Resistance Exercise Training Improves Metabolic and Inflammatory Control in Adipose and Muscle Tissues in Mice Fed a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
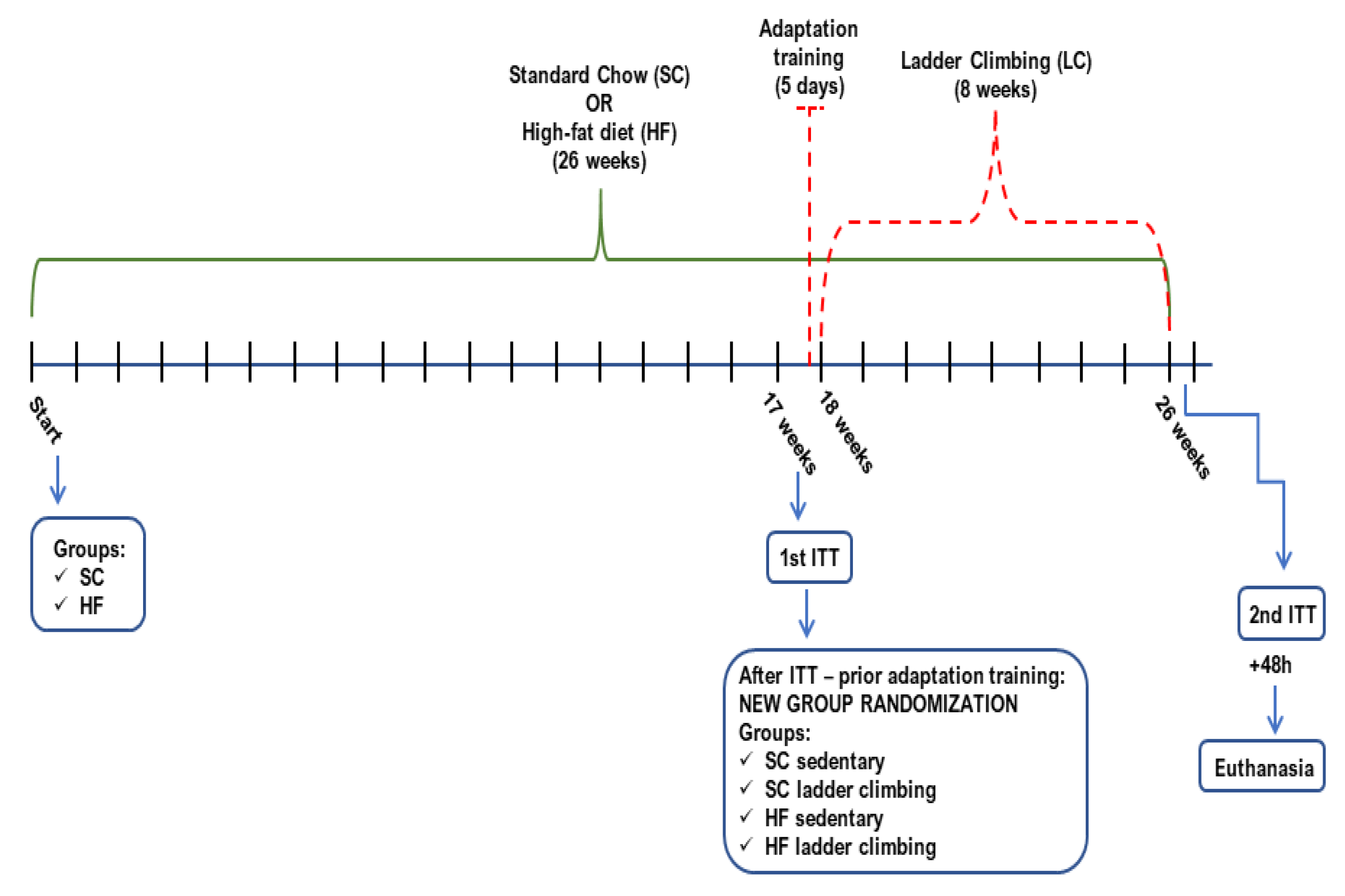
2.1. Animals, Diet, and Ethics Approval
2.2. Resistance Training—Ladder Climbing Protocol
2.3. Body Weight (BW) and Insulin Tolerance Test (ITT)
2.4. Euthanasia and Sample Processing
2.5. Western Blot Analysis
2.6. RNA Isolation and Quantitative PCR (RT-qPCR)
2.7. Histological Analysis
2.8. Inflammatory Parameters
2.9. Statistical Analyses
3. Results
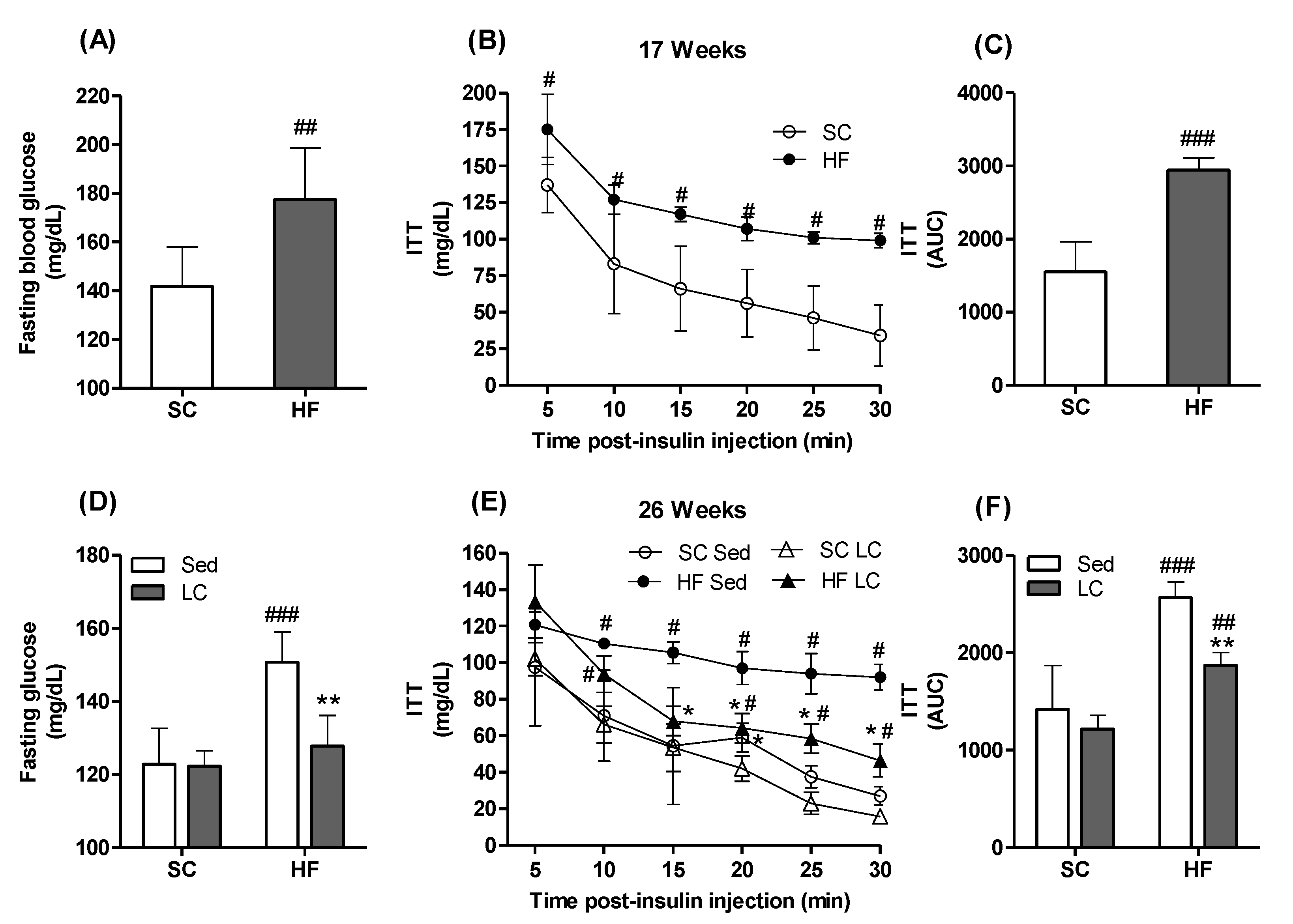
3.1. Effects of Diet on Body Weight (BW), Fasting Blood Glucose, and Insulin Tolerance Parameters
3.2. Effects of LC on BW
3.3. Effects of LC on Adiposity and WAT Morphology
3.4. Effects of LC on Metabolic Markers
3.5. Effects of LC on the Phosphorylation of AKT, GSK-3β, and AMPK in the Gastrocnemius Muscle
3.6. Effects of LC on the Gene Expression of Rac1, AMPK, and CaMKK2β
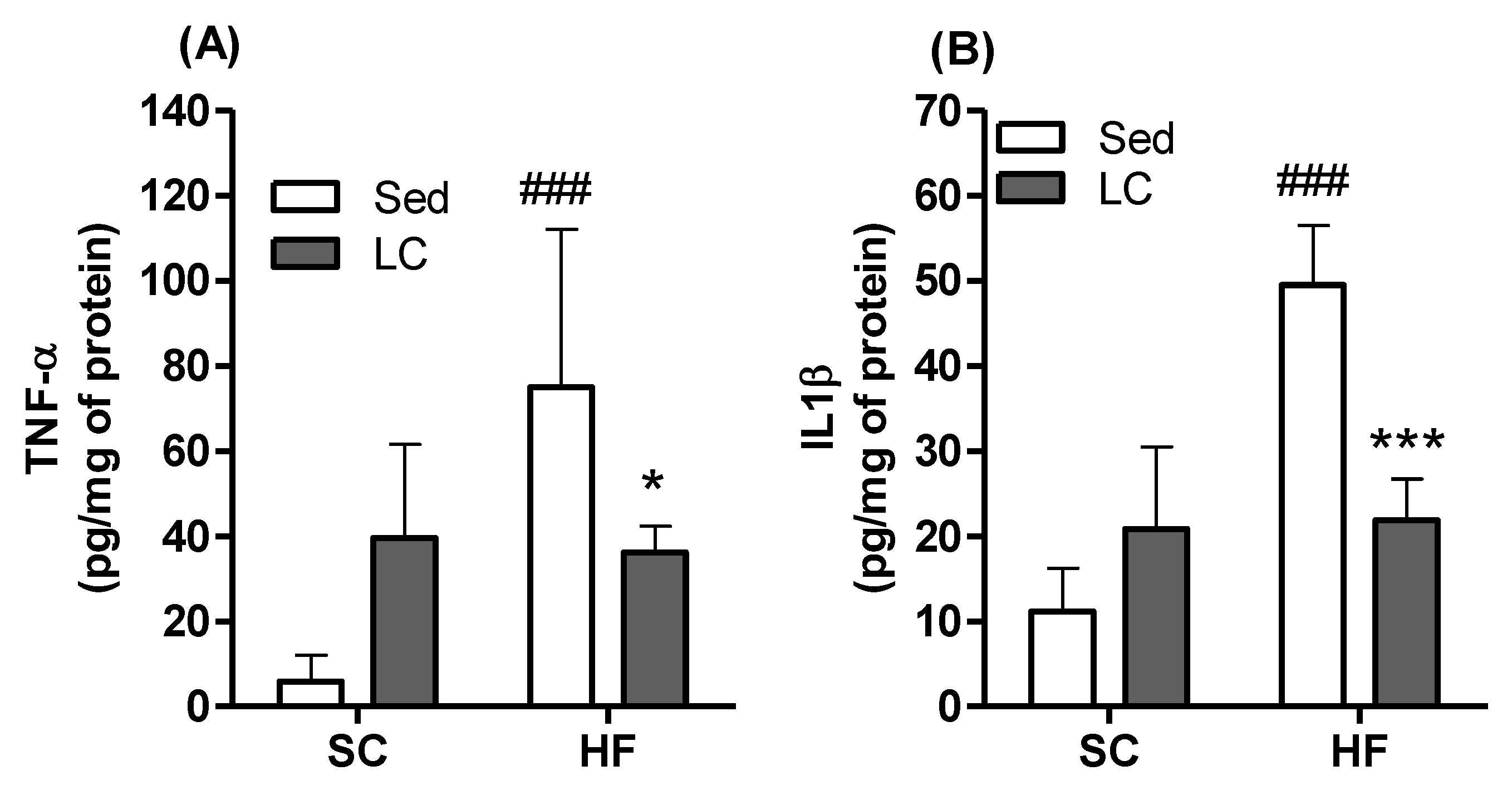
3.7. Effects of HF and LC on TNF-α and IL1β Contents and Expression of NADPH Subunits in Quadriceps Muscles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Luz, G.; Frederico, M.J.S.; Da Silva, S.; Vitto, M.F.; Cesconetto, P.A.; De Pinho, R.A.; Pauli, J.R.; Silva, A.S.R.; Cintra, D.E.; Ropelle, E.R.; et al. Endurance exercise training ameliorates insulin resistance and reticulum stress in adipose and hepatic tissue in obese rats. Eur. J. Appl. Physiol. 2011, 111, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.; Maggi, R.; Tromm, C.; Silva, L.; Luciano, T.; Marques, S.; Lira, F.; De Souza, C.; Pinho, R.A. Exercise training performed simultaneously to a high-fat diet reduces the degree of insulin resistance and improves adipoR1-2/APPL1 protein levels in mice. Lipids Health Dis. 2012, 11, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias, J.M.; Bom, K.F.; Tromm, C.B.; Luciano, T.F.; Marques, S.O.; Tuon, T.; Silva, L.A.; Lira, F.S.; de Souza, C.T.; Pinho, R.A. Effect of physical training on the adipose tissue of diet-induced obesity mice: Interaction between reactive oxygen species and lipolysis. Horm. Metab. Res. 2013, 45, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenster, C.P.; Weinsier, R.L.; Darley-Usmar, V.M.; Patel, R.P. Obesity, aerobic exercise, and vascular disease: The role of oxidant stress. Obes. Res. 2002, 10, 964–968. [Google Scholar] [CrossRef]
- Okura, T.; Nakata, Y.; Lee, D.J.; Ohkawara, K.; Tanaka, K. Effects of aerobic exercise and obesity phenotype on abdominal fat reduction in response to weight loss. Int. J. Obes. 2005, 29, 1259–1266. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.-H.; Paik, I.-Y.; Jacobs, K. Regulation of blood glucose homeostasis during prolonged exercise. Mol. Cells 2007, 23, 272–279. [Google Scholar]
- Di Meo, S.; Iossa, S.; Venditti, P. Improvement of obesity-linked skeletal muscle insulin resistance by strength and endurance training. J. Endocrinol. 2017, 234, R159–R181. [Google Scholar] [CrossRef] [Green Version]
- LeBrasseur, N.K.; Walsh, K.; Arany, Z. Metabolic benefits of resistance training and fast glycolytic skeletal muscle. AJP Endocrinol. Metab. 2011, 300, E3–E10. [Google Scholar] [CrossRef] [Green Version]
- Strasser, B.; Arvandi, M.; Siebert, U. Resistance training, visceral obesity and inflammatory response: A review of the evidence. Obes. Rev. 2012, 13, 578–591. [Google Scholar] [CrossRef]
- O’Donoghue, G.; Blake, C.; Cunningham, C.; Lennon, O.; Perrotta, C. What exercise prescription is optimal to improve body composition and cardiorespiratory fitness in adults living with obesity? A network meta-analysis. Obes. Rev. 2021, 22, 19. [Google Scholar] [CrossRef]
- Codella, R.; Ialacqua, M.; Terruzzi, I.; Luzi, L. May the force be with you: Why resistance training is essential for subjects with type 2 diabetes mellitus without complications. Endocrine 2018, 62, 14–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walberg, J.L. Aerobic Exercise and Resistance Weight-Training During Weight Reduction. Sport. Med. 1989, 7, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Vandenborne, K.; Goldsmith, R.; Simoneau, J.-A.; Heymsfield, S.; Joanisse, D.R.; Hirsch, J.; Murphy, E.; Matthews, D.; Segal, K.R.; et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am. J. Physiol. 2003, 285, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, J.; Taimela, S.; Eriksson, K.; Parviainen, S.; Peltonen, J.; Kujala, U. Resistance training in the treatment of non-insulin-dependent diabetes mellitus. Int. J. Sports Med. 1997, 18, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.E.; Richter, E.A. Regulation of glucose and glycogen metabolism during and after exercise. J. Physiol. 2012, 590, 1069–1076. [Google Scholar] [CrossRef] [Green Version]
- Sylow, L.; Nielsen, I.L.; Kleinert, M.; Møller, L.L.V.; Ploug, T.; Schjerling, P.; Bilan, P.J.; Klip, A.; Jensen, T.E.; Richter, E.A. Rac1 governs exercise-stimulated glucose uptake in skeletal muscle through regulation of GLUT4 translocation in mice. J. Physiol. 2016, 594, 4997–5008. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Manzano, P.; Rodriguez-Ayllon, M.; Acosta, F.M.; Niederseer, D.; Niebauer, J. Beyond general resistance training. Hypertrophy versus muscular endurance training as therapeutic interventions in adults with type 2 diabetes mellitus: A systematic review and meta-analysis. Obes. Rev. 2020, 21, 35. [Google Scholar] [CrossRef]
- Effting, P.S.; Brescianini, S.M.S.; Sorato, H.R.; Fernandes, B.B.; Fidelis, G.D.S.P.; Silva, P.R.L.d.; Silveira, P.C.L.; Nesi, R.T.; Ceddia, R.B.; Pinho, R.A. Resistance Exercise Modulates Oxidative Stress Parameters and TNF-α Content in the Heart of Mice with Diet-Induced Obesity. Arq. Bras. Cardiol. 2019, 112, 545–552. [Google Scholar] [CrossRef]
- Leite, R.D.; Durigan, R.D.C.M.; De Souza Lino, A.D.; De Souza Campos, M.V.; Souza, M.D.G.; Selistre-De-Araújo, H.S.; Bouskela, E.; Kraemer-Aguiar, L.G. Resistance training may concomitantly benefit body composition, blood pressure and muscle MMP-2 activity on the left ventricle of high-fat fed diet rats. Metabolism. 2013, 62, 1477–1484. [Google Scholar] [CrossRef]
- Mardare, C.; Krüger, K.; Liebisch, G.; Seimetz, M.; Couturier, A.; Ringseis, R.; Wilhelm, J.; Weissmann, N.; Eder, K.; Mooren, F.C. Endurance and resistance training affect high fat diet-induced increase of ceramides, inflammasome expression, and systemic inflammation in mice. J. Diabetes Res. 2016, 2016, 4536470. [Google Scholar] [CrossRef] [Green Version]
- Talebi-Garakani, E.; Safarzade, A. Resistance training decreases serum inflammatory markers in diabetic rats. Endocrine 2013, 43, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Vilela, T.C.; Effting, P.S.; Dos Santos, P.G.; Farias, H.; Paganini, L.; Rebelo, H.S.; Nesi, R.T.; de Andrade, V.M.; de Pinho, R.A. Aerobic and strength training induce changes in oxidative stress parameters and elicit modifications of various cellular components in skeletal muscle of aged rats. Exp.Gerontol. 2018, 106, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Inoguchi, T.; Sonta, T.; Tsubouchi, H.; Etoh, T.; Kakimoto, M.; Sonoda, N.; Sato, N.; Sekiguchi, N.; Kobayashi, K.; Sumimoto, H.; et al. Protein Kinase C-Dependent Increase in Reactive Oxygen Species (ROS) Production in Vascular Tissues of Diabetes: Role of Vascular NAD(P)H Oxidase. J. Am. Soc. Nephrol. 2003, 14, S227–S232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JeBailey, L.; Wanono, O.; Niu, W.; Roessler, J.; Rudich, A.; Klip, A. Ceramide- and oxidant-induced insulin resistance involve loss of insulin-dependent Rac-activation and actin remodeling in muscle cells. Diabetes 2007, 56, 394–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elia, M. Organ and Tissue Contribution to Metabolic Rate. In Energy Metabolism: Tissue Determinants and Cellular Corollaries; Kinney, J.M., Tucker, H.N., Eds.; Raven Press: New York, NY, USA, 1992; pp. 61–79. [Google Scholar]
- Mizgier, M.L.; Casas, M.; Contreras-Ferrat, A.; Llanos, P.; Galgani, J.E. Potential role of skeletal muscle glucose metabolism on the regulation of insulin secretion. Obes. Rev. 2014, 15, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, V.; Yaligar, J.; Michael, N.; Kaur, K.; Anantharaj, R.; Verma, S.K.; Sadananthan, S.A.; Le, G.T.T.; Goh, J.; Velan, S.S. A 12-week aerobic exercise intervention results in improved metabolic function and lower adipose tissue and ectopic fat in high-fat diet fed rats. Biosci. Rep. 2021, 41, BSR20201707. [Google Scholar] [CrossRef]
- Cordeira, J.; Monahan, D. Voluntary wheel running reduces weight gain in mice by decreasing high-fat food consumption. Physiol. Behav. 2019, 207, 1–6. [Google Scholar] [CrossRef]
- Motta, V.F.; Aguila, M.B.; Mandarin-de-Lacerda, C.A. High-intensity interval training (swimming) significantly improves the adverse metabolism and comorbidities in diet-induced obese mice. J. Sport. Med. Phys. Fit. 2016, 56, 655–663. [Google Scholar]
- Hornberger, T.; Farrar, R. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef]
- Scheffer, D.L.; Silva, L.A.; Tromm, C.B.; da Rosa, G.L.; Silveira, P.C.L.; de Souza, C.T.; Latini, A.; Pinho, R.A. Impact of different resistance training protocols on muscular oxidative stress parameters. Appl. Physiol. Nutr. Metab. 2012, 37, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; dos Santos Pedroso, G.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats Through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Ayala, J.E.; Samuel, V.T.; Morton, G.J.; Obici, S.; Croniger, C.M.; Shulman, G.I.; Wasserman, D.H.; McGuinness, O.P. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis. Model. Mech. 2010, 3, 525–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.Y.; Liao, J.K. A mouse model of diet-induced obesity and insulin resistance. Methods Mol. Biol. 2012, 821, 421–433. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.; et al. The need for transparency and good practices in the qPCR literature. Nat. Methods 2013, 10, 1063–1067. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Nikawa, T.; Kawano, Y.; Nakayama, M.; Ikeda, M. Effects of dimethyl sulfoxide and dexamethasone on mRNA expression of housekeeping genes in cultures of C2C12 myotubes. Biochem. Biophys. Res. Commun. 2008, 367, 603–608. [Google Scholar] [CrossRef]
- Pernot, F.; Dorandeu, F.; Beaup, C.; Peinnequin, A. Selection of reference genes for real-time quantitative reverse transcription-polymerase chain reaction in hippocampal structure in a murine model of temporal lobe epilepsy with focal seizures. J. Neurosci. Res. 2010, 88, 1000–1008. [Google Scholar] [CrossRef]
- Tatsumi, K.; Ohashi, K.; Taminishi, S.; Okano, T.; Yoshioka, A.; Shima, M. Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem. Biophys. Res. Commun. 2008, 374, 106–110. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhao, J.-X.; Zhu, M.-J.; Foretz, M.; Viollet, B.; Dodson, M.V.; Du, M. AMP-Activated Protein Kinase α1 but Not α2 Catalytic Subunit Potentiates Myogenin Expression and Myogenesis. Mol. Cell. Biol. 2013, 33, 4517–4525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Zhu, M.; Zhang, S.; Foretz, M.; Viollet, B.; Du, M. Obesity impairs skeletal muscle regeneration through inhibition of AMPK. Diabetes 2016, 65, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, Y.; Donley, J.; Seletskaia, E.; Shingare, S.; Kamerud, J.; Gorovits, B. A Simple Approach to Determine a Curve Fitting Model with a Correct Weighting Function for Calibration Curves in Quantitative Ligand Binding Assays. AAPS J. 2018, 20, 45. [Google Scholar] [CrossRef]
- García-Hermoso, A.; Ramírez-Vélez, R.; Ramírez-Campillo, R.; Peterson, M.D.; Martínez-Vizcaíno, V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: A systematic review and meta-Analysis. Br. J. Sports Med. 2018, 52, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Scott, C.A.; Mao, C.; Tang, J.; Farmer, A.J. Resistance exercise versus aerobic exercise for type 2 diabetes: A systematic review and meta-analysis. Sport. Med. 2014, 44, 487–499. [Google Scholar] [CrossRef]
- Leontieva, O.V.; Paszkiewicz, G.; Demidenko, Z.N.; Blagosklonny, M.V. Resveratrol potentiates rapamycin to prevent hyperinsulinemia and obesity in male mice on high fat diet. Cell Death Dis. 2013, 4, e472. [Google Scholar] [CrossRef]
- Hoffler, U.; Hobbie, K.; Wilson, R.; Bai, R.; Rahman, A.; Malarkey, D.; Travlos, G.; Ghanayem, B.I. Diet-induced obesity is associated with hyperleptinemia, hyperinsulinemia, hepatic steatosis, and glomerulopathy in C57Bl/6J mice. Endocrine 2009, 36, 311–325. [Google Scholar] [CrossRef] [Green Version]
- Krisan, A.D.; Collins, D.E.; Crain, A.M.; Kwong, C.C.; Singh, M.K.; Bernard, J.R.; Yaspelkis, B.B., III. Resistance training enhances components of the insulin signaling cascade in normal and high-fat-fed rodent skeletal muscle. J. Appl. Physiol. 2004, 96, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- Yaspelkis, B.B.; Singh, M.K.; Trevino, B.; Krisan, A.D.; Collins, D.E. Resistance training increases glucose uptake and transport in rat skeletal muscle. Acta Physiol. Scand. 2002, 175, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bronczek, G.A.; Soares, G.M.; de Barros, J.F.; Vettorazzi, J.F.; Kurauti, M.A.; Marconato-Júnior, E.; Zangerolamo, L.; Marmentini, C.; Boschero, A.C.; Costa-Júnior, J.M. Resistance exercise training improves glucose homeostasis by enhancing insulin secretion in C57BL/6 mice. Sci. Rep. 2021, 11, 8574. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, S.; Takeda, T.; Kitaura, T.; Takenaka, N.; Kataoka, T.; Satoh, T. Akt2 regulates Rac1 activity in the insulin-dependent signaling pathway leading to GLUT4 translocation to the plasma membrane in skeletal muscle cells. Cell. Signal. 2013, 25, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Luo, K.; Liu, C.; Wang, X.; Zhang, D.; Chi, A.; Zhang, J.; Sun, L. Decrease in myostatin by ladder-climbing training is associated with insulin resistance in diet-induced obese rats. Chin. Med. J. 2014, 127, 2342–2349. [Google Scholar]
- Henríquez-Olguín, C.; Boronat, S.; Cabello-Verrugio, C.; Jaimovich, E.; Hidalgo, E.; Jensen, T.E. The Emerging Roles of Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 in Skeletal Muscle Redox Signaling and Metabolism. Antioxid. Redox Signal. 2019, 31, 1371–1410. [Google Scholar] [CrossRef]
- Marko, D.M.; Shamshoum, H. Rac1 supports muscle glucose uptake independently of Akt. J. Physiol. 2018, 596, 4815–4816. [Google Scholar] [CrossRef]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [Green Version]
- Kluth, O.; Mirhashemi, F.; Scherneck, S.; Kaiser, D.; Kluge, R.; Neschen, S.; Joost, H.G.; Schürmann, A. Dissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: Role of forkhead box O1 (FOXO1) in glucose-induced beta cell failure. Diabetologia 2011, 54, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Saghizadeh, M.; Ong, J.M.; Garvey, W.T.; Henry, R.R.; Kern, P.A. The Expression of TNF alpha by Human Muscle: Relationship to Insulin Resistance. J. Clin. Investig. 1996, 97, 1111–1116. [Google Scholar] [CrossRef]
- da Luz Scheffer, D.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Fischer, C.P.; Keller, C.; Møller, K.; Pedersen, B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E433–E437. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gao, X.; Yang, X.; Liu, C.; Wang, X.; Han, Y.; Zhao, X.; Chi, A.; Sun, L. Ladder-Climbing Training Prevents Bone Loss and Microarchitecture Deterioration in Diet-Induced Obese Rats. Calcif. Tissue Int. 2016, 98, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Dokken, B.B.; Saengsirisuwan, V.; Kim, J.S.; Teachey, M.K.; Henriksen, E.J. Oxidative stress-induced insulin resistance in rat skeletal muscle: Role of glycogen synthase kinase-3. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E615–E621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Saengsirisuwan, V.; Sloniger, J.A.; Teachey, M.K.; Henriksen, E.J. Oxidant stress and skeletal muscle glucose transport: Roles of insulin signaling and p38 MAPK. Free Radic. Biol. Med. 2006, 41, 818–824. [Google Scholar] [CrossRef]
- Pinho, R.A.; Sepa-Kishi, D.M.; Bikopoulos, G.; Wu, M.V.; Uthayakumar, A.; Mohasses, A.; Hughes, M.C.; Perry, C.G.R.; Ceddia, R.B. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic. Biol. Med. 2017, 110, 381–389. [Google Scholar] [CrossRef]
- Apostolopoulou, M.; Gordillo, R.; Koliaki, C.; Gancheva, S.; Jelenik, T.; De Filippo, E.; Herder, C.; Markgraf, D.; Jankowiak, F.; Esposito, I.; et al. Specific hepatic sphingolipids relate to insulin resistance, oxidative stress, and inflammation in nonalcoholic steato hepatitis. Diabetes Care 2018, 41, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef]
- Ferreira, L.F.; Laitano, O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016, 98, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Campos, C.; Díaz-Vegas, A.; Galgani, J.E.; Juretic, N.; Osorio-Fuentealba, C.; Bucarey, J.L.; Tapia, G.; Valenzuela, R.; Contreras-Ferrat, A.; et al. Insulin-dependent H2O2 production is higher in muscle fibers of mice fed with a high-fat diet. Int. J. Mol. Sci. 2013, 14, 15740–15754. [Google Scholar] [CrossRef]
- Wei, Y.; Sowers, J.R.; Clark, S.E.; Li, W.; Ferrario, C.M.; Stump, C.S. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kB activation via NADPH oxidase. Am. J. Physiol. Metab. 2008, 85724, 345–351. [Google Scholar]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, A.S.P.; Salmon, A.B.; Bruno, F.; Jimenez, F.; Martinez, H.G.; Halade, G.V.; Ahuja, S.S.; Clark, R.A.; DeFronzo, R.A.; Abboud, H.E.; et al. Nox2 Mediates Skeletal Muscle Insulin Resistance Induced by a High-Fat Diet. J. Biol. Chem. 2015, 290, 13427–13439. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene | Gene Bank | Sequence (5′→3′) | |
|---|---|---|---|
| Ppia [40] | NM_008907 | Forward | TATCTGCACTGCCAAGACTGAATG |
| Reverse | CTTCTTGCTGGTCTTGCCATTCC | ||
| gp91phox | NM_007807.5 | Forward | CCAAAACCATTCGGAGGTCTTATTT |
| Reverse | TGGTACTGGGCACTCCTTTATTT | ||
| Nox 4 | NM_015760.5 | Forward | CCGGGATTTGCTACTGCCTCCATC |
| Reverse | ACTCCAATGCCTCCAGCCACAC | ||
| p22phox | NM_001301284.1 | Forward | GCAGAGGTCCGAAAGAAGCCGA |
| Reverse | ACAGCCACTGAAGGTCACACGA | ||
| Rac1 | NM_009007.2 | Forward | CCATCAAGTGTGTGGTGGTGGGA |
| Reverse | AACACGTCTGTCTGCGGGTAGG | ||
| p47phox | NM_001286037.1 | Forward | CGCAGGTGAACCGTATGTAA |
| Reverse | CAGGAGCTTATGAATGACCTCAA | ||
| p67phox | NM_010877.5 | Forward | CATGCCTGGGAACATCGTCTTT |
| Reverse | GGGTGAATCCGAAGCTCAACTG | ||
| AMPKα2 [43] | NM_178143.2 | Forward | CATGGCTGAGAAGCAGAAGCAC |
| Reverse | CTTAACTGCCACTTTATGGCCTG | ||
| CaMKK2β [44] | NM_001199676.1 | Forward | CCAGGATTGTGGTGCCTGAAATC |
| Reverse | ATTCTCGACCTCCTCTTCGGTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Effting, P.S.; Thirupathi, A.; Müller, A.P.; Pereira, B.C.; Sepa-Kishi, D.M.; Marqueze, L.F.B.; Vasconcellos, F.T.F.; Nesi, R.T.; Pereira, T.C.B.; Kist, L.W.; et al. Resistance Exercise Training Improves Metabolic and Inflammatory Control in Adipose and Muscle Tissues in Mice Fed a High-Fat Diet. Nutrients 2022, 14, 2179. https://doi.org/10.3390/nu14112179
Effting PS, Thirupathi A, Müller AP, Pereira BC, Sepa-Kishi DM, Marqueze LFB, Vasconcellos FTF, Nesi RT, Pereira TCB, Kist LW, et al. Resistance Exercise Training Improves Metabolic and Inflammatory Control in Adipose and Muscle Tissues in Mice Fed a High-Fat Diet. Nutrients. 2022; 14(11):2179. https://doi.org/10.3390/nu14112179
Chicago/Turabian StyleEffting, Pauline S., Anand Thirupathi, Alexandre P. Müller, Bárbara C. Pereira, Diane M. Sepa-Kishi, Luis F. B. Marqueze, Franciane T. F. Vasconcellos, Renata T. Nesi, Talita C. B. Pereira, Luiza W. Kist, and et al. 2022. "Resistance Exercise Training Improves Metabolic and Inflammatory Control in Adipose and Muscle Tissues in Mice Fed a High-Fat Diet" Nutrients 14, no. 11: 2179. https://doi.org/10.3390/nu14112179
APA StyleEffting, P. S., Thirupathi, A., Müller, A. P., Pereira, B. C., Sepa-Kishi, D. M., Marqueze, L. F. B., Vasconcellos, F. T. F., Nesi, R. T., Pereira, T. C. B., Kist, L. W., Bogo, M. R., Ceddia, R. B., & Pinho, R. A. (2022). Resistance Exercise Training Improves Metabolic and Inflammatory Control in Adipose and Muscle Tissues in Mice Fed a High-Fat Diet. Nutrients, 14(11), 2179. https://doi.org/10.3390/nu14112179








