Current Hydration Habits: The Disregarded Factor for the Development of Renal and Cardiometabolic Diseases
Abstract
:1. Introduction
2. Types of Dehydration
3. What Constitutes an Adequate Water Intake?
4. Intake of Sweetened Beverages
5. Metabolic, Cardiovascular, and Renal Effects Resulting from Poor Hydration Habits
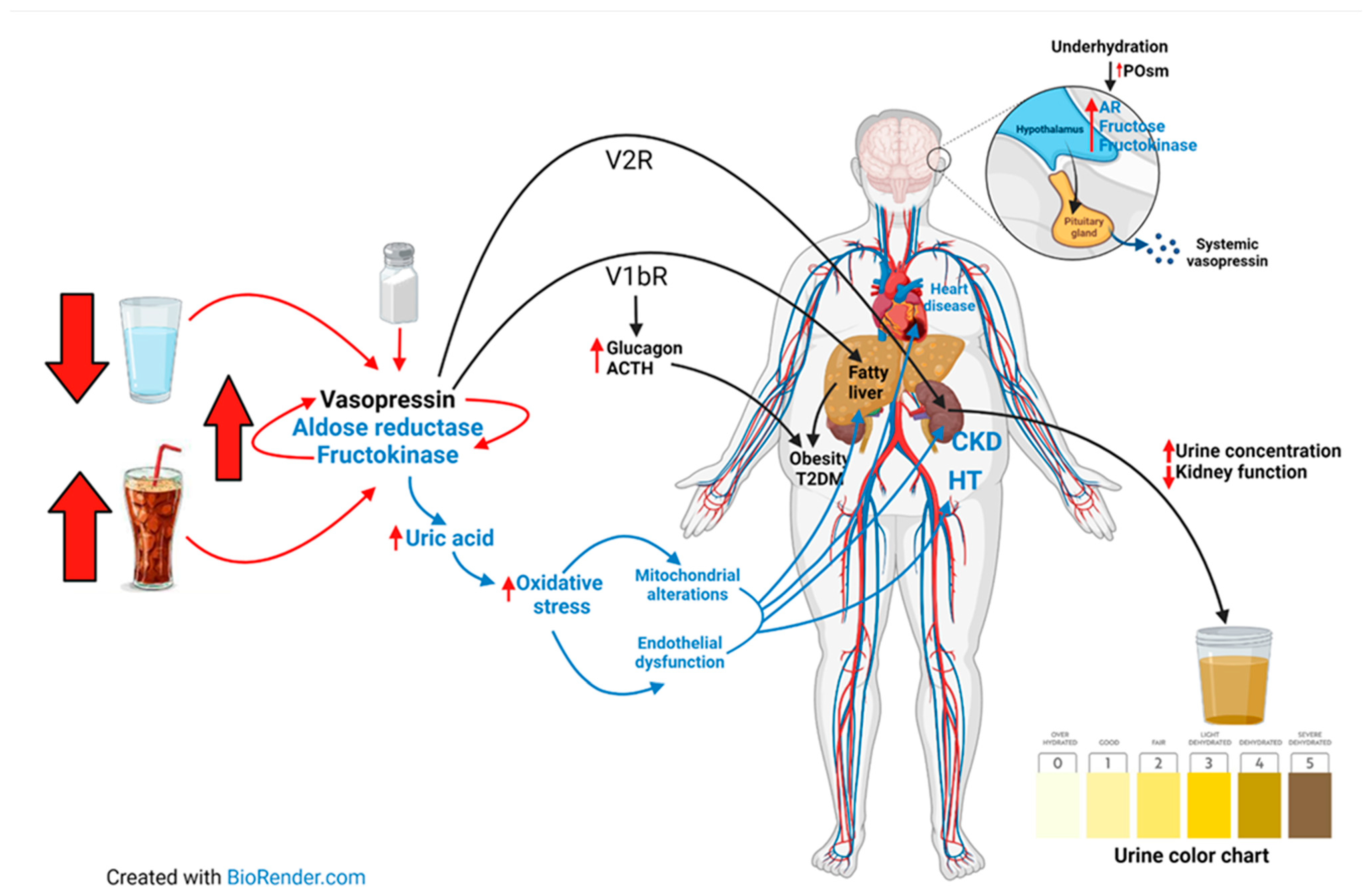
5.1. Vasopressin Pathway
5.2. Aldose Reductase-Fructokinase (AR-F) Pathway
5.3. Synergy of the Vasopressin and AR-F Pathways
6. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nyberg, S.T.; Singh-Manoux, A.; Pentti, J.; Madsen, I.E.H.; Sabia, S.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Goldberg, M.; et al. Association of Healthy Lifestyle with Years Lived Without Major Chronic Diseases. JAMA Intern. Med. 2020, 180, 760–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, E.C. Water: Neglected, unappreciated and under researched. Eur. J. Clin. Nutr. 2013, 67, 492–495. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, S.M. Water: An Essential but Overlooked Nutrient. J. Am. Diet. Assoc. 1999, 99, 200–206. [Google Scholar] [CrossRef]
- Bouby, N.; Fernandes, S. Mild dehydration, vasopressin and the kidney: Animal and human studies. Eur. J. Clin. Nutr. 2003, 57, S39–S46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaecke, T.; Perrier, E.T.; Melander, O. A Journey through the Early Evidence Linking Hydration to Metabolic Health. Ann. Nutr. Metab. 2020, 76, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Stookey, J.J.D. Negative, Null and Beneficial Effects of Drinking Water on Energy Intake, Energy Expenditure, Fat Oxidation and Weight Change in Randomized Trials: A Qualitative Review. Nutrients 2016, 8, 19. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, Assessment, and Performance Effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.J.; Stenvinkel, P.; Andrews, P.; Sánchez-Lozada, L.G.; Nakagawa, T.; Gaucher, E.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Jimenez, C.R.; Garcia, G.; et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J. Interm. Med. 2020, 287, 252–262. [Google Scholar] [CrossRef] [Green Version]
- Kavouras, S.A. Hydration, dehydration, underhydration, optimal hydration: Are we barking up the wrong tree? Eur. J. Nutr. 2019, 58, 471–473. [Google Scholar] [CrossRef]
- Stookey, J.D. Analysis of 2009–2012 Nutrition Health and Examination Survey (NHANES) Data to Estimate the Median Water Intake Associated with Meeting Hydration Criteria for Individuals Aged 12–80 Years in the US Population. Nutrients 2019, 11, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stookey, J.D.; Kavouras, S.A.; Suh, H.; Lang, F. Underhydration Is Associated with Obesity, Chronic Diseases, and Death within 3 to 6 Years in the U.S. Population Aged 51–70 Years. Nutrients 2020, 12, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stookey, J.D. High Prevalence of Plasma Hypertonicity among Community-Dwelling Older Adults: Results from NHANES III. J. Am. Diet. Assoc. 2005, 105, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Gandy, J. Water intake: Validity of population assessment and recommendations. Eur. J. Nutr. 2015, 54, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA. Panel on Dietetic Products, Nutrition, and Allergies (NDA); Scientific Opinion on Dietary reference values for water. EFSA J. 2010, 8, 1459. [Google Scholar]
- Institute of Medicine FaNB. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride and Sulfate; National Academies Press: Washington, DC, USA, 2005; p. 638. [Google Scholar]
- Pêgo, C.; Guelinckx, I.; Moreno, L.A.; Kavouras, S.; Gandy, J.J.; Martinez, H.; Bardosono, S.; Abdollahi, M.; Nasseri, E.; Jarosz, A.; et al. Total fluid intake and its determinants: Cross-sectional surveys among adults in 13 countries worldwide. Eur. J. Nutr. 2015, 54 (Suppl. 2), 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, H.; Guelinckx, I.; Salas-Salvadó, J.; Gandy, J.; Kavouras, S.A.; Moreno, L.A. Harmonized Cross-Sectional Surveys Focused on Fluid Intake in Children, Adolescents and Adults: The Liq.In7 Initiative. Ann. Nutr. Metab. 2016, 68 (Suppl. 2), 12–18. [Google Scholar] [CrossRef]
- Stookey, J.D.; Barclay, D.; Arieff, A.; Popkin, B.M. The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur. J. Clin. Nutr. 2007, 61, 190–199. [Google Scholar] [CrossRef] [Green Version]
- Stookey, J.D.; Purser, J.L.; Pieper, C.F.; Cohen, H.J. Plasma hypertonicity: Another marker of frailty? J. Am. Geriatr. Soc. 2004, 52, 1313–1320. [Google Scholar] [CrossRef]
- Stookey, J.D.; Brass, B.; Holliday, A.; Arieff, A. What is the cell hydration status of healthy children in the USA? Preliminary data on urine osmolality and water intake. Public Health Nutr. 2012, 15, 2148–2156. [Google Scholar] [CrossRef] [Green Version]
- Bar-David, Y.; Urkin, J.; Landau, D.; Bar-David, Z.; Pilpel, D. Voluntary dehydration among elementary school children residing in a hot arid environment. J. Hum. Nutr. Diet. 2009, 22, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Manz, F.; Wentz, A. 24-h hydration status: Parameters, epidemiology and recommendations. Eur. J. Clin. Nutr. 2003, 57 (Suppl. 2), S10–S18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, L.E.; Herrera Soto, J.A.; Hacker, F.T.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary indices during dehydration, exercise, and rehydration. Int. J. Sport Nutr. 1998, 8, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kavouras, S.A. Assessing hydration status. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 519–524. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Maresh, C.M.; Castellani, J.W.; Bergeron, M.F.; Kenefick, R.W.; LaGasse, K.E.; Riebe, D. Urinary indices of hydration status. Int. J. Sport Nutr. 1994, 4, 265–279. [Google Scholar] [CrossRef]
- Kavouras, S.A.; Johnson, E.C.; Bougatsas, D.; Arnaoutis, G.; Panagiotakos, D.B.; Perrier, E.; Klein, A. Validation of a urine color scale for assessment of urine osmolality in healthy children. Eur. J. Nutr. 2016, 55, 907–915. [Google Scholar] [CrossRef] [Green Version]
- Urkin, J.; Bar-David, Y. Voluntary, Nonintentional Dehydration and Health. Am. J. Public Health 2015, 105, e7. [Google Scholar] [CrossRef]
- Greenleaf, J.E. Problem: Thirst, drinking behavior, and involuntary dehydration. Med. Sci. Sports Exerc. 1992, 24, 645–656. [Google Scholar] [CrossRef]
- Rodger, A.; Wehbe, L.H.; Papies, E.K. “I know it’s just pouring it from the tap, but it’s not easy”: Motivational processes that underlie water drinking. Appetite 2021, 164, 105249. [Google Scholar] [CrossRef]
- Piil, J.F.; Lundbye-Jensen, J.; Christiansen, L.; Ioannou, L.; Tsoutsoubi, L.; Dallas, C.N.; Mantzios, K.; Flouris, A.D.; Nybo, L. High prevalence of hypohydration in occupations with heat stress-Perspectives for performance in combined cognitive and motor tasks. PLoS ONE 2018, 13, e0205321. [Google Scholar] [CrossRef] [Green Version]
- Venugopal, V.; Rekha, S.; Manikandan, K.; Latha, P.K.; Vennila, V.; Ganesan, N.; Kumaravel, P.; Chinnadurai, S.J. Heat stress and inadequate sanitary facilities at workplaces—An occupational health concern for women? Glob. Health Action 2016, 9, 31945. [Google Scholar] [CrossRef] [PubMed]
- Faidah, N.; Soraya, G.V.; Erlichster, M.; Natzir, R.; Chana, G.; Skafidas, E.; Hardjo, M.; Ganda, I.J.; Bahar, B. Detection of voluntary dehydration in paediatric populations using non-invasive point-of-care saliva and urine testing. J. Paediatr. Child Health 2021, 57, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Garneau, N.L.; Nuessle, T.M.; Mendelsberg, B.J.; Shepard, S.; Tucker, R.M. Sweet liker status in children and adults consequences for beverage intake in adults. Food Qual. Prefer. 2018, 65, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.S. Dehydration: Isonatremic, Hyponatremic, and Hypernatremic Recognition and Management. Pediatr. Rev. 2015, 36, 274–283. [Google Scholar] [CrossRef]
- Brooks, C.J.; Gortmaker, S.L.; Long, M.W.; Cradock, A.L.; Kenney, E.L. Racial ethnic and socioeconomic disparities in hydration status among us adults and the role of tap water and other beverage intake. Am. J. Public Health 2017, 107, 1387–1394. [Google Scholar] [CrossRef]
- Phillips, P.A.; Rolls, B.J.; Ledingham, J.G.G.; Forsling, M.L.; Morton, J.J.; Crowe, M.J.; Wollner, L. Reduced thirst after water deprivation in healthy elderly men. N. Engl. J. Med. 1984, 311, 753–759. [Google Scholar] [CrossRef]
- Block, J.P.; Gillman, M.W.; Linakis, S.K.; Goldman, R.E. “If it tastes good, I’m drinking it”: Qualitative study of beverage consumption among college students. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2013, 52, 702–706. [Google Scholar] [CrossRef] [Green Version]
- Meyer, F.; Bar-Or, O.; Salsberg, A.; Passe, D. Hypohydration during exercise in children: Effect on thirst, drink preferences, and rehydration. Int. J. Sport Nutr. 1994, 4, 22–35. [Google Scholar] [CrossRef]
- Battram, D.S.; Piche, L.; Beynon, C.; Kurtz, J.; He, M. Sugar-Sweetened Beverages: Children’s Perceptions, Factors of Influence, and Suggestions for Reducing Intake. J. Nutr. Educ. Behav. 2015, 48, 27–34. [Google Scholar] [CrossRef]
- Vennerød, F.F.F.; Almli, V.L.; Berget, I.; Lien, N. Do parents form their children s sweet preference the role of parents and taste sensitivity on preferences for sweetness in pre schoolers. Food Qual. Prefer. 2017, 62, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Pan, L.; Sherry, B.; Li, R. The association of sugar sweetened beverage intake during infancy with sugar sweetened beverage intake at 6 years of age. Pediatrics 2014, 134, S56–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Arroyo, F.E.; Pérez-Estévez, H.E.; Tapia, E.; Gonzaga, G.; Muñoz-Jiménez, I.; Soto, V.; Sánchez-Lozada, L.G. Restricted Water Intake and Hydration with Fructose-Containing Beverages during Infancy Predispose to Aggravate an Acute Renal Ischemic Insult in Adolescent Rats. Biomed. Res. Int. 2020, 2020, 4281802. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Li, Y.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogart, L.M.; Cowgill, B.O.; Sharma, A.J.; Uyeda, K.; Sticklor, L.A.; Alijewicz, K.E.; Schuster, M.A. Parental and home environmental facilitators of sugar-sweetened beverage consumption among overweight and obese Latino youth. Acad. Pediatr. 2013, 13, 348–355. [Google Scholar] [CrossRef] [Green Version]
- Popkin, B.M.; Hawkes, C. Sweetening of the global diet, particularly beverages: Patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016, 4, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Bergallo, P.; Castagnari, V.; Fernández, A.; Mejía, R. Regulatory initiatives to reduce sugar-sweetened beverages (SSBs) in Latin America. PLoS ONE 2018, 13, e0205694. [Google Scholar] [CrossRef]
- Sigala, D.M.; Stanhope, K.L. An Exploration of the Role of Sugar-Sweetened Beverage in Promoting Obesity and Health Disparities. Curr. Obes. Rep. 2021, 10, 39–52. [Google Scholar] [CrossRef]
- Kumar, G.S.; Pan, L.; Park, S.; Leekwan, S.H.; Onufrak, S.; Blanck, H.M. Sugar sweetened beverage consumption among adults 18 states 2012. Morb. Mortal. Wkly. Rep. 2014, 63, 686–690. [Google Scholar]
- Han, E.; Powell, L.M. Consumption patterns of sugar sweetened beverages in the united states. J. Acad. Nutr. Diet. 2013, 113, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Ogden, C.L.; Kit, B.K.; Carroll, M.D.; Park, S. Consumption of sugar drinks in the united states 2005–2008. In NCHS Data Brief; NCH: Highlandville, MD, USA, 2011; pp. 1–8. [Google Scholar]
- Martinez, H.; Morin, C.; Gandy, J.; Carmuega, E.; Arredondo, J.L.; Pimentel, C.; Moreno, L.A.; Kavouras, S.A.; Salas-Salvadó, J.; Guelinckx, I. Fluid intake of Latin American adults: Results of four 2016 Liq.In 7 national cross-sectional surveys. Eur. J. Nutr. 2018, 57, 65–75. [Google Scholar] [CrossRef] [Green Version]
- Redondo, M.; Hernández-Aguado, I.; Lumbreras, B. The impact of the tax on sweetened beverages: A systematic review. Am. J. Clin. Nutr. 2018, 108, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Colchero, M.A.; Rivera-Dommarco, J.; Popkin, B.M.; Ng, S.W. In Mexico, Evidence of Sustained Consumer Response Two Years After Implementing A Sugar-Sweetened Beverage Tax. Health Aff. (Millwood) 2017, 36, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Falbe, J.; Thompson, H.R.; Becker, C.M.; Rojas, N.; McCulloch, C.E.; Madsen, K.A. Impact of the Berkeley Excise Tax on Sugar-Sweetened Beverage Consumption. Am. J. Public Health 2016, 106, 1865–1871. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Ng, S.W. Sugar-sweetened beverage taxes: Lessons to date and the future of taxation. PLoS Med. 2021, 18, e1003412. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Hu, F.B. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat. Rev. Endocrinol. 2022, 18, 205–218. [Google Scholar] [CrossRef]
- De Ruyter, J.C.; Olthof, M.R.; Seidell, J.C.; Katan, M.B. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N. Engl. J. Med. 2012, 367, 1397–1406. [Google Scholar] [CrossRef] [Green Version]
- García-Arroyo, F.E.; Tapia, E.; Muñoz-Jiménez, I.; Gonzaga-Sánchez, G.; Arellano-Buendía, A.S.; Osorio-Alonso, H.; Manterola-Romero, L.; Roncal-Jiménez, C.A.; Johnson, R.J.; Sánchez-Lozada, L.G. Fluid Intake Restriction Concomitant to Sweetened Beverages Hydration Induce Kidney Damage. Oxid. Med. Cell. Longev. 2020, 2020, 8850266. [Google Scholar] [CrossRef]
- Garcia, F.; Cristóbal, M.; Arellano-Buendía, A.S.; Osorio, H.; Tapia, E.; Soto, V.; Madero, M.; Lanaspa, M.A.; Roncal-Jiménez, C.; Bankir, L.; et al. Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R57–R65. [Google Scholar] [CrossRef] [Green Version]
- Watso, J.C.; Farquhar, W.B. Hydration Status and Cardiovascular Function. Nutrients 2019, 11, 1866. [Google Scholar] [CrossRef] [Green Version]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Fay, M.J.; Du, J.; Yu, X.; North, W.G. Evidence for expression of vasopressin V2 receptor mRNA in human lung. Peptides 1996, 17, 477–481. [Google Scholar] [CrossRef]
- Andres-Hernando, A.; Jensen, T.J.; Kuwabara, M.; Orlicky, D.J.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Garcia, G.E.; Ishimoto, T.; Maclean, P.S.; et al. Vasopressin mediates fructose-induced metabolic syndrome by activating the V1b receptor. JCI Insight. 2021, 6, e140848. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Stenvinkel, P.; Jensen, T.; Lanaspa, M.A.; Roncal, C.; Song, Z.; Bankir, L.; Sanchez-Lozada, L.-G. Metabolic and Kidney Diseases in the Setting of Climate Change, Water Shortage, and Survival Factors. J. Am. Soc. Nephrol. 2016, 27, 2247–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasevska, I.; Enhörning, S.; Christensson, A.; Persson, M.; Nilsson, P.M.; Melander, O. Increased Levels of Copeptin, a Surrogate Marker of Arginine Vasopressin, Are Associated with an Increased Risk of Chronic Kidney Disease in a General Population. Am. J. Nephrol. 2016, 44, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Roussel, R.; El Boustany, R.; Bouby, N.; Potier, L.; Fumeron, F.; Mohammedi, K.; Balkau, B.; Tichet, J.; Bankir, L.; Marre, M.; et al. Plasma copeptin avp gene variants and incidence of type 2 diabetes in a cohort from the community. J. Clin. Endocrinol. Metab. 2016, 101, 2432–2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasevska, I.; Enhorning, S.; Persson, M.; Nilsson, P.M.; Melander, O. Copeptin predicts coronary artery disease cardiovascular and total mortality. Heart 2016, 102, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Butler-Dawson, J.; Dally, M.; Johnson, R.J.; Johnson, E.C.; Krisher, L.; Sánchez-Lozada, L.-G.; Griffin, B.R.; Brindley, S.; Newman, L.S. Association of Copeptin, a Surrogate Marker of Arginine Vasopressin, with Decreased Kidney Function in Sugarcane Workers in Guatemala. Ann. Nutr. Metab. 2020, 76, 30–36. [Google Scholar] [CrossRef]
- Perrier, E.; Vergne, S.; Klein, A.; Poupin, M.; Rondeau, P.; Le Bellego, L.; Armstrong, L.E.; Lang, F.; Stookey, J.; Tack, I. Hydration biomarkers in free-living adults with different levels of habitual fluid consumption. Br. J. Nutr. 2013, 109, 1678–1687. [Google Scholar] [CrossRef] [Green Version]
- Roussel, R.; Fezeu, L.; Bouby, N.; Balkau, B.; Lantieri, O.; Alhenc-Gelas, F.; DESIR Study Group. Low water intake and risk for new-onset hyperglycemia. Diabetes Care 2011, 34, 2551–2554. [Google Scholar] [CrossRef] [Green Version]
- Lemetais, G.; Melander, O.; Vecchio, M.; Bottin, J.H.; Enhörning, S.; Perrier, E.T. Effect of increased water intake on plasma copeptin in healthy adults. Eur. J. Nutr. 2018, 57, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Taveau, C.; Chollet, C.; Waeckel, L.; Desposito, D.; Bichet, D.-G.; Arthus, M.-F.; Magnan, C.; Philippe, E.; Paradis, V.; Foufelle, F.; et al. Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia 2015, 58, 1081–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enhörning, S.; Brunkwall, L.; Tasevska, I.; Ericson, U.; Tholin, J.P.; Persson, M.; Lemetais, G.; Vanhaecke, T.; Dolci, A.; Perrier, E.T.; et al. Water Supplementation Reduces Copeptin and Plasma Glucose in Adults with High Copeptin: The H2O Metabolism Pilot Study. J. Clin. Endocrinol. Metab. 2019, 1045, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Carroll, H.A.; Betts, J.A.; Johnson, L. An investigation into the relationship between plain water intake and glycated Hb (HbA1c): A sex-stratified, cross-sectional analysis of the UK National Diet and Nutrition Survey (2008-2012). Br. J. Nutr. 2016, 116, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, H.A.; Davis, M.G.; Papadaki, A. Higher plain water intake is associated with lower type 2 diabetes risk: A cross-sectional study in humans. Nutr. Res. 2015, 35, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Stookey, J.D.; Constant, F.; Popkin, B.M.; Gardner, C.D. Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 2008, 16, 2481–2488. [Google Scholar] [CrossRef]
- Bouby, N.; Bachmann, S.; Bichet, D.; Bankir, L. Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am. J. Physiol. 1990, 258 Pt 2, F973–F979. [Google Scholar] [CrossRef]
- Bardoux, P.; Bichet, D.G.; Martin, H.; Gallois, Y.; Marre, M.; Arthus, M.-F.; Lonergan, M.; Ruel, N.; Bouby, N.; Bankir, L. Vasopressin increases urinary albumin excretion in rats and humans: Involvement of V2 receptors and the renin-angiotensin system. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2003, 18, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Roncal-Jimenez, C.A.; Milagres, T.; Andres-Hernando, A.; Kuwabara, M.; Jensen, T.; Song, Z.; Bjornstad, P.; Garcia, G.E.; Sato, Y.; Sanchez-Lozada, L.G.; et al. Effects of exogenous desmopressin on a model of heat stress nephropathy in mice. Am. J. Physiol. Ren. Physiol. 2017, 312, F418–F426. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.R.; Childs, M.E.; Adams, E.M. Direct cardiac effects of vasopressin role of v1 and v2 vasopressinergic receptors. Am. J. Physiol. Heart Circ. Physiol. 1988, 255, H261–H265. [Google Scholar] [CrossRef]
- Wang, H.W.; Jiang, M.Y. Higher volume of water intake is associated with lower risk of albuminuria and chronic kidney disease. Medicine 2021, 21, e26009. [Google Scholar] [CrossRef]
- Clark, W.F.; Sontrop, J.M.; Macnab, J.J.; Suri, R.; Moist, L.; Salvadori, M.; Garg, A.X. Urine volume and change in estimated GFR in a community-based cohort study. Clin. J. Am. Soc. Nephrol. CJASN 2011, 6, 2634–2641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sontrop, J.M.; Dixon, S.N.; Garg, A.X.; Buendia-Jimenez, I.; Dohein, O.; Huang, S.-H.S.; Clark, W.F. Association between water intake, chronic kidney disease, and cardiovascular disease: A cross-sectional analysis of NHANES data. Am. J. Nephrol. 2013, 37, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Enhörning, S. High water intake and low urine osmolality are associated with favorable metabolic profile at a population level: Low vasopressin secretion as a possible explanation. Eur. J. Nutr. 2020, 59, 3715–3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, W.F.; Sontrop, J.M.; Huang, S.H.; Gallo, K.; Moist, L.; House, A.A.; Cuerden, M.S.; Weir, M.A.; Bagga, A.; Brimble, S.; et al. Effect of Coaching to Increase Water Intake on Kidney Function Decline in Adults with Chronic Kidney Disease: The CKD WIT Randomized Clinical Trial. JAMA 2018, 319, 1870–1879. [Google Scholar] [CrossRef]
- Andres-Hernando, A.; Johnson, R.J.; Lanaspa, M.A. Endogenous fructose production: What do we know and how relevant is it? Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 289–294. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.-G.; Andres-Hernando, A.; Garcia, F.; Cicerchi, C.; Li, N.; Kuwabara, M.; Roncal-Jimenez, C.A.; Johnson, R.J.; Lanaspa, M.A. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J. Biol. Chem. 2019, 294, 4272–4281. [Google Scholar] [CrossRef]
- Huang, Z.; Hong, Q.; Zhang, X.; Xiao, W.; Wang, L.; Cui, S.; Feng, Z.; Lv, Y.; Cai, G.; Chen, X.; et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun. Signal 2017, 15, 3. [Google Scholar] [CrossRef] [Green Version]
- Lanaspa, M.A.; Ishimoto, T.; Cicerchi, C.; Tamura, Y.; Roncal-Jimenez, C.A.; Chen, W.; Tanabe, K.; Andres-Hernando, A.; Orlicky, D.J.; Finol, E.; et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 25, 2526–2538. [Google Scholar] [CrossRef] [Green Version]
- Hallfrisch, J. Metabolic effects of dietary fructose. FASEB J. 1990, 4, 2652–2660. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Cicerchi, C.; Garcia, G.; Li, N.; Roncal-Jimenez, C.A.; Rivard, C.J.; Hunter, B.; Andrés-Hernando, A.; Ishimoto, T.; Sánchez-Lozada, L.G.; et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS ONE 2012, 7, e48801. [Google Scholar] [CrossRef] [Green Version]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirillo, P.; Gersch, M.S.; Mu, W.; Scherer, P.M.; Kim, K.M.; Gesualdo, L.; Henderson, G.N.; Johnson, R.J.; Sautin, Y.Y. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J. Am. Soc. Nephrol. 2009, 20, 545–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncal Jimenez, C.A.; Ishimoto, T.; Lanaspa, M.A.; Rivard, C.J.; Nakagawa, T.; Ejaz, A.A.; Cicerchi, C.; Inaba, S.; Le, M.; Miyazaki, M.; et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014, 86, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Glaser, J.; Lemery, J.; Rajagopalan, B.; Diaz, H.F.; García-Trabanino, R.; Taduri, G.; Madero, M.; Amarasinghe, M.; Abraham, G.; Anutrakulchai, S.; et al. Climate Change and the Emergent Epidemic of CKD from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy. Clin. J. Am. Soc. Nephrol. CJASN 2016, 11, 1472–1483. [Google Scholar] [CrossRef] [Green Version]
- Andres-Hernando, A.; Li, N.; Cicerchi, C.; Inaba, S.; Chen, W.; Roncal-Jimenez, C.; Le, M.T.; Wempe, M.F.; Milagres, T.; Ishimoto, T.; et al. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat. Commun. 2017, 8, 14181. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric Acid-Induced C-Reactive Protein Expression: Implication on Cell Proliferation and Nitric Oxide Production of Human Vascular Cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.C.; Sato, S.; Tsai, J.Y.; Yan, S.; Bakr, S.; Zhang, H.; Oates, P.J.; Ramasamy, R. Aldose reductase activation is a key component of myocardial response to ischemia. FASEB J. 2002, 16, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.C.; Bakr, S.; Ellery, C.A.; Oates, P.J. Ramasamy, R. Sorbitol dehydrogenase: A novel target for adjunctive protection of ischemic myocardium. FASEB J. 2003, 17, 2331–2333. [Google Scholar] [CrossRef]
- Mirtschink, P.; Krishnan, J.; Grimm, F.; Sarre, A.; Hörl, M.; Kayikci, M.; Fankhauser, N.; Christinat, Y.; Cortijo, C.; Feehan, O.; et al. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 2015, 522, 444–449. [Google Scholar] [CrossRef]
- Wolf, J.P.; Nguyen, N.U.; Dumoulin, G.; Berthelay, S. Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm. Metab. Res. = Hormon- und Stoffwechselforschung = Horm. Metab. 1992, 24, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Roncal-Jimenez, C.A.; Lanaspa-Garcia, M.A.; Oppelt, S.A.; Kuwabara, M.; Jensen, T.; Milagres, T.; Andres-Hernando, A.; Ishimoto, T.; Garcia, G.E.; et al. Role of fructose and fructokinase in acute dehydration-induced vasopressin gene expression and secretion in mice. J. Neurophysiol. 2017, 117, 646–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, C.L.; Johnson, B.D.; Sackett, J.R.; Parker, M.D.; Schlader, Z.J. Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R189–R198. [Google Scholar] [CrossRef] [PubMed]
- García-Arroyo, F.E.; Tapia, E.; Blas-Marron, M.G.; Gonzaga, G.; Silverio, O.; Cristóbal, M.; Osorio, H.; Arellano-Buendía, A.S.; Zazueta, C.; Aparicio-Trejo, O.E.; et al. Vasopressin Mediates the Renal Damage Induced by Limited Fructose Rehydration in Recurrently Dehydrated Rats. Int. J. Biol. Sci. 2017, 13, 961–975. [Google Scholar] [CrossRef] [Green Version]
- Kanbay, M.; Guler, B.; Ertuglu, L.; Dagel, T.; Afsar, B.; Incir, S.; Baygul, A.; Covic, A.; Andres-Hernando, A.; Sánchez-Lozada, L.; et al. The Speed of Ingestion of a Sugary Beverage Has an Effect on the Acute Metabolic Response to Fructose. Nutrients 2021, 13, 1916. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; García-Arroyo, F.E.; Gonzaga, G.; Blas-Marron, M.G.; Silverio, O.; Muñoz, I.; Tapia, E.; Johnson, R.J. Heat-Induced Renal Damage (HRD) Is Dose-Dependently Worsened by Fructose (F)Concentration in Rehydration Fluid. J. Am. Soc. Nephrol. 2017, 28, 772. [Google Scholar]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence from Sugar-Sweetened Beverages Tells Us. J. Am. Coll Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef] [Green Version]
- Libuda, L.; Kersting, M.; Alexy, U. Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr. 2012, 15, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, M.; Hisatome, I.; Roncal-Jimenez, C.A.; Niwa, K.; Andres-Hernando, A.; Jensen, T.; Bjornstad, P.; Milagres, T.; Cicerchi, C.; Song, Z.; et al. Increased Serum Sodium and Serum Osmolarity Are Independent Risk Factors for Developing Chronic Kidney Disease; 5 Year Cohort Study. PLoS ONE 2017, 12, e0169137. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, M.; Kanbay, M.; Niwa, K.; Ae, R.; Andres-Hernando, A.; Roncal-Jimenez, C.; Garcia, G.; Sánchez-Lozada, L.; Rodriguez-Iturbe, B.; Hisatome, I.; et al. Hyperosmolarity and Increased Serum Sodium Concentration Are Risks for Developing Hypertension Regardless of Salt Intake: A Five-Year Cohort Study in Japan. Nutrients 2020, 12, 1422. [Google Scholar] [CrossRef]
- Jalal, D.I.; Smits, G.; Johnson, R.J.; Chonchol, M. Increased fructose associates with elevated blood pressure. J. Am. Soc. Nephrol. 2010, 21, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Madero, M.; Jalal, D.I.; Sanchez-Lozada, L.G.; Johnson, R.J. Uric acid and the origins of hypertension. J. Pediatr. 2013, 162, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pozo, S.E.; Schold, J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Johnson, R.J.; Lillo, J.L. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: Role of uric acid in the hypertensive response. Int. J. Obes. (Lond.) 2010, 34, 454–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 2008, 300, 924–932. [Google Scholar] [CrossRef] [Green Version]
- Soletsky, B.; Feig, D.I. Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 2012, 60, 1148–1156. [Google Scholar] [CrossRef] [Green Version]
- García-Arroyo, F.E.; Muñoz-Jiménez, I.; Gonzaga, G.; Tapia, E.; Osorio-Alonso, H.; A Roncal-Jiménez, C.; Iroz, A.; Vecchio, M.; Reyes-García, J.G.; Johnson, R.J.; et al. A Role for Both V1a and V2 Receptors in Renal Heat Stress Injury Amplified by Rehydration with Fructose. Int. J. Mol. Sci. 2019, 20, 5764. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, J.D.; Persaud, P.; Williams, C.K.; Chen, Y.; Burg, M.B. cAMP-independent role of PKA in tonicity-induced transactivation of tonicity-responsive enhancer/ osmotic response element-binding protein. Proc. Natl. Acad. Sci. USA 2002, 99, 16800–16805. [Google Scholar] [CrossRef] [Green Version]
- García-Arroyo, F.E.; Gonzaga, G.; Muñoz-Jiménez, I.; Osorio-Alonso, H.; Iroz, A.; Vecchio, M.; Tapia, E.; Roncal-Jiménez, C.A.; Johnson, R.J.; Sánchez-Lozada, L.G. Antioxidant supplements as a novel mean for blocking recurrent heat stress-induced kidney damage following rehydration with fructose-containing beverages. Free Radic. Biol. Med. 2019, 141, 182–191. [Google Scholar] [CrossRef]
- Fujiki, T.; Ando, F.; Murakami, K.; Isobe, K.; Mori, T.; Susa, K.; Nomura, N.; Sohara, E.; Rai, T.; Uchida, S. Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci. Rep. 2019, 9, 9245. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, R.J.; García-Arroyo, F.E.; Gonzaga-Sánchez, G.; Vélez-Orozco, K.A.; Álvarez-Álvarez, Y.Q.; Aparicio-Trejo, O.E.; Tapia, E.; Osorio-Alonso, H.; Andrés-Hernando, A.; Nakagawa, T.; et al. Current Hydration Habits: The Disregarded Factor for the Development of Renal and Cardiometabolic Diseases. Nutrients 2022, 14, 2070. https://doi.org/10.3390/nu14102070
Johnson RJ, García-Arroyo FE, Gonzaga-Sánchez G, Vélez-Orozco KA, Álvarez-Álvarez YQ, Aparicio-Trejo OE, Tapia E, Osorio-Alonso H, Andrés-Hernando A, Nakagawa T, et al. Current Hydration Habits: The Disregarded Factor for the Development of Renal and Cardiometabolic Diseases. Nutrients. 2022; 14(10):2070. https://doi.org/10.3390/nu14102070
Chicago/Turabian StyleJohnson, Richard J., Fernando E. García-Arroyo, Guillermo Gonzaga-Sánchez, Kevin A. Vélez-Orozco, Yamnia Quetzal Álvarez-Álvarez, Omar Emiliano Aparicio-Trejo, Edilia Tapia, Horacio Osorio-Alonso, Ana Andrés-Hernando, Takahiko Nakagawa, and et al. 2022. "Current Hydration Habits: The Disregarded Factor for the Development of Renal and Cardiometabolic Diseases" Nutrients 14, no. 10: 2070. https://doi.org/10.3390/nu14102070








