zhao, J.; Balzar, S.; Chu, H.W.; Wenzel, S.E. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin. Exp. Allergy 2008, 38, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Redington, A.E.; Meng, Q.H.; Springall, D.R.; Evans, T.J.; Créminon, C.; Maclouf, J.; Holgate, S.T.; Howarth, P.H.; Polak, J.M. Increased expression of inducible nitric oxide synthase and cyclo-oxygenase-2 in the airway epithelium of asthmatic subjects and regulation by corticosteroid treatment. Thorax 2001, 56, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Rumzhum, N.N.; Ammit, A.J. Cyclooxygenase 2: Its regulation, role and impact in airway inflammation. Clin. Exp. Allergy 2016, 46, 397–410. [Google Scholar] [CrossRef]
- Sung, J.E.; Lee, H.A.; Kim, J.E.; Yun, W.B.; An, B.S.; Yang, S.Y.; Kim, D.S.; Lee, C.Y.; Lee, H.S.; Bae, C.J.; et al. Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int. J. Mol. Med. 2017, 40, 1365–1376. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.; Dwivedi, U.N.; Kakkar, P. Tinospora cordifolia extract modulates COX-2, iNOS, ICAM-1, pro-inflammatory cytokines and redox status in murine model of asthma. J. Ethnopharmacol. 2014, 153, 326–337. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell. Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [Green Version]
- Ingram, J.L.; Kraft, M. Metalloproteinases as modulators of allergic asthma: Therapeutic perspectives. Met. Med. 2015, 2, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Cataldo, D.; Munaut, C.; Noël, A.; Frankenne, F.; Bartsch, P.; Foidart, J.M.; Louis, R. MMP-2-and MMP-9-linked gelatinolytic activity in the sputum from patients with asthma and chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 2000, 123, 259–267. [Google Scholar] [CrossRef]
- McMillan, S.; Kearley, J.; Campbell, J.D.; Zhu, X.W.; Larbi, K.Y.; Shipley, J.M.; Senior, R.M.; Nourshargh, S.; Lloyd, C.M. Matrix metalloproteinase-9 deficiency results in enhanced allergen-induced airway inflammation. J. Immunol. 2004, 172, 2586–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbel, M.; Belleguic, C.; Boichot, E.; Lagente, V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol. Toxicol. 2002, 18, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Deng, J.S.; Chiu, C.S.; Liao, J.C.; Hsieh, W.T.; Sheu, M.J.; Wu, C.H. Hispolon Protects against Acute Liver Damage in the Rat by Inhibiting Lipid Peroxidation, Proinflammatory Cytokine, and Oxidative Stress and Downregulating the Expressions of iNOS, COX-2, and MMP-9. Evid. Based Complement Alternat. Med. 2012, 2012, 480714. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-Y.; Kim, J.-W.; Kim, J.-H.; Jeong, J.-S.; Lim, J.-O.; Ko, J.-W.; Kim, T.-W. Inner Shell of the Chestnut (Castanea crenatta) Suppresses Inflammatory Responses in Ovalbumin-Induced Allergic Asthma Mouse Model. Nutrients 2022, 14, 2067. https://doi.org/10.3390/nu14102067
Kim C-Y, Kim J-W, Kim J-H, Jeong J-S, Lim J-O, Ko J-W, Kim T-W. Inner Shell of the Chestnut (Castanea crenatta) Suppresses Inflammatory Responses in Ovalbumin-Induced Allergic Asthma Mouse Model. Nutrients. 2022; 14(10):2067. https://doi.org/10.3390/nu14102067
Chicago/Turabian StyleKim, Chang-Yeop, Jeong-Won Kim, Jin-Hwa Kim, Ji-Soo Jeong, Je-Oh Lim, Je-Won Ko, and Tae-Won Kim. 2022. "Inner Shell of the Chestnut (Castanea crenatta) Suppresses Inflammatory Responses in Ovalbumin-Induced Allergic Asthma Mouse Model" Nutrients 14, no. 10: 2067. https://doi.org/10.3390/nu14102067
APA StyleKim, C. -Y., Kim, J. -W., Kim, J. -H., Jeong, J. -S., Lim, J. -O., Ko, J. -W., & Kim, T. -W. (2022). Inner Shell of the Chestnut (Castanea crenatta) Suppresses Inflammatory Responses in Ovalbumin-Induced Allergic Asthma Mouse Model. Nutrients, 14(10), 2067. https://doi.org/10.3390/nu14102067





