Diet Modification before or during Pregnancy on Maternal and Foetal Outcomes in Rodent Models of Maternal Obesity
Abstract
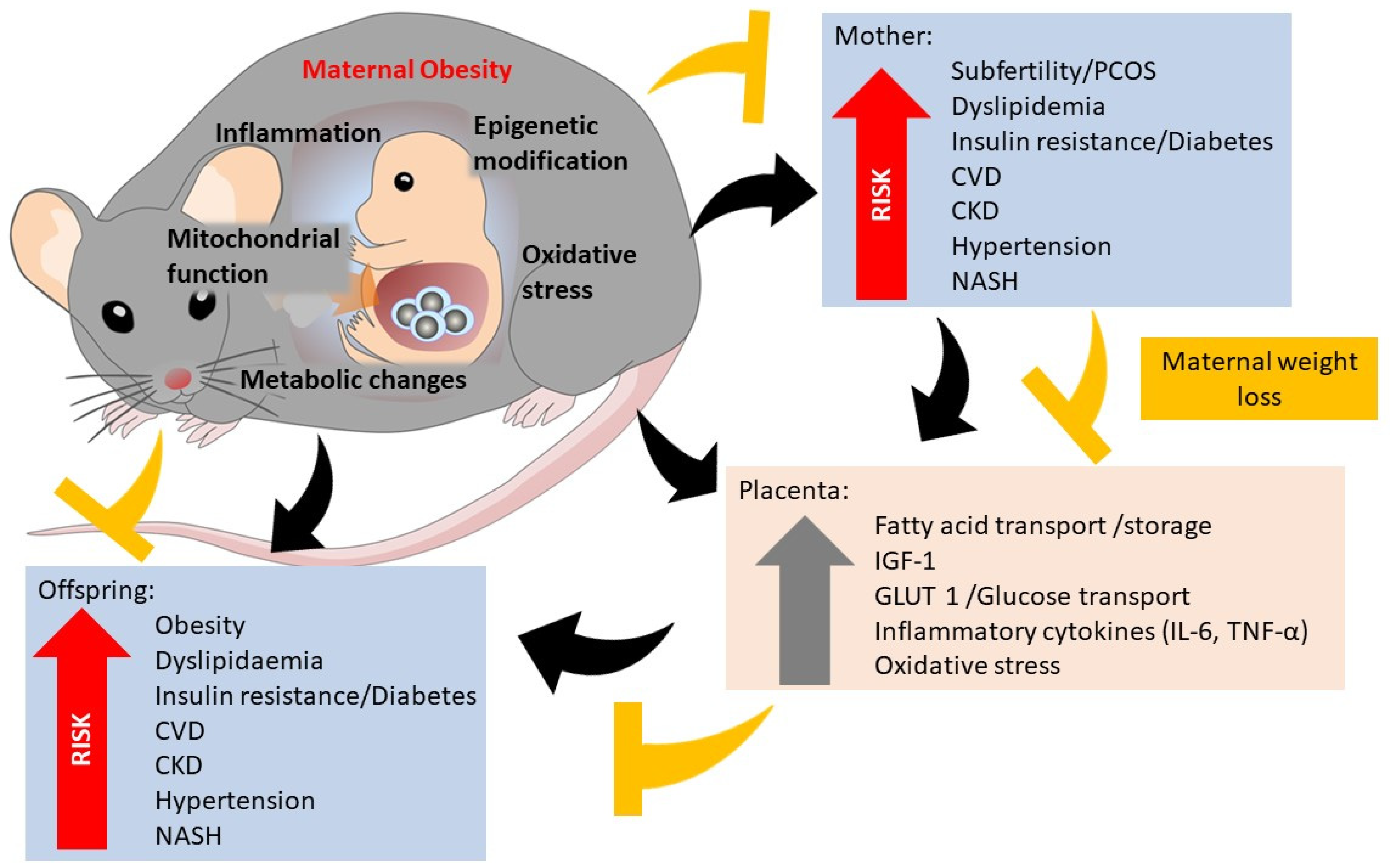
:1. Introduction
1.1. Effects of Maternal Obesity on Maternal Outcomes
1.1.1. Rodent Studies
1.1.2. Human Studies
1.2. Effects of Maternal Obesity on Foetal and Offspring Outcomes
1.2.1. Rodent Studies
1.2.2. Human Studies Supporting the Effect of Maternal Obesity on the Offspring
1.3. Diet Modulation in Pregnancy and Its Impact on Obesity-Related Maternal and Perinatal Outcomes
1.3.1. Rodent Studies
1.3.2. Human Studies
1.4. Pre-Pregnancy Weight Reduction and Its Impact on Obesity-Related Maternal, Perinatal and Offspring Outcomes
1.4.1. Rodent Studies
1.4.2. Human Studies
2. Future Directions
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Metsälä, J.; Stach-Lempinen, B.; Gissler, M.; Eriksson, J.G.; Koivusalo, S. Risk of Pregnancy Complications in Relation to Maternal Prepregnancy Body Mass Index: Population-Based Study from Finland 2006-10. Paediatr. Perinat. Epidemiol. 2016, 30, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Ruager-Martin, R.; Hyde, M.J.; Modi, N. Maternal obesity and infant outcomes. Early Hum. Dev. 2010, 86, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Dodd, J.M.; Turnbull, D.; McPhee, A.J.; Deussen, A.R.; Grivell, R.; Yelland, L.N.; Crowther, C.A.; Wittert, G.; Owens, J.A.; Robinson, J.S.; et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ Br. Med. J. 2014, 348, g1285. [Google Scholar] [CrossRef] [Green Version]
- Opray, N.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M. Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese. Cochrane Database Syst. Rev. 2015, 7, Cd010932. [Google Scholar] [CrossRef]
- Ainge, H.; Thompson, C.; Ozanne, S.E.; Rooney, K.B. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int. J. Obes. 2011, 35, 325–335. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, N.; Chen, H.; Pollock, C.; Glastras, S. Pre-Conception Weight Loss Improves Reproductive, Metabolic and Kidney Health in Obese Mice and Their Offspring. J. Endocr. Soc. 2021, 5 (Suppl. 1), A322–A323. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Mahany, E.B.; Han, X.; Borges, B.C.; Cruz-Machado, S.D.S.; Allen, S.J.; Galiano, D.G.; Hoenerhoff, M.J.; Bellefontaine, N.H.; Elias, C.F. Obesity and High-Fat Diet Induce Distinct Changes in Placental Gene Expression and Pregnancy Outcome. Endocrinology 2018, 159, 1718–1733. [Google Scholar] [CrossRef] [Green Version]
- Zain, M.M.; Norman, R.J. Impact of Obesity on Female Fertility and Fertility Treatment. Women’s Health 2008, 4, 183–194. [Google Scholar] [CrossRef]
- Rautureau, G.J.; Morio, B.; Guibert, S.; Lefevre, C.; Perrier, J.; Alves, A.; Chauvin, M.A.; Pinteur, C.; Monet, M.A.; Godet, M.; et al. Dietary obesity in mice is associated with lipid deposition and metabolic shifts in the lungs sharing features with the liver. Sci. Rep. 2021, 11, 8712. [Google Scholar] [CrossRef] [PubMed]
- Chaar, L.J.; Coelho, A.; Silva, N.M.; Festuccia, W.L.; Antunes, V.R. High-fat diet-induced hypertension and autonomic imbalance are associated with an upregulation of CART in the dorsomedial hypothalamus of mice. Physiol. Rep. 2016, 4, e12811. [Google Scholar] [CrossRef] [Green Version]
- Che, C.; Dudick, K.; Shoemaker, R. Cardiac hypertrophy with obesity is augmented after pregnancy in C57BL/6 mice. Biol. Sex Differ. 2019, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Dudick, K.; Che, C.; Shoemaker, R. Differential cardiac geometry during pregnancy in lean versus obese mice. Rev. Cardiovasc. Med. 2022, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Bytautiene, E.; Tamayo, E.; Kechichian, T.; Drever, N.; Gamble, P.; Hankins, G.D.; Saade, G.R. Prepregnancy obesity and sFlt1-induced preeclampsia in mice: Developmental programming model of metabolic syndrome. Am. J. Obstet. Gynecol. 2011, 204, 398.e1–398.e8. [Google Scholar] [CrossRef] [PubMed]
- Chechi, K.; Cheema, S.K. Maternal diet rich in saturated fats has deleterious effects on plasma lipids of mice. Exp. Clin. Cardiol. 2006, 11, 129–135. [Google Scholar] [PubMed]
- Kita, T.; Kume, N.; Minami, M.; Hayashida, K.; Murayama, T.; Sano, H.; Moriwaki, H.; Kataoka, H.; Nishi, E.; Horiuchi, H.; et al. Role of oxidized LDL in atherosclerosis. Ann. N. Y. Acad. Sci. 2001, 947, 199–205, discussion 205–206. [Google Scholar] [CrossRef]
- Jump, D.B.; Tripathy, S.; Depner, C.M. Fatty acid–regulated transcription factors in the liver. Annu. Rev. Nutr. 2013, 33, 249–269. [Google Scholar] [CrossRef] [Green Version]
- Masi, L.N.; Rodrigues, A.C.; Curi, R. Fatty acids regulation of inflammatory and metabolic genes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 418–424. [Google Scholar] [CrossRef]
- Heerwagen, M.J.R.; Miller, M.R.; Barbour, L.A.; Friedman, J.E. Maternal obesity and fetal metabolic programming: A fertile epigenetic soil. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2010, 299, R711–R722. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Dahlman, I.; Kaaman, M.; Olsson, T.; Tan, G.D.; Bickerton, A.S.; Wåhlén, K.; Andersson, J.; Nordström, E.A.; Blomqvist, L.; Sjögren, A.; et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J. Clin. Endocrinol. Metab. 2005, 90, 5834–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma interleukin-8 concentrations are increased in obese subjects and related to fat mass and tumor necrosis factor-α system. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef] [PubMed]
- Small, L.; Brandon, A.E.; Turner, N.; Cooney, G.J. Modeling insulin resistance in rodents by alterations in diet: What have high-fat and high-calorie diets revealed? Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E251–E265. [Google Scholar] [CrossRef] [PubMed]
- de Barros Mucci, D.; Kusinski, L.C.; Wilsmore, P.; Loche, E.; Pantaleão, L.C.; Ashmore, T.J.; Blackmore, H.L.; Fernandez-Twinn, D.S.; do Carmo, M.d.G.T.; Ozanne, S.E. Impact of maternal obesity on placental transcriptome and morphology associated with fetal growth restriction in mice. Int. J. Obes. 2020, 44, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, T.; Schulze-Edinghausen, M.; Turnwald, E.-M.; Janoschek, R.; Bae-Gartz, I.; Zentis, P.; Handwerk, M.; Wohlfarth, M.; Schauss, A.; Hucklenbruch-Rother, E.; et al. Effect of Maternal Obesity in Mice on IL-6 Levels and Placental Endothelial Cell Homeostasis. Nutrients 2020, 12, 296. [Google Scholar] [CrossRef] [Green Version]
- Ingvorsen, C.; Thysen, A.H.; Fernandez-Twinn, D.; Nordby, P.; Nielsen, K.F.; Ozanne, S.E.; Brix, S.; Hellgren, L.I. Effects of pregnancy on obesity-induced inflammation in a mouse model of fetal programming. Int. J. Obes. 2014, 38, 1282–1289. [Google Scholar] [CrossRef]
- Kim, D.W.; Young, S.L.; Grattan, D.R.; Jasoni, C.L. Obesity during pregnancy disrupts placental morphology, cell proliferation, and inflammation in a sex-specific manner across gestation in the mouse. Biol. Reprod. 2014, 90, 130. [Google Scholar] [CrossRef] [Green Version]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Hauguel-de Mouzon, S. Obesity in Pregnancy Stimulates Macrophage Accumulation and Inflammation in the Placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Gohir, W.; Kennedy, K.M.; Wallace, J.G.; Saoi, M.; Bellissimo, C.J.; Britz-McKibbin, P.; Petrik, J.J.; Surette, M.G.; Sloboda, D.M. High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. J. Physiol. 2019, 597, 3029–3051. [Google Scholar] [CrossRef]
- Parker, V.J.; Solano, M.E.; Arck, P.C.; Douglas, A.J. Diet-induced obesity may affect the uterine immune environment in early-mid pregnancy, reducing NK-cell activity and potentially compromising uterine vascularization. Int. J. Obes. 2014, 38, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Baltayeva, J.; Konwar, C.; Castellana, B.; Mara, D.L.; Christians, J.K.; Beristain, A.G. Obesogenic diet exposure alters uterine natural killer cell biology and impairs vasculature remodeling in mice. Biol. Reprod. 2020, 102, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Schumacher, M.A.; Jiang, J.; Kanai, Y.; Powell, T.L.; Jansson, T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J. Physiol. 2012, 590, 1495–1509. [Google Scholar] [CrossRef]
- Aye, I.L.; Rosario, F.J.; Powell, T.L.; Jansson, T. Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl. Acad. Sci. USA 2015, 112, 12858–12863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godfrey, K.M.; Costello, P.M.; Lillycrop, K.A. The developmental environment, epigenetic biomarkers and long-term health. J. Dev. Orig. Health Dis. 2015, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.E.; Voisin, S.; Jouin, M.; Jouneau, L.; Prézelin, A.; Lecoutre, S.; Breton, C.; Jammes, H.; Junien, C.; Gabory, A. Expression of epigenetic machinery genes is sensitive to maternal obesity and weight loss in relation to fetal growth in mice. Clin. Epigenetics 2016, 8, 22. [Google Scholar] [CrossRef]
- Eriksson, U.J.; Bone, A.J.; Turnbull, D.M.; Baird, J.D. Timed interruption of insulin therapy in diabetic BB/E rat pregnancy: Effect on maternal metabolism and fetal outcome. Acta Endocrinol. 1989, 120, 800–810. [Google Scholar] [CrossRef]
- Capobianco, E.; Jawerbaum, A.; Romanini, M.C.; White, V.; Pustovrh, C.; Higa, R.; Martinez, N.; Mugnaini, M.T.; Sonez, C.; Gonzalez, E. 15-Deoxy-Delta12,14-prostaglandin J2 and peroxisome proliferator-activated receptor gamma (PPARgamma) levels in term placental tissues from control and diabetic rats: Modulatory effects of a PPARgamma agonist on nitridergic and lipid placental metabolism. Reprod. Fertil. Dev. 2005, 17, 423–433. [Google Scholar] [CrossRef]
- Diamant, Y.Z.; Metzger, B.E.; Freinkel, N.; Shafrir, E. Placental lipid and glycogen content in human and experimental diabetes mellitus. Am. J. Obstet. Gynecol. 1982, 144, 5–11. [Google Scholar] [CrossRef]
- Shafrir, E.; Barash, V. Placental function in maternal-fetal fat transport in diabetes. Biol. Neonate 1987, 51, 102–112. [Google Scholar] [CrossRef]
- Herrera, E.; Palacin, M.; Martin, A.; Lasuncion, M.A. Relationship between maternal and fetal fuels and placental glucose transfer in rats with maternal diabetes of varying severity. Diabetes 1985, 34 (Suppl. 2), 42–46. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity 2015, 23, 1663–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosario, F.J.; Powell, T.L.; Jansson, T. Activation of placental insulin and mTOR signaling in a mouse model of maternal obesity associated with fetal overgrowth. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R87–R93. [Google Scholar] [CrossRef] [Green Version]
- Gaccioli, F.; White, V.; Capobianco, E.; Powell, T.L.; Jawerbaum, A.; Jansson, T. Maternal overweight induced by a diet with high content of saturated fat activates placental mTOR and eIF2alpha signaling and increases fetal growth in rats. Biol. Reprod. 2013, 89, 96. [Google Scholar] [CrossRef]
- Rosario, F.J.; Jansson, N.; Kanai, Y.; Prasad, P.D.; Powell, T.L.; Jansson, T. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology 2011, 152, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Metzer, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar]
- Metzger, B.E.; Coustan, D.R. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care 1998, 21 (Suppl. 2), B161–B167. [Google Scholar]
- Chu, S.Y.; Callaghan, W.M.; Kim, S.Y.; Schmid, C.H.; Lau, J.; England, L.J.; Dietz, P.M. Maternal Obesity and Risk of Gestational Diabetes Mellitus. Diabetes Care 2007, 30, 2070–2076. [Google Scholar] [CrossRef] [Green Version]
- Hod, M.; Kapur, A.; Sacks, D.A.; Hadar, E.; Agarwal, M.; Di Renzo, G.C.; Roura, L.C.; McIntyre, H.D.; Morris, J.L.; Divakar, H. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: A pragmatic guide for first-trimester screening and prevention. Int. J. Gynaecol. Obstet. 2019, 145 (Suppl. 1), 1–33. [Google Scholar]
- Madan, J.; Chen, M.; Goodman, E.; Davis, J.; Allan, W.; Dammann, O. Maternal obesity, gestational hypertension, and preterm delivery. J. Matern. Fetal. Neonatal. Med. 2010, 23, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.C.; Davis, J.M.; Craig, W.Y.; Collins, M.; Allan, W.; Quinn, R.; Dammann, O. Maternal obesity and markers of inflammation in pregnancy. Cytokine 2009, 47, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, J.E.; Ferrell, W.R.; Crawford, L.; Wallace, A.M.; Greer, I.A.; Sattar, N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J. Clin. Endocrinol. Metab. 2002, 87, 4231–4237. [Google Scholar] [CrossRef]
- Stewart, F.M.; Freeman, D.J.; Ramsay, J.E.; Greer, I.A.; Caslake, M.; Ferrell, W.R. Longitudinal assessment of maternal endothelial function and markers of inflammation and placental function throughout pregnancy in lean and obese mothers. J. Clin. Endocrinol. Metab. 2007, 92, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Ehrenberg, H.M.; Mercer, B.M.; Catalano, P.M. The influence of obesity and diabetes on the prevalence of macrosomia. Am. J. Obstet. Gynecol. 2004, 191, 964–968. [Google Scholar] [CrossRef]
- Dosch, N.C.; Guslits, E.F.; Weber, M.B.; Murray, S.E.; Ha, B.; Coe, C.L.; Auger, A.P.; Kling, P.J. Maternal Obesity Affects Inflammatory and Iron Indices in Umbilical Cord Blood. J. Pediatr. 2016, 172, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef]
- James-Allan, L.B.; Arbet, J.; Teal, S.B.; Powell, T.L.; Jansson, T. Insulin stimulates GLUT4 trafficking to the syncytiotrophoblast basal plasma membrane in the human placenta. J. Clin. Endocrinol. Metab. 2019, 104, 4225–4238. [Google Scholar] [CrossRef]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 42–49. [Google Scholar]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Haghiac, M.; Surace, P. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity 2011, 19, 476–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Greenwald, E.; Jack-Roberts, C.; Ajeeb, T.T.; Malysheva, O.V.; Caudill, M.A.; Axen, K.; Saxena, A.; Semernina, E.; Nanobashvili, K.; et al. Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J. Nutr. Biochem. 2017, 49, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Liu, X.; Tian, Q.; Zhao, L.; Chen, Y.; Hu, Y.; Chae, S.A.; De Avilla, J.M.; Zhu, M.; Du, M. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J. Physiol. 2019, 597, 3333–3347. [Google Scholar] [CrossRef] [PubMed]
- Bariani, M.V.; Correa, F.; Rubio, A.P.D.; Marvaldi, C.; Schander, J.A.; Beltrame, J.S.; Cella, M.; Silberman, D.M.; Aisemberg, J.; Franchi, A.M. Maternal obesogenic diet combined with postnatal exposure to high-fat diet induces metabolic alterations in offspring. J. Cell. Physiol. 2020, 235, 8260–8269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Guan, H.; Guo, Y.; Zhu, L.; Liu, X. Maternal High-Fat Diet Programs Renal Peroxisomes and Activates NLRP3 Inflammasome-Mediated Pyroptosis in the Rat Fetus. J. Inflamm. Res. 2021, 14, 5095–5110. [Google Scholar] [CrossRef]
- Helle, E.; Priest, J.R. Maternal Obesity and Diabetes Mellitus as Risk Factors for Congenital Heart Disease in the Offspring. J. Am. Heart Assoc. 2020, 9, e011541. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Dev. Neurosci. 2009, 27, 627–633. [Google Scholar] [CrossRef] [Green Version]
- Jawerbaum, A.; Gonzalez, E. Diabetic pregnancies: The challenge of developing in a pro-inflammatory environment. Curr. Med. Chem. 2006, 13, 2127–2138. [Google Scholar]
- Perez, P.A.; DiPatrizio, N.V. Impact of maternal western diet-induced obesity on offspring mortality and peripheral endocannabinoid system in mice. PLoS ONE 2018, 13, e0205021. [Google Scholar] [CrossRef]
- Mouralidarane, A.; Soeda, J.; Visconti-Pugmire, C.; Samuelsson, A.-M.; Pombo, J.; Maragkoudaki, X.; Butt, A.; Saraswati, R.; Novelli, M.; Fusai, G.; et al. Maternal obesity programs offspring nonalcoholic fatty liver disease by innate immune dysfunction in mice. Hepatology 2013, 58, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.Y.; Taylor, P.D.; Dekou, V.; Seed, P.T.; Lakasing, L.; Graham, D.; Dominiczak, A.F.; Hanson, M.A.; Poston, L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 2003, 41, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, P.; Bitsanis, D.; Ghebremeskel, K.; Crawford, M.A.; Poston, L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J. Physiol. 2001, 533 Pt 3, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. Maternal High-Fat Diet Disturbs the DNA Methylation Profile in the Brown Adipose Tissue of Offspring Mice. Front. Endocrinol. 2021, 12, 705827. [Google Scholar] [CrossRef]
- Loche, E.; Blackmore, H.L.; Carpenter, A.A.; Beeson, J.; Pinnock, A.; Ashmore, T.J.; Aiken, C.; De Almeida-Faria, J.; Schoonejans, J.; Giussani, D.; et al. Maternal diet-induced obesity programmes cardiac dysfunction in male mice independently of post-weaning diet. Cardiovasc. Res. 2018, 114, 1372–1384. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Oest, M.E.; Prater, M.R. Intrauterine exposure to high saturated fat diet elevates risk of adult-onset chronic diseases in C57BL/6 mice. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2009, 86, 377–384. [Google Scholar] [CrossRef]
- Larkin, B.P.; Nguyen, L.T.; Hou, M.; Glastras, S.J.; Chen, H.; Wang, R.; Pollock, C.A.; Saad, S. Novel Role of Gestational Hydralazine in Limiting Maternal and Dietary Obesity-Related Chronic Kidney Disease. Front. Cell Dev. Biol. 2021, 9, 705263. [Google Scholar] [CrossRef]
- Glastras, S.J.; Chen, H.; McGrath, R.T.; Zaky, A.A.; Gill, A.J.; Pollock, C.A.; Saad, S. Effect of GLP-1 Receptor Activation on Offspring Kidney Health in a Rat Model of Maternal Obesity. Sci. Rep. 2016, 6, 23525. [Google Scholar] [CrossRef] [Green Version]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. Biomed. Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef]
- Meneghini, M.A.; Galarza, R.A.; Flores Quiroga, J.P.; Faletti, A.G. Diet-induced maternal obesity and overnutrition cause a decrease in the sperm quality of the offspring. J. Nutr. Biochem. 2022, 103, 108966. [Google Scholar] [CrossRef]
- Andreas, E.; Reid, M.; Zhang, W.; Moley, K.H. The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development. Mol. Hum. Reprod. 2019, 25, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Durmuş, B.; Hofman, A.; Mackenbach, J.P.; Steegers, E.A.; Jaddoe, V.W. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obes. (Silver Spring) 2013, 21, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.K.; Emmett, P.M.; Noble, S.; Ness, A.; Dunger, D.B.; ALSPAC Study Team. Dietary Energy Intake at the Age of 4 Months Predicts Postnatal Weight Gain and Childhood Body Mass Index. Pediatrics 2006, 117, e503–e508. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.; Larkin, B.; Wang, R.; Faiz, A.; Pollock, C.; Saad, S. Blood DNA Methylation Predicts Diabetic Kidney Disease Progression in High Fat Diet-Fed Mice. Nutrients 2022, 14, 785. [Google Scholar] [CrossRef] [PubMed]
- Larkin, B.; Glastras, S.; Chen, H.; Pollock, C.; Saad, S. DNA methylation and the potential role of demethylating agents in prevention of progressive chronic kidney disease. FASEB J. 2018, 32, 5215–5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tajaddini, A.; Kendig, M.D.; Prates, K.V.; Westbrook, R.F.; Morris, M.J. Male Rat Offspring Are More Impacted by Maternal Obesity Induced by Cafeteria Diet than Females—Additive Effect of Postweaning Diet. Int. J. Mol. Sci. 2022, 23, 1442. [Google Scholar] [CrossRef] [PubMed]
- Akhaphong, B.; Gregg, B.; Kumusoglu, D.; Jo, S.; Singer, K.; Scheys, J.; DelProposto, J.; Lumeng, C.; Bernal-Mizrachi, E.; Alejandro, E.U. Maternal High-Fat Diet During Pre-Conception and Gestation Predisposes Adult Female Offspring to Metabolic Dysfunction in Mice. Front. Endocrinol. 2022, 12, 1716. [Google Scholar] [CrossRef]
- Casasnovas, J.; Damron, C.L.; Jarrell, J.; Orr, K.S.; Bone, R.N.; Archer-Hartmann, S.; Azadi, P.; Kua, K.L. Offspring of Obese Dams Exhibit Sex-Differences in Pancreatic Heparan Sulfate Glycosaminoglycans and Islet Insulin Secretion. Front. Endocrinol. 2021, 12, 658439. [Google Scholar] [CrossRef]
- Reynolds, R.M.; Allan, K.M.; Raja, E.A.; Bhattacharya, S.; McNeill, G.; Hannaford, P.C.; Sarwar, N.; Lee, A.J.; Norman, J. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: Follow-up of 1 323 275 person years. BMJ 2013, 347, f4539. [Google Scholar] [CrossRef] [Green Version]
- Gaillard, R.; Steegers, E.A.; Duijts, L.; Felix, J.F.; Hofman, A.; Franco, O.H.; Jaddoe, V.W. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: The Generation R Study. Hypertension 2014, 63, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Aris, I.M.; Bernard, J.Y.; Chen, L.-W.; Tint, M.T.; Pang, W.W.; E Soh, S.; Saw, S.-M.; Shek, L.P.-C.; Godfrey, K.M.; Gluckman, P.D.; et al. Modifiable risk factors in the first 1000 days for subsequent risk of childhood overweight in an Asian cohort: Significance of parental overweight status. Int. J. Obes. 2018, 42, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, M.; Cain, M.A.; Brumley, J. Excessive gestational weight gain. J. Midwifery Women’s Health 2019, 64, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dello Russo, M.; Ahrens, W.; De Vriendt, T.; Marild, S.; Molnar, D.; Moreno, L.A.; Reeske, A.; Veidebaum, T.; Kourides, Y.A.; Barba, G.; et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: The IDEFICS project. Int. J. Obes. 2013, 37, 914–919. [Google Scholar] [CrossRef] [Green Version]
- Ensenauer, R.; Chmitorz, A.; Riedel, C.; Fenske, N.; Hauner, H.; Nennstiel-Ratzel, U.; Von Kries, R. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: Results from a retrospective cohort study. Int. J. Obes. 2013, 37, 505–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaillard, R.; Steegers, E.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int. J. Obes. 2015, 39, 677–685. [Google Scholar] [CrossRef]
- Oostvogels, A.J.; Stronks, K.; Roseboom, T.J.; van der Post, J.A.; van Eijsden, M.; Vrijkotte, T.G. Maternal prepregnancy BMI, offspring’s early postnatal growth, and metabolic profile at age 5–6 years: The ABCD Study. J. Clin. Endocrinol. Metab. 2014, 99, 3845–3854. [Google Scholar] [CrossRef] [Green Version]
- Hochner, H.; Friedlander, Y.; Calderon-Margalit, R.; Meiner, V.; Sagy, Y.; Avgil-Tsadok, M.; Burger, A.; Savitsky, B.; Siscovick, D.S.; Manor, O. Associations of maternal prepregnancy body mass index and gestational weight gain with cardio-metabolic risk factors in adolescent offspring: A prospective cohort study. BJOG 2016, 123, 207–216. [Google Scholar]
- Eriksson, J.G.; Sandboge, S.; Salonen, M.; Kajantie, E.; Osmond, C. Maternal weight in pregnancy and offspring body composition in late adulthood: Findings from the Helsinki Birth Cohort Study (HBCS). Ann. Med. 2015, 47, 94–99. [Google Scholar] [CrossRef]
- Forsén, T.; Eriksson, J.G.; Tuomilehto, J.; Teramo, K.; Osmond, C.; Barker, D.J. Mother’s weight in pregnancy and coronary heart disease in a cohort of Finnish men: Follow up study. BMJ 1997, 315, 837–840. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, K.M.; Sheppard, A.; Gluckman, P.D.; Lillycrop, K.A.; Burdge, G.C.; McLean, C.; Rodford, J.; Slater-Jefferies, J.L.; Garratt, E.; Crozier, S.R.; et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 2011, 60, 1528–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobi, E.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.; Slagboom, P.; Heijmans, B.T. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef] [PubMed]
- Sletner, L.; Moen, A.E.F.; Yajnik, C.S.; Lekanova, N.; Sommer, C.; Birkeland, K.I.; Jenum, A.K.; Böttcher, Y. Maternal Glucose and LDL-Cholesterol Levels Are Related to Placental Leptin Gene Methylation, and, Together With Nutritional Factors, Largely Explain a Higher Methylation Level Among Ethnic South Asians. Front. Endocrinol. 2021, 12, 809916. [Google Scholar] [CrossRef] [PubMed]
- Gimpfl, M.; Rozman, J.; Dahlhoff, M.; Kübeck, R.; Blutke, A.; Rathkolb, B.; Klingenspor, M.; de Angelis, M.H.; Öner-Sieben, S.; Seibt, A.; et al. Modification of the fatty acid composition of an obesogenic diet improves the maternal and placental metabolic environment in obese pregnant mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1605–1614. [Google Scholar] [CrossRef]
- Ramalingam, L.; Menikdiwela, K.R.; Clevenger, S.; Eboh, T.; Allen, L.; Koboziev, I.; Scoggin, S.; Rashid, A.M.; Moussa, H.; Moustaid-Moussa, N. Maternal and postnatal supplementation of fish oil improves metabolic health of mouse male offspring. Obesity 2018, 26, 1740–1748. [Google Scholar] [CrossRef] [Green Version]
- Carter, L.G.; Qi, N.R.; De Cabo, R.; Pearson, K.J. Maternal exercise improves insulin sensitivity in mature rat offspring. Med. Sci. Sports Exerc. 2013, 45, 832. [Google Scholar] [CrossRef] [Green Version]
- Vega, C.C.; Reyes-Castro, L.A.; Bautista, C.J.; Larrea, F.; Nathanielsz, P.W.; Zambrano, E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int. J. Obes. 2015, 39, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Poston, L.; Bell, R.; Croker, H.; Flynn, A.C.; Godfrey, K.M.; Goff, L.; Hayes, L.; Khazaezadeh, N.; Nelson, S.M.; Oteng-Ntim, E.; et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): A multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 767–777. [Google Scholar] [CrossRef]
- Norman, J.E.; Reynolds, R.M. The consequences of obesity and excess weight gain in pregnancy. Proc. Nutr. Soc. 2011, 70, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Smits, A.; Marei, W.F.A.; De Neubourg, D.; Leroy, J.L.M.R. Diet normalization or caloric restriction as a preconception care strategy to improve metabolic health and oocyte quality in obese outbred mice. Reprod. Biol. Endocrinol. 2021, 19, 166. [Google Scholar] [CrossRef]
- Rodrigo, N.; Chen, H.; Pollock, C.; Glastras, S.J. Preconception weight loss improves fertility and maternal outcomes in obese mice. J. Endocrinol. 2022, 253, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, H.; Xu, H.; Li, J.; Golovko, M.; Cheng, H.; Lynch, E.C.; Liu, L.; McCauley, N.; Kennedy, L.; et al. Maternal diet intervention before pregnancy primes offspring lipid metabolism in liver. Lab. Investig. 2020, 100, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, M.; Zhou, Y.; Zhou, T.; Wu, C.; Alpini, G.; Zhang, K.K.; Xie, L. A long-term maternal diet transition from high-fat diet to normal fat diet during pre-pregnancy avoids adipose tissue inflammation in next generation. PLoS ONE 2018, 13, e0209053. [Google Scholar] [CrossRef] [PubMed]
- Dennison, C.A.; Eslinger, A.J.; Reimer, R.A. Preconception Prebiotic and Sitagliptin Treatment in Obese Rats Affects Pregnancy Outcomes and Offspring Microbiota, Adiposity, and Glycemia. Front. Endocrinol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.I.; Lee, M.-Y.; Getchell, K.M.; So, K.; Hirshman, M.F.; Goodyear, L.J. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015, 64, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayson, B.E.; Schneider, K.M.; Woods, S.C.; Seeley, R.J. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci. Transl. Med. 2013, 5, 199ra112. [Google Scholar] [CrossRef] [Green Version]
- Ceglarek, V.M.; Bertasso, I.M.; Pietrobon, C.B.; Scomazzon, S.P.; Leite, N.C.; Bonfleur, M.L.; Araújo, A.C.F.; Balbo, S.L.; Grassiolli, S. Maternal Roux-en-Y gastric bypass surgery reduces lipid deposition and increases UCP1 expression in the brown adipose tissue of male offspring. Sci. Rep. 2021, 11, 1158. [Google Scholar] [CrossRef]
- Sim, K.A.; Dezarnaulds, G.M.; Denyer, G.S.; Skilton, M.R.; Caterson, I.D. Weight loss improves reproductive outcomes in obese women undergoing fertility treatment: A randomized controlled trial. Clin. Obes. 2014, 4, 61–68. [Google Scholar] [CrossRef]
- Muirhead, R.; Kizirian, N.; Lal, R.; Black, K.; Prys-Davies, A.; Nassar, N.; Baur, L.; Sainsbury, A.; Sweeting, A.; Markovic, T.; et al. A Pilot Randomized Controlled Trial of a Partial Meal Replacement Preconception Weight Loss Program for Women with Overweight and Obesity. Nutrients 2021, 13, 3200. [Google Scholar] [CrossRef]
- Guzick, D.S.; Wing, R.; Smith, D.; Berga, S.L.; Winters, S.J. Endocrine consequences of weight loss in obese, hyperandrogenic, anovulatory women. Fertil. Steril. 1994, 61, 598–604. [Google Scholar] [CrossRef]
- Clark, A.; Ledger, W.; Galletly, C.; Tomlinson, L.; Blaney, F.; Wang, X.; Norman, R. Weight loss results in significant improvement in pregnancy and ovulation rates in anovulatory obese women. Hum. Reprod. 1995, 10, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Women’s and Children’s Health. National Institute for Health and Clinical Excellence: Guidance. Fertility: Assessment and Treatment for People with Fertility Problems; Royal College of Obstetricians & Gynaecologists Copyright© 2022, National Collaborating Centre for Women’s and Children’s Health: London, UK, 2013. [Google Scholar]
- Boedt, T.; Vanhove, A.C.; Vercoe, M.A.; Matthys, C.; Dancet, E.; Lie Fong, S. Preconception lifestyle advice for people with infertility. Cochrane Database Syst. Rev. 2021, 4, Cd008189. [Google Scholar] [PubMed]
- Abodeely, A.; Roye, G.D.; Harrington, D.T.; Cioffi, W.G. Pregnancy outcomes after bariatric surgery: Maternal, fetal, and infant implications. Surg. Obes. Relat. Dis. 2008, 4, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity 2013, 21 (Suppl. 1), S1–S27. [Google Scholar]
- Snoek, K.M.; Steegers-Theunissen, R.P.; Hazebroek, E.J.; Willemsen, S.P.; Galjaard, S.; Laven, J.S.; Schoenmakers, S. The effects of bariatric surgery on periconception maternal health: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 1030–1055. [Google Scholar] [CrossRef]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Ruban, A.; Ashrafian, H.; Teare, J.P. The EndoBarrier: Duodenal-Jejunal Bypass Liner for Diabetes and Weight Loss. Gastroenterol. Res. Pract. 2018, 2018, 7823182. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigo, N.; Saad, S.; Pollock, C.; Glastras, S.J. Diet Modification before or during Pregnancy on Maternal and Foetal Outcomes in Rodent Models of Maternal Obesity. Nutrients 2022, 14, 2154. https://doi.org/10.3390/nu14102154
Rodrigo N, Saad S, Pollock C, Glastras SJ. Diet Modification before or during Pregnancy on Maternal and Foetal Outcomes in Rodent Models of Maternal Obesity. Nutrients. 2022; 14(10):2154. https://doi.org/10.3390/nu14102154
Chicago/Turabian StyleRodrigo, Natassia, Sonia Saad, Carol Pollock, and Sarah J. Glastras. 2022. "Diet Modification before or during Pregnancy on Maternal and Foetal Outcomes in Rodent Models of Maternal Obesity" Nutrients 14, no. 10: 2154. https://doi.org/10.3390/nu14102154
APA StyleRodrigo, N., Saad, S., Pollock, C., & Glastras, S. J. (2022). Diet Modification before or during Pregnancy on Maternal and Foetal Outcomes in Rodent Models of Maternal Obesity. Nutrients, 14(10), 2154. https://doi.org/10.3390/nu14102154






