Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition
2.3. T1-Weighted Image Processing
2.4. QSM Reconstruction
2.5. Behavioural Measures
2.6. Statistical Analysis
3. Results
3.1. Demographics
3.2. Diagnostic Group Differences in Susceptibility and Volume
3.3. Behavioural Outcomes
3.4. Magnetic Susceptibility and Behavioural Outcomes
3.5. Brain Volume and Behavioural Outcomes
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, M.; Gibbard, B. Overview of Fetal Alcohol Spectrum Disorders for Mental Health Professionals. Can. Child Adolesc. Psychiatry Rev. 2003, 12, 57–63. [Google Scholar]
- Cook, J.L.; Green, C.R.; Lilley, C.M.; Anderson, S.M.; Baldwin, M.E.; Chudley, A.E.; Conry, J.L.; LeBlanc, N.; Loock, C.A.; Lutke, J.; et al. Fetal alcohol spectrum disorder: A guideline for diagnosis across the lifespan. CMAJ 2016, 188, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.F.; Smith, V.C. Fetal alcohol spectrum disorders. Pediatrics 2015, 136, e1395–e1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donald, K.A.; Eastman, E.; Howells, F.M.; Adnams, C.; Riley, E.P.; Woods, R.P.; Narr, K.L.; Stein, D.J. Neuroimaging effects of prenatal alcohol exposure on the developing human brain: A magnetic resonance imaging review. Acta Neuropsychiatr. 2015, 27, 251–269. [Google Scholar] [CrossRef] [Green Version]
- Lebel, C.; Roussotte, F.; Sowell, E.R. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol. Rev. 2011, 21, 102–118. [Google Scholar] [CrossRef] [Green Version]
- Sowell, E.R.; Mattson, S.N.; Kan, E.; Thompson, P.M.; Riley, E.P.; Toga, A.W. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb. Cortex 2008, 18, 136–144. [Google Scholar] [CrossRef]
- Lebel, C.; Rasmussen, C.; Wyper, K.; Walker, L.; Andrew, G.; Yager, J.; Beaulieu, C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2008, 32, 1732–1740. [Google Scholar] [CrossRef]
- Wozniak, J.R.; Muetzel, R.L. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol. Rev. 2011, 21, 133–147. [Google Scholar] [CrossRef]
- Coles, C.D.; Li, Z. Functional neuroimaging in the examination of effects of prenatal alcohol exposure. Neuropsychol. Rev. 2011, 21, 119–132. [Google Scholar] [CrossRef]
- Pei, J.; Denys, K.; Hughes, J.; Rasmussen, C. Mental health issues in fetal alcohol spectrum disorder. J. Ment. Health 2011, 20, 473–483. [Google Scholar] [CrossRef]
- O’Leary, C.M.; Nassar, N.; Zubrick, S.R.; Kurinczuk, J.J.; Stanley, F.; Bower, C. Evidence of a complex association between dose, pattern and timing of prenatal alcohol exposure and child behaviour problems. Addiction 2010, 105, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.E.; Jamieson, B.; Milligan, K. Risk for Childhood Internalizing and Externalizing Behavior Problems in the Context of Prenatal Alcohol Exposure: A Meta-Analysis and Comprehensive Examination of Moderators. Alcohol. Clin. Exp. Res. 2018, 42, 1358–1377. [Google Scholar] [CrossRef] [PubMed]
- Weyrauch, D.; Schwartz, M.; Hart, B.; Klug, M.G.; Burd, L. Comorbid mental disorders in fetal alcohol spectrum disorders: A systematic review. J. Dev. Behav. Pediatr. 2017, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.; Lichter, R.; Dennison, M.; Vijayakumar, N.; Schwartz, O.; Byrne, M.L.; Simmons, J.G.; Yücel, M.; Pantelis, C.; McGorry, P.; et al. Structural brain development and depression onset during adolescence: A prospective longitudinal study. Am. J. Psychiatry 2014, 171, 564–571. [Google Scholar] [CrossRef]
- Nogovitsyn, N.; Souza, R.; Muller, M.; Srajer, A.; Metzak, P.D.; Hassel, S.; Ismail, Z.; Protzner, A.; Bray, S.L.; Lebel, C.; et al. Aberrant limbic brain structures in young individuals at risk for mental illness. Psychiatry Clin. Neurosci. 2020, 74, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, K.; Rosenberg, D.R.; Easter, P.C.; MacMaster, F.P.; Chen, H.H.; Nicoletti, M.; Caetano, S.C.; Hatch, J.P.; Soares, J.C. Striatal volume abnormalities in treatment-naive patients diagnosed with pediatric major depressive disorder. J. Child Adolesc. Psychopharmacol. 2008, 18, 121–131. [Google Scholar] [CrossRef]
- Koolschijn, P.C.M.P.; Van Haren, N.E.M.; Lensvelt-Mulders, G.J.L.M.; Hulshoff Pol, H.E.; Kahn, R.S. Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009, 30, 3719–3735. [Google Scholar] [CrossRef]
- Moon, C.M.; Kim, G.W.; Jeong, G.W. Whole-brain gray matter volume abnormalities in patients with generalized anxiety disorder: Voxel-based morphometry. Neuroreport 2014, 25, 184–189. [Google Scholar] [CrossRef]
- Qin, S.; Young, C.B.; Duan, X.; Chen, T.; Supekar, K.; Menon, V. Amygdala subregional structure and intrinsic functional connectivity predicts individual differences in anxiety during early childhood. Biol. Psychiatry 2014, 75, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Hilbert, K.; Pine, D.S.; Muehlhan, M.; Lueken, U.; Steudte-Schmiedgen, S.; Beesdo-Baum, K. Gray and white matter volume abnormalities in generalized anxiety disorder by categorical and dimensional characterization. Psychiatry Res. Neuroimaging 2015, 234, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Yang, F.; Zhang, Y.; He, Z.; Song, M.; Jiang, T.; Li, Z.; Lu, S.; Wu, W.; Su, L.; et al. Childhood Maltreatment Is Associated with Larger Left Thalamic Gray Matter Volume in Adolescents with Generalized Anxiety Disorder. PLoS ONE 2013, 8, e71898. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; De Rossi, P.; Watson, B.; Wharton, A.; Greenstein, D.; Raznahan, A.; Sharp, W.; Lerch, J.P.; Chakravarty, M.M. Mapping the development of the basal ganglia in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 780–789.e11. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, A.; Lebel, C.; Rasmussen, C.; Andrew, G.; Beaulieu, C. Extensive deep gray matter volume reductions in children and adolescents with fetal alcohol spectrum disorders. Alcohol. Clin. Exp. Res. 2011, 35, 1404–1417. [Google Scholar] [CrossRef] [PubMed]
- Spadoni, A.; McGee, C.; Fryer, S.; Riley, E.P. Neuroimaging and fetal alcohol spectrum disorders. Neuroscience 2007, 31, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Archibald, S.; Fennema-Notestine, C.; Gamst, A.; Riley, E.; Mattson, S.; Jernigan, T. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev. Med. Child Neurol. 2001, 43, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.H.; Su, T.P.; Chen, Y.S.; Hsu, J.W.; Huang, K.L.; Chang, W.H.; Chen, T.J.; Bai, Y.M. Association between psychiatric disorders and iron deficiency anemia among children and adolescents: A nationwide population-based study. BMC Psychiatry 2013, 13, 161. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Chao, H.H.; Huang, W.T.; Chen, S.C.C.; Yang, H.Y. Psychiatric disorders risk in patients with iron deficiency anemia and association with iron supplementation medications: A nationwide database analysis. BMC Psychiatry 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Kwik-Uribe, C.L.; Gietzen, D.; German, J.B.; Golub, M.S.; Keen, C.L. Chronic Marginal Iron Intakes during Early Development in Mice Result in Persistent Changes in Dopamine Metabolism and Myelin Composition. J. Nutr. 2000, 130, 2821–2830. [Google Scholar] [CrossRef] [Green Version]
- Beard, J.L.; Connor, J.R. Iron status and neural functioning. Annu. Rev. Nutr. 2003, 23, 41–58. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Green, A.R. Iron deficiency and neurotransmitter synthesis and function. Proc. Nutr. Soc. 1978, 37, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Carter, R.C.; Jacobson, S.W.; Molteno, C.D.; Jacobson, J.L. Fetal alcohol exposure, iron-deficiency anemia, and infant growth. Pediatrics 2007, 120, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Georgieff, M.K. Perinatal aspects of iron metabolism. Acta Paediatr. 2002, 91, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Jacobson, J.L.; Burden, M.J.; Armony-Sivan, R.; Dodge, N.C.; Angelilli, M.L.; Lozoff, B.; Jacobson, S.W. Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010, 126, e427–e434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozoff, B.; Corapci, F.; Burden, M.J.; Kaciroti, N.; Angulo-Barroso, R.; Sazawal, S.; Black, M. Preschool-aged children with iron deficiency anemia show altered affect and behavior. J. Nutr. 2007, 137, 683–689. [Google Scholar] [CrossRef] [Green Version]
- Lozoff, B.; Jimenez, E.; Hagen, J.; Mollen, E.; Wolf, A.W. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics 2000, 105, e51. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.W.; Roskams, A.J.I.; Connor, J.R. Iron Regulation in the Developing Rat Brain: Effect of In Utero Ethanol Exposure. J. Neurochem. 1995, 65, 373–380. [Google Scholar] [CrossRef]
- Huebner, S.M.; Blohowiak, S.E.; Kling, P.J.; Smith, S.M. Prenatal alcohol exposure alters fetal iron distribution and elevates hepatic hepcidin in a rat model of fetal alcohol spectrum disorders. J. Nutr. 2016, 146, 1180–1188. [Google Scholar] [CrossRef]
- Huebner, S.M.; Tran, T.D.; Rufer, E.S.; Crump, P.M.; Smith, S.M. Maternal Iron Deficiency Worsens the Associative Learning Deficits and Hippocampal and Cerebellar Losses in a Rat Model of Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2015, 39, 2097–2107. [Google Scholar] [CrossRef]
- Molteno, C.D.; Jacobson, J.L.; Carter, R.C.; Dodge, N.C.; Jacobson, S.W. Infant Emotional Withdrawal: A Precursor of Affective and Cognitive Disturbance in Fetal Alcohol Spectrum Disorders. Alcohol. Clin. Exp. Res. 2014, 38, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Adisetiyo, V.; Jensen, J.H.; Tabesh, A.; Deardorff, R.L.; Fieremans, E.; Di Martino, A.; Gray, K.M.; Castellanos, F.X.; Helpern, J.A. Multimodal MR imaging of brain iron in attention deficit hyperactivity disorder: A noninvasive biomarker that responds to psychostimulant treatment? Radiology 2014, 272, 524–532. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Xu, Y.; Liu, X.; Chen, Z.; Zhou, Y.; Nie, L.; He, L. Quantitative susceptibility mapping shows lower brain iron content in children with autism. Eur. Radiol. 2021, 31, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Azoulay, R.; Castellanos, F.X.; Chalard, F.; Lecendreux, M.; Chechin, D.; Delorme, R.; Sebag, G.; Sbarbati, A.; Mouren, M.C.; et al. Brain iron levels in attention-deficit/hyperactivity disorder: A pilot MRI study. World J. Biol. Psychiatry 2012, 13, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Péran, P.; Hagberg, G.; Luccichenti, G.; Cherubini, A.; Brainovich, V.; Celsis, P.; Caltagirone, C.; Sabatini, U. Voxel-based analysis of R2* maps in the healthy human brain. J. Magn. Reson. Imaging 2007, 26, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Drayer, B.; Burger, P.; Darwin, R.; Riederer, S.; Herfkens, R.; Johnson, G.A. MRI of brain iron. Am. J. Roentgenol. 1986, 147, 103–110. [Google Scholar] [CrossRef]
- Haacke, E.M.; Ayaz, M.; Khan, A.; Manova, E.S.; Krishnamurthy, B.; Gollapalli, L.; Ciulla, C.; Kim, I.; Petersen, F.; Kirsch, W. Establishing a Baseline Phase Behavior in Magnetic Resonance Imaging to Determine Normal vs. Abnormal Iron Content in the Brain. J. Magn. Reson. Imaging 2007, 26, 256–264. [Google Scholar] [CrossRef]
- Langkammer, C.; Krebs, N.; Goessler, W.; Scheurer, E.; Ebner, F.; Yen, K.; Fazekas, F.; Ropele, S. Quantitative MR imaging of brain iron: A postmortem validation study. Radiology 2010, 257, 455–462. [Google Scholar] [CrossRef]
- Mitsumori, F.; Watanabe, H.; Takaya, N.; Garwood, M.; Auerbach, E.J.; Michaeli, S.; Mangia, S. Toward understanding transverse relaxation in human brain through its field dependence. Magn. Reson. Med. 2012, 68, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Shmueli, K. Quantitative Susceptibility Mapping, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 1, ISBN 9780128170571. [Google Scholar]
- Sun, H.; Walsh, A.J.; Lebel, R.M.; Blevins, G.; Catz, I.; Lu, J.Q.; Johnson, E.S.; Emery, D.J.; Warren, K.G.; Wilman, A.H. Validation of quantitative susceptibility mapping with Perls’ iron staining for subcortical gray matter. Neuroimage 2015, 105, 486–492. [Google Scholar] [CrossRef]
- Langkammer, C.; Schweser, F.; Krebs, N.; Deistung, A.; Goessler, W.; Scheurer, E.; Sommer, K.; Reishofer, G.; Yen, K.; Fazekas, F.; et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage 2012, 62, 1593–1599. [Google Scholar] [CrossRef] [Green Version]
- Bilgic, B.; Pfefferbaum, A.; Rohlfing, T.; Sullivan, E.V.; Adalsteinsson, E. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage 2012, 59, 2625–2635. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Zhong, Y.; Xu, Y.; Qin, J.; Zhang, N.; Zhu, X.; Li, Y. Quantitative susceptibility mapping reveals an association between brain iron load and depression severity. Front. Hum. Neurosci. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, Y.; Li, Q.; Xu, J.; Yan, S.; Cai, J.; Jiaerken, Y.; Lou, M. Brain Iron Deposits in Thalamus Is an Independent Factor for Depressive Symptoms Based on Quantitative Susceptibility Mapping in an Older Adults Community Population. Front. Psychiatry 2019, 10, 734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, A.; Roediger, D.; Mueller, B.; Boys, C.; Hendrickson, T.; Schumacher, M.; Mattson, S.; Jones, K.; Riley, E.; Lim, K.; et al. Para-limbic Structural Abnormalities are Associated with Internalizing Symptoms in Children with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2020, 44, 1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [Green Version]
- Dale, A.M.; Fischl, B.; Sereno, M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999, 9, 179–194. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Rahman, H.; Arevalillo-Herráez, M.; Gdeisat, M.; Burton, D.; Lalor, M.; Lilley, F.; Moore, C.; Sheltraw, D.; Qudeisat, M. Robust three-dimensional best-path phase-unwrapping algorithm that avoids singularity loops. Appl. Opt. 2009, 48, 4582–4596. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wilman, A.H. Background field removal using spherical mean value filtering and Tikhonov regularization. Magn. Reson. Med. 2013, 71, 1151–1157. [Google Scholar] [CrossRef]
- Li, W.; Wang, N.; Yu, F.; Han, H.; Cao, W.; Romero, R.; Tantiwongkosi, B.; Duong, T.Q.; Liu, C. A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. Neuroimage 2015, 108, 111–122. [Google Scholar] [CrossRef] [Green Version]
- MATLAB, version R2018a. 9.7.0.1190202 (R2018a). The MathWorks Inc.: Natick, MA, USA, 2018.
- Li, W.; Wu, B.; Batrachenko, A.; Bancroft-Wu, V.; Morey, R.A.; Shashi, V.; Langkammer, C.; De Bellis, M.D.; Ropele, S.; Song, A.W.; et al. Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum. Brain Mapp. 2014, 35, 2698–2713. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Shi, J.; Wei, H.; Han, V.; Zhu, W.Z.; Liu, C. Neonate and infant brain development from birth to 2 years assessed using MRI-based quantitative susceptibility mapping. Neuroimage 2019, 185, 349–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, H.; Cronin, M.J.; He, N.; Yan, F.; Liu, C. Longitudinal data for magnetic susceptibility of normative human brain development and aging over the lifespan. Data Br. 2018, 20, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly accurate inverse consistent registration: A robust approach. Neuroimage 2010, 53, 1181–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.R.; Kamphaus, R.W.; Vannest, K.J. Behavior Assessment System for Children (BASC) BT—Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 366–371. ISBN 978-0-387-79948-3. [Google Scholar]
- R Core Team, version 4.0.0. R: A Language and Environment for Statistical Computing; Boston, MA, USA, 2020. [Google Scholar]
- Li, W.; Wu, B.; Avram, A.V.; Liu, C. Magnetic susceptibility anisotropy of human brain in vivo and its molecular underpinnings. Neuroimage 2012, 59, 2088–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweser, F.; Deistung, A.; Lehr, B.W.; Reichenbach, J.R. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: An approach to in vivo brain iron metabolism? Neuroimage 2011, 54, 2789–2807. [Google Scholar] [CrossRef] [PubMed]
- Dlouhy, A.C.; Outten, C.E. The iron metallome in Eukaryotic organisms. Met. Ions Life Sci. 2013, 12, 241–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Double, K.L.; Maywald, M.; Schmittel, M.; Riederer, P.; Gerlach, M. In vitro studies of ferritin iron release and neurotoxicity. J. Neurochem. 1998, 70, 2492–2499. [Google Scholar] [CrossRef]
- Raghunathan, R.; Liu, C.-H.; Kouka, A.; Singh, M.; Miranda, R.C.; Larin, K.V. Dose-response analysis of microvasculature changes in the murine fetal brain and the maternal extremities due to prenatal ethanol exposure. J. Biomed. Opt. 2020, 25, 126001. [Google Scholar] [CrossRef]
- Schenck, J.F. Health and Physiological Effects of Human Exposure to Whole-Body Four-Tesla Magnetic Fields during MRI. Ann. N. Y. Acad. Sci. 1992, 649, 285–301. [Google Scholar] [CrossRef]
- Floresco, S.B. The nucleus accumbens: An interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015, 66, 25–32. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Erikson, K.M.; Jones, B.C.; Beard, J.L. Iron Deficiency Alters Dopamine Transporter Functioning in Rat Striatum. J. Nutr. 2000, 130, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H. Neuropharmacological and neurobiological aspects of iron deficiency. In Brain, Behaviour, and Iron in the Infant Diet; Springer: Manchester, UK, 1990; pp. 83–106. ISBN 9781447117667. [Google Scholar]
- Alcantara, A.A.; Chen, V.; Herring, B.E.; Mendenhall, J.M.; Berlanga, M.L. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain Res. 2003, 986, 22–29. [Google Scholar] [CrossRef]
- Ressler, K.J. Amygdala Activity, Fear, and Anxiety: Modulation by Stress. Biol. Psychiatry 2010, 67, 1117–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaev, O.; Piletti Chatain, C.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Cabronero, J.; Betts, M.J.; Cardenas-Blanco, A.; Yang, S.; Nestor, P.J. In vivo MRI mapping of brain iron deposition across the adult lifespan. J. Neurosci. 2016, 36, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, T.; Hikida, T. Role of basal ganglia neurocircuitry in the pathology of psychiatric disorders. Psychiatry Clin. Neurosci. 2019, 73, 289–301. [Google Scholar] [CrossRef] [Green Version]
- Adisetiyo, V.; Gray, K.M.; Jensen, J.H.; Helpern, J.A. Brain iron levels in attention-deficit/hyperactivity disorder normalize as a function of psychostimulant treatment duration. NeuroImage Clin. 2019, 24, 6076–6083. [Google Scholar] [CrossRef]
- Sánchez-González, M.Á.; García-Cabezas, M.Á.; Rico, B.; Cavada, C. The primate thalamus is a key target for brain dopamine. J. Neurosci. 2005, 25, 6076–6083. [Google Scholar] [CrossRef]
- Haber, S.N. Corticostriatal circuitry. In Neuroscience in the 21st Century: From Basic to Clinical, 2nd ed.; Springer: New York, NY, USA, 2016; pp. 1721–1741. ISBN 9781493934744. [Google Scholar]
- Larsen, B.; Bourque, J.; Moore, T.M.; Adebimpe, A.; Calkins, M.E.; Elliott, M.A.; Gur, R.C.; Gur, R.E.; Moberg, P.J.; Roalf, D.R.; et al. Longitudinal development of brain iron is linked to cognition in youth. J. Neurosci. 2020, 40, 1810–1818. [Google Scholar] [CrossRef]
- Kim, J.; Wessling-Resnick, M. Iron and mechanisms of emotional behavior. J. Nutr. Biochem. 2014, 25, 1101–1107. [Google Scholar] [CrossRef] [Green Version]
- Georgieff, M.K. The role of iron in neurodevelopment: Fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008, 36, 1267–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, Q.R.; McMorris, C.A.; Kar, P.; Ritter, C.; Gibbard, W.B.; Tortorelli, C.; Lebel, C. Different brain profiles in children with prenatal alcohol exposure with or without early adverse exposures. Hum. Brain Mapp. 2020, 41, 4375–4385. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, J.R.; Fink, B.A.; Fuglestad, A.J.; Eckerle, J.K.; Boys, C.J.; Sandness, K.E.; Radke, J.P.; Miller, N.C.; Lindgren, C.; Brearley, A.M.; et al. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J. Neurodev. Disord. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Ernst, A.M.; Gimbel, B.A.; De Water, E.; Eckerle, J.K.; Radke, J.P.; Georgieff, M.K.; Wozniak, J.R. Prenatal and Postnatal Choline Supplementation in Fetal Alcohol Spectrum Disorder. Nutrients 2022, 14, 688. [Google Scholar] [CrossRef] [PubMed]
- Huebner, S.M.; Helfrich, K.K.; Saini, N.; Blohowiak, S.E.; Cheng, A.A.; Kling, P.J.; Smith, S.M. Dietary Iron Fortification Normalizes Fetal Hematology, Hepcidin, and Iron Distribution in a Rat Model of Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2018, 42, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, K.K.; Saini, N.; Kwan, S.T.C.; Rivera, O.C.; Hodges, R.; Smith, S.M. Gestational Iron Supplementation Improves Fetal Outcomes in a Rat Model of Prenatal Alcohol Exposure. Nutrients 2022, 14, 1653. [Google Scholar] [CrossRef] [PubMed]
- Astley Hemingway, S.J.; Davies, J.K.; Jirikowic, T.; Olson, E.M. What proportion of the brain structural and functional abnormalities observed among children with fetal alcohol spectrum disorder is explained by their prenatal alcohol exposure and their other prenatal and postnatal risks? Adv. Pediatric Res. 2020, 7, 41. [Google Scholar]
- Bauer, C.R.; Shankaran, S.; Bada, H.S.; Lester, B.; Wright, L.L.; Krause-Steinrauf, H.; Smeriglio, V.L.; Finnegan, L.P.; Maza, P.L.; Verter, J. The Maternal Lifestyle Study: Drug exposure during pregnancy and short-term maternal outcomes. Am. J. Obstet. Gynecol. 2002, 186, 487–495. [Google Scholar] [CrossRef]
- Lebel, C.A.; McMorris, C.A.; Kar, P.; Ritter, C.; Andre, Q.; Tortorelli, C.; Gibbard, W. Ben Characterizing adverse prenatal and postnatal experiences in children. Birth Defects Res. 2019, 111, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.D.; Martinez, Y.J.; Shumka, E.; Baker, H. Multiple informant agreement of child, parent, and teacher ratings of child anxiety within community samples. Can. J. Psychiatry 2014, 59, 34–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
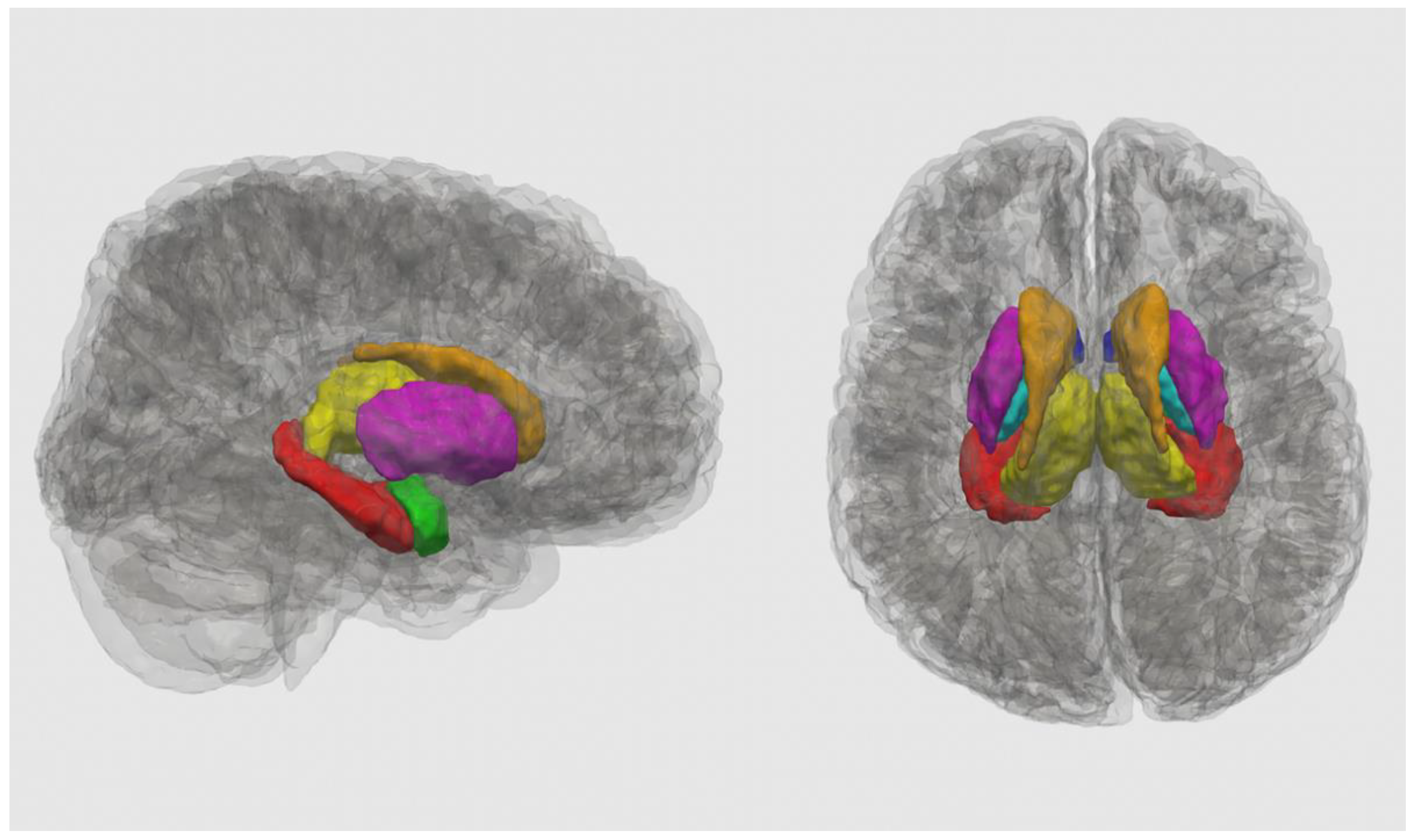

| Prenatal Alcohol Exposure (PAE) | Unexposed | Statistical Analysis | |
|---|---|---|---|
| Age ± SD | 11.18 ± 2.16 | 11.18 ± 2.27 | t (37.057) = −0.005, p = 0.996 |
| Parent/caregiver reported gender | 53%B 1, 47%G (10B, 9G) | 58%B, 43%G 2 (23B, 17G) | X2 (1) = 0.124, p = 0.725 |
| Mean Susceptibility ± SD (ppm) | ||||
|---|---|---|---|---|
| Brain Region | PAE | Control | F | p(q) |
| Caudate | 0.0236 ± 0.00565 | 0.0215 ± 0.00682 | 1.45 | 0.234 (0.546) |
| Putamen | 0.0233 ± 0.00563 | 0.0240 ± 0.00634 | 0.114 | 0.737 (0.737) |
| Pallidum | 0.0831 ± 0.0147 | 0.0816 ± 0.0182 | 0.175 | 0.677 (0.737) |
| Thalamus | 0.00322 ± 0.00374 | 0.000945 ± 0.00519 | 2.71 | 0.105 (0.368) |
| Amygdala | 0.0171 ± 0.00926 | 0.0186 ± 0.00971 | 0.367 | 0.547 (0.737) |
| Hippocampus | −0.00177 ± 0.00977 | 0.00340 ± 0.00814 | 4.57 | 0.037 * (0.259) |
| Nucleus accumbens | −0.0336 ± 0.0154 | −0.0384 ± 0.0200 | 0.918 | 0.342 (0.598) |
| Mean Volume ± SD mm3 | ||||
|---|---|---|---|---|
| Brain Region | PAE | Control | F | p(q) |
| Caudate | 6801 ± 686 | 7592 ± 738 | 16.7 | <0.001 ** (<0.001 **) |
| Putamen | 9812 ± 955 | 10,548 ± 1128 | 5.92 | 0.018 * (0.042 *) |
| Pallidum | 3189 ± 356 | 3639 ± 401 | 18.2 | <0.001 ** (<0.001 **) |
| Thalamus | 15,227± 1707 | 15,830 ± 1442 | 1.963 | 0.167 (0.234) |
| Amygdala | 3143 ± 338 | 3281 ± 396 | 1.68 | 0.201 (0.235) |
| Hippocampus | 7912 ± 915 | 8277 ± 652 | 2.90 | 0.094 (0.164) |
| Nucleus accumbens | 1212 ± 214 | 1239 ± 192 | 0.180 | 0.673 (0.673) |
| Diagnostic Group | Wilcoxon Rank Test | |||
|---|---|---|---|---|
| PAE (n = 19) | Unexposed Controls (n = 40) | |||
| Externalizing Problems composite score | Median score (IQR) 1 | 68 (10) | 50 (9) | W = 604, p < 0.001 ** |
| At risk (60–69) | 9 (47%) | 4 (10%) | ||
| Clinically significant ≥ 70 | 5 (26%) | 2 (5%) | ||
| Total | 14 (73%) | 6 (15%) | ||
| Aggression subscale | Median score (IQR) | 60 (11) | 50 (8) | W = 604, p < 0.001 ** |
| At risk (60–69) | 8 (42%) | 2 (5%) | ||
| Clinically significant ≥ 70 | 2 (11%) | 2 (5%) | ||
| Total | 10 (53%) | 4 (10%) | ||
| Conduct subscale | Median score (IQR) | 60 (16) | 51 (14) | W = 575, p = 0.002 * |
| At risk (60–69) | 5 (26%) | 5 (12.5%) | ||
| Clinically significant ≥ 70 | 5 (26%) | 3 (7.5%) | ||
| Total | 10 (52%) | 8 (20%) | ||
| Hyperactivity subscale | Median score (IQR) | 69 (14) | 50 (10) | W = 626, p < 0.001 ** |
| At risk (60–69) | 8 (42%) | 5 (12.5%) | ||
| Clinically significant ≥ 70 | 6 (32%) | 2 (5%) | ||
| Total | 14 (74%) | 8 (17.5%) | ||
| Internalizing Problems composite score | Median score (IQR) | 50 (19) | 50 (15) | W = 423, p = 0.490 |
| At risk (60–69) | 2 (11%) | 3 (7.5%) | ||
| Clinically significant ≥ 70 | 4 (21%) | 4 (10%) | ||
| Total | 6 (32%) | 7 (17.5%) | ||
| Anxiety subscale | Median score (IQR) | 49 (23) | 49 (16) | W = 372, p = 0.900 |
| At risk (60–69) | 5 (26%) | 3 (8%) | ||
| Clinically significant ≥ 70 | 2 (11%) | 5 (13%) | ||
| Total | 7 (37%) | 8 (20%) | ||
| Depression subscale | Median score (IQR) | 58 (15) | 51 (11) | W = 477, p = 0.119 |
| At risk (60–69) | 4 (21%) | 3 (8%) | ||
| Clinically significant ≥ 70 | 4 (21%) | 4 (10%) | ||
| Total | 8 (42%) | 7 (18%) | ||
| Somatization subscale | Median score (IQR) | 50 (27) | 48 (14) | W = 428, p = 0.445 |
| At risk (60–69) | 1 (5%) | 6 (15%) | ||
| Clinically significant ≥ 70 | 5 (26%) | 1 (3%) | ||
| Total | 6 (32%) | 7 (18%) | ||
| Brain Region | Main Effect of Diagnostic Group | Main Effect of Susceptibility | Group–Susceptibility Interaction 1 | ||||
|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | ||
| Internalizing Problems composite score | Caudate | 1.66 | 0.203 | 0.213 | 0.646 | ||
| Putamen | 1.87 | 0.177 | 0.048 | 0.828 | |||
| Pallidum | 1.73 | 0.194 | 3.28 | 0.076 | |||
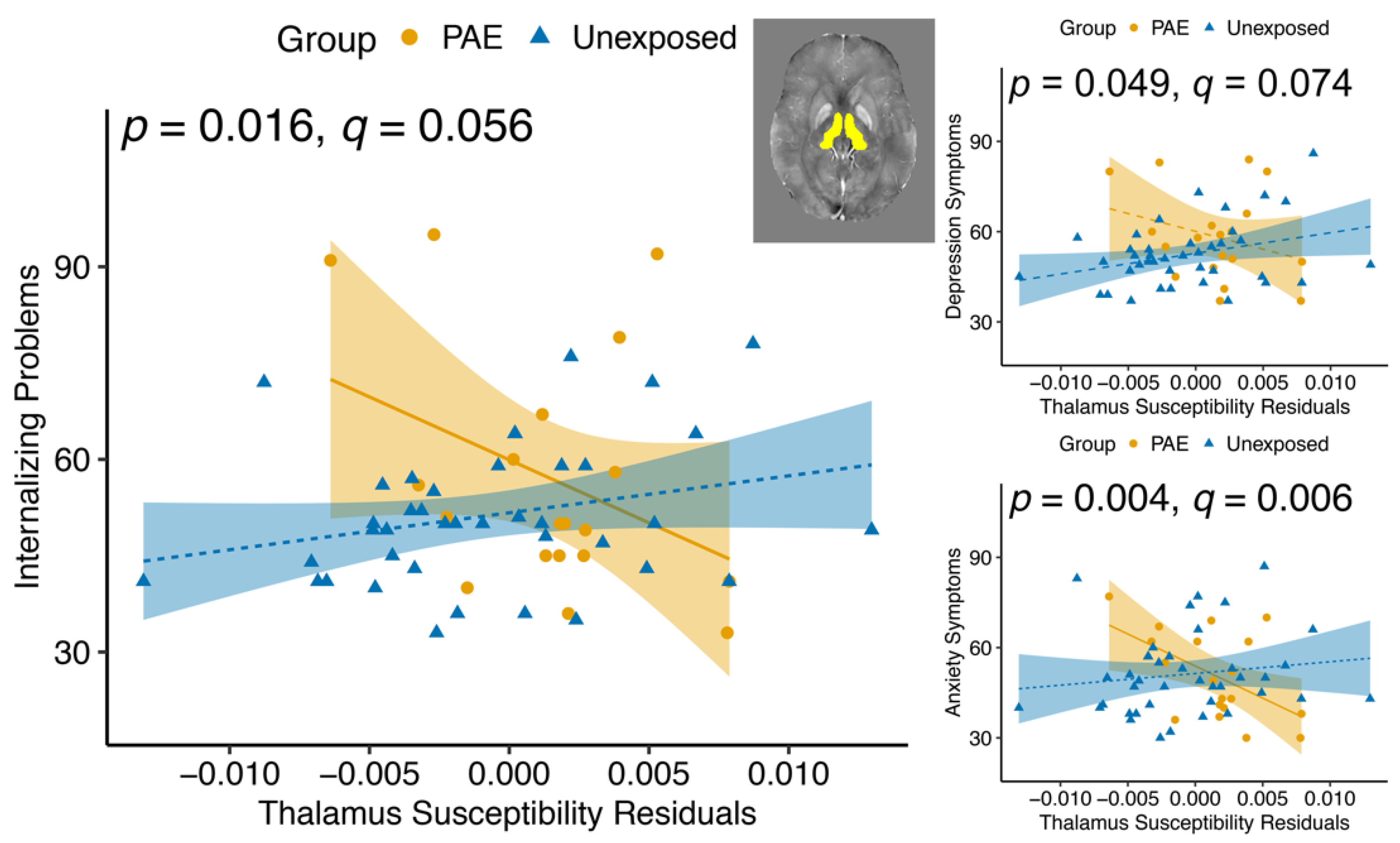
| Thalamus | 1.81 | 0.185 | 0.091 | 0.764 | 6.16 | 0.016 * | |
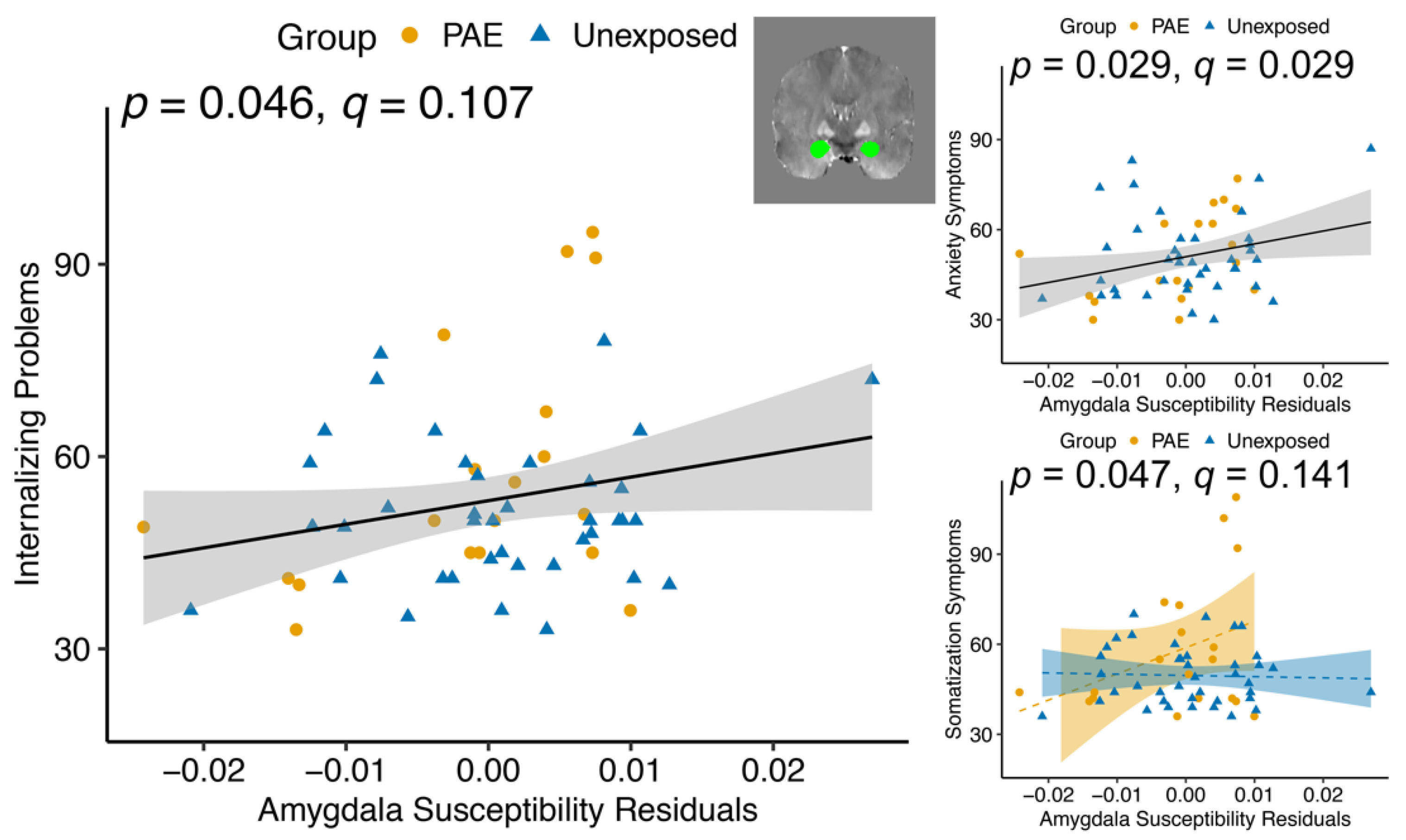
| Amygdala | 2.54 | 0.117 | 4.17 | 0.046 * | |||
| Hippocampus | 2.39 | 0.128 | 0.598 | 0.443 | |||
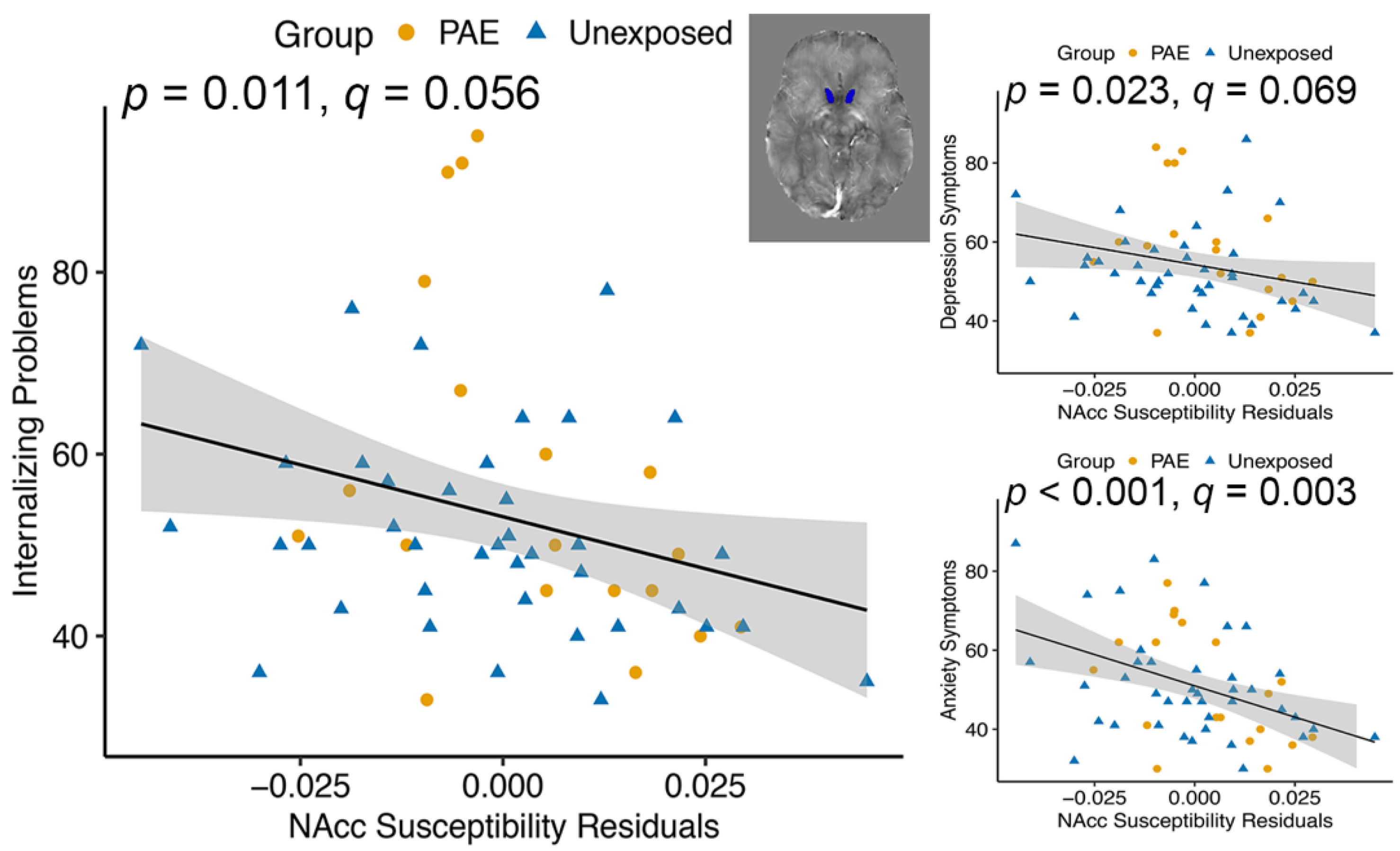
| Nucleus accumbens | 3.21 | 0.079 | 7.02 | 0.011 * | |||
| Anxiety subscale | Thalamus | 0.024 | 0.879 | 0.043 | 0.837 | 9.31 | 0.004 * |
| Amygdala | .000215 | 0.988 | 5.01 | 0.029 * | |||
| Nucleus accumbens | 0.060 | 0.808 | 12.46 | <0.001 ** | |||
| Depression subscale | Thalamus | 2.24 | 0.140 | 1.10 | 0.300 | 4.04 | 0.049 * |
| Nucleus accumbens | 4.29 | 0.043 * | 5.45 | 0.023 * | |||
| Somatization subscale | Amygdala | 4.54 | 0.038 * | 1.52 | 0.222 | 4.12 | 0.047 * |
| Externalizing Problems composite score | Caudate | 13.5 | <0.001 ** | 0.911 | 0.344 | ||
| Putamen | 16.1 | <0.001 ** | 0.432 | 0.514 | 4.50 | 0.039 * | |
| Pallidum | 14.6 | <0.001 ** | 0.225 | 0.637 | |||
| Thalamus | 12.2 | <0.001 ** | 2.64 | 0.110 | |||
| Amygdala | 14.4 | <0.001 ** | 0.471 | 0.495 | |||
| Hippocampus | 14.2 | <0.001 ** | 0.061 | 0.806 | |||
| Nucleus accumbens | 14.4 | <0.001 ** | 0.014 | 0.907 | |||
| Aggression subscale | Putamen | 14.8 | <0.001 ** | 0.332 | 0.567 | ||
| Conduct subscale | Putamen | 12.0 | 0.001 * | 3.57 | 0.062 | ||
| Hyperactivity subscale | Putamen | 20.2 | <0.001 ** | 0.883 | 0.352 | ||
| Brain Region | Main Effect of Diagnostic Group | Main Effect of Volume | |||
|---|---|---|---|---|---|
| F | p | F | p | ||
| Internalizing Problems composite score | Caudate | 1.90 | 0.174 | 0.124 | 0.728 |
| Putamen | 2.19 | 0.145 | 0.282 | 0.598 | |
| Pallidum | 1.46 | 0.232 | 0.000713 | 0.979 | |
| Thalamus | 1.55 | 0.218 | 0.370 | 0.546 | |
| Amygdala | 1.90 | 0.174 | 0.012 | 0.912 | |
| Hippocampus | 2.12 | 0.152 | 0.233 | 0.631 | |
| Nucleus accumbens | 2.33 | 0.132 | 3.52 | 0.066 | |
| Externalizing Problems composite score | Caudate | 12.4 | <0.001 ** | 0.102 | 0.750 |
| Putamen | 11.6 | 0.001 ** | 0.760 | 0.387 | |
| Pallidum | 6.04 | 0.017 * | 3.99 | 0.051 | |
| Thalamus | 12.8 | <0.001 ** | 2.39 | 0.128 | |
| Amygdala | 14.4 | <0.001 ** | 0.003 | 0.955 | |
| Hippocampus | 13.7 | <0.001 ** | 0.039 | 0.844 | |
| Nucleus accumbens | 15.6 | <0.001 ** | 1.32 | 0.256 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhid, D.; McMorris, C.A.; Sun, H.; Gibbard, B.; Tortorelli, C.; Lebel, C. Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure. Nutrients 2022, 14, 2213. https://doi.org/10.3390/nu14112213
Nakhid D, McMorris CA, Sun H, Gibbard B, Tortorelli C, Lebel C. Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure. Nutrients. 2022; 14(11):2213. https://doi.org/10.3390/nu14112213
Chicago/Turabian StyleNakhid, Daphne, Carly A. McMorris, Hongfu Sun, Ben Gibbard, Christina Tortorelli, and Catherine Lebel. 2022. "Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure" Nutrients 14, no. 11: 2213. https://doi.org/10.3390/nu14112213
APA StyleNakhid, D., McMorris, C. A., Sun, H., Gibbard, B., Tortorelli, C., & Lebel, C. (2022). Brain Iron and Mental Health Symptoms in Youth with and without Prenatal Alcohol Exposure. Nutrients, 14(11), 2213. https://doi.org/10.3390/nu14112213









