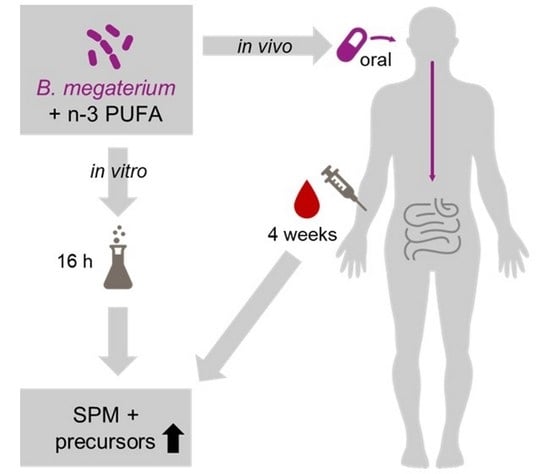
Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Cell Culture
Preparation of Liposomal n-3 PUFA
2.2. Lipid Mediator Metabololipidomics by UPLC-MS-MS
2.3. Genome Analysis
2.4. Clinical Trial—Study Design & Objective
2.4.1. Study Subjects
2.4.2. Study Product
2.4.3. Methods for Samples and Data Collection
2.4.4. Safety (Adverse Events, Concomitant Medication, and Tolerability)
2.4.5. Statistical Analysis
3. Results
3.1. Bacillus Megaterium Strains Harbor CYP102A1 (BM3) Gene Variants and Produce SPM and SPM Precursors from n-3 PUFA Lysine Salt
3.2. Human Study
3.2.1. Subject Characteristics
3.2.2. SPM Levels in Plasma after a 4-Week Intervention with B. megaterium and n-3 PUFA-Lysine Salt
3.2.3. Safety and Tolerability
4. Discussion
5. Conclusions
6. Patent Applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balk, E.M.; Lichtenstein, A.H. Omega-3 fatty acids and cardiovascular disease: Summary of the 2016 agency of healthcare research and quality evidence review. Nutrients 2017, 9, 865. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L., 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health Stat. Rep. 2015, 79, 1–16. [Google Scholar]
- Schunck, W.H.; Konkel, A.; Fischer, R.; Weylandt, K.H. Therapeutic potential of omega-3 fatty acid-derived epoxyeicosanoids in cardiovascular and inflammatory diseases. Pharmacol. Ther. 2018, 183, 177–204. [Google Scholar] [CrossRef]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel anti-inflammatory-pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution pharmacology: Opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]
- Werz, O.; Gerstmeier, J.; Libreros, S.; De la Rosa, X.; Werner, M.; Norris, P.C.; Chiang, N.; Serhan, C.N. Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat. Commun. 2018, 9, 59. [Google Scholar] [CrossRef]
- Jordan, P.M.; Werz, O. Specialized pro-resolving mediators: Biosynthesis and biological role in bacterial infections. FEBS J. 2021. [Google Scholar] [CrossRef]
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72. [Google Scholar] [CrossRef] [Green Version]
- Pochard, C.; Coquenlorge, S.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Meurette, G.; Freyssinet, M.; Neunlist, M.; Rolli-Derkinderen, M. Defects in 15-HETE production and control of epithelial permeability by human enteric glial cells from patients with Crohn’s disease. Gastroenterology 2016, 150, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Vance, R.E.; Hong, S.; Gronert, K.; Serhan, C.N.; Mekalanos, J.J. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc. Natl. Acad. Sci. USA 2004, 101, 2135–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, T.; Shibata, D.; Kino, K. Phylogenetic analysis of Bacillus P450 monooxygenases and evaluation of their activity towards steroids. Steroids 2009, 74, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, T.; Nzuza, N.; Chen, W.; Nelson, D.R.; Syed, K. Impact of lifestyle on cytochrome P450 monooxygenase repertoire is clearly evident in the bacterial phylum Firmicutes. Sci. Rep. 2020, 10, 13982. [Google Scholar] [CrossRef]
- Capdevila, J.H.; Wei, S.; Helvig, C.; Falck, J.R.; Belosludtsev, Y.; Truan, G.; Graham-Lorence, S.E.; Peterson, J.A. The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450BM-3. J. Biol. Chem. 1996, 271, 22663–22671. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Jain, A.; Marleau, S.; Clish, C.; Kantarci, A.; Behbehani, B.; Colgan, S.P.; Stahl, G.L.; Merched, A.; Petasis, N.A.; et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J. Immunol. 2003, 171, 6856–6865. [Google Scholar] [CrossRef] [Green Version]
- Prescott, D.; McKay, D.M. Aspirin-triggered lipoxin enhances macrophage phagocytosis of bacteria while inhibiting inflammatory cytokine production. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G487–G497. [Google Scholar] [CrossRef]
- Rajasagi, N.K.; Reddy, P.B.; Mulik, S.; Gjorstrup, P.; Rouse, B.T. Neuroprotectin D1 reduces the severity of herpes simplex virus-induced corneal immunopathology. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6269–6279. [Google Scholar] [CrossRef] [Green Version]
- Baillie, J.K.; Digard, P. Influenza--time to target the host? N. Engl. J. Med. 2013, 369, 191–193. [Google Scholar] [CrossRef] [Green Version]
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Wang, X.; Sun, L.; Schultzberg, M.; Hjorth, E. Can inflammation be resolved in Alzheimer’s disease? Ther. Adv. Neurol. Disord. 2018, 11, 1756286418791107. [Google Scholar] [CrossRef] [PubMed]
- Kasikara, C.; Doran, A.C.; Cai, B.; Tabas, I. The role of non-resolving inflammation in atherosclerosis. J. Clin. Investig. 2018, 128, 2713–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livne-Bar, I.; Wei, J.; Liu, H.H.; Alqawlaq, S.; Won, G.J.; Tuccitto, A.; Gronert, K.; Flanagan, J.G.; Sivak, J.M. Astrocyte-derived lipoxins A4 and B4 promote neuroprotection from acute and chronic injury. J. Clin. Investig. 2017, 127, 4403–4414. [Google Scholar] [CrossRef] [Green Version]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, H.; Hisada, T.; Ishizuka, T.; Utsugi, M.; Kawata, T.; Shimizu, Y.; Okajima, F.; Dobashi, K.; Mori, M. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem. Biophys. Res. Commun. 2008, 367, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Periz, A.; Horrillo, R.; Ferre, N.; Gronert, K.; Dong, B.; Moran-Salvador, E.; Titos, E.; Martinez-Clemente, M.; Lopez-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [Green Version]
- Perretti, M.; Norling, L.V. Actions of SPM in regulating host responses in arthritis. Mol. Asp. Med. 2017, 58, 57–64. [Google Scholar] [CrossRef]
- Perna, E.; Aguilera-Lizarraga, J.; Florens, M.V.; Jain, P.; Theofanous, S.A.; Hanning, N.; De Man, J.G.; Berg, M.; De Winter, B.; Alpizar, Y.A.; et al. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70, 1275–1286. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, X.; Zhang, X.; Huang, J.; He, J.; Peng, L.; Ye, C.; Wang, Y.; Xue, F.; Ai, D.; et al. Metabolic profiling of murine plasma reveals eicosapentaenoic acid metabolites protecting against endothelial activation and atherosclerosis. Br. J. Pharmacol. 2018, 175, 1190–1204. [Google Scholar] [CrossRef] [Green Version]
- Endo, J.; Sano, M.; Isobe, Y.; Fukuda, K.; Kang, J.X.; Arai, H.; Arita, M. 18-HEPE, an n-3 fatty acid metabolite released by macrophages, prevents pressure overload-induced maladaptive cardiac remodeling. J. Exp. Med. 2014, 211, 1673–1687. [Google Scholar] [CrossRef]
- Aung, T.; Halsey, J.; Kromhout, D.; Gerstein, H.C.; Marchioli, R.; Tavazzi, L.; Geleijnse, J.M.; Rauch, B.; Ness, A.; Galan, P.; et al. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: Meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018, 3, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Zhang, M.J.; Hellmann, J.; Kosuri, M.; Bhatnagar, A.; Spite, M. Proresolution therapy for the treatment of delayed healing of diabetic wounds. Diabetes 2013, 62, 618–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barden, A.E.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Specialized proresolving lipid mediators in humans with the metabolic syndrome after n-3 fatty acids and aspirin. Am. J. Clin. Nutr. 2015, 102, 1357–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ungaro, F.; Tacconi, C.; Massimino, L.; Corsetto, P.A.; Correale, C.; Fonteyne, P.; Piontini, A.; Garzarelli, V.; Calcaterra, F.; Della Bella, S.; et al. MFSD2A promotes endothelial generation of inflammation-resolving lipid mediators and reduces colitis in mice. Gastroenterology 2017, 153, 1363–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coquenlorge, S.; Van Landeghem, L.; Jaulin, J.; Cenac, N.; Vergnolle, N.; Duchalais, E.; Neunlist, M.; Rolli-Derkinderen, M. The arachidonic acid metabolite 11beta-ProstaglandinF2alpha controls intestinal epithelial healing: Deficiency in patients with Crohn’s disease. Sci. Rep. 2016, 6, 25203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangino, M.J.; Brounts, L.; Harms, B.; Heise, C. Lipoxin biosynthesis in inflammatory bowel disease. Prostaglandins Other Lipid Mediat. 2006, 79, 84–92. [Google Scholar] [CrossRef]
- Ringholz, F.C.; Buchanan, P.J.; Clarke, D.T.; Millar, R.G.; McDermott, M.; Linnane, B.; Harvey, B.J.; McNally, P.; Urbach, V. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur. Respir. J. 2014, 44, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Werner, M.; Jordan, P.M.; Romp, E.; Czapka, A.; Rao, Z.; Kretzer, C.; Koeberle, A.; Garscha, U.; Pace, S.; Claesson, H.E.; et al. Targeting biosynthetic networks of the proinflammatory and proresolving lipid metabolome. FASEB J. 2019, 33, 6140–6153. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Horn, T.; Adel, S.; Schumann, R.; Sur, S.; Kakularam, K.R.; Polamarasetty, A.; Redanna, P.; Kuhn, H.; Heydeck, D. Evolutionary aspects of lipoxygenases and genetic diversity of human leukotriene signaling. Prog. Lipid Res. 2015, 57, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Thistlethwaite, S.; Jeffreys, L.N.; Girvan, H.M.; McLean, K.J.; Munro, A.W. A promiscuous bacterial P450: The unparalleled diversity of BM3 in pharmaceutical metabolism. Int. J. Mol. Sci. 2021, 22, 11380. [Google Scholar] [CrossRef] [PubMed]
- Hazards, E.P.o.B.; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e06174. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Gupta, S.K.; Perretti, M.; Godson, C.; Brennan, E.; Li, Y.; Soehnlein, O.; Shimizu, T.; Werz, O.; Chiurchiu, V.; et al. The Atlas of Inflammation Resolution (AIR). Mol. Asp. Med. 2020, 74, 100894. [Google Scholar] [CrossRef]
- Ajabnoor, S.M.; Thorpe, G.; Abdelhamid, A.; Hooper, L. Long-term effects of increasing omega-3, omega-6 and total polyunsaturated fats on inflammatory bowel disease and markers of inflammation: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 2293–2316. [Google Scholar] [CrossRef]
- Mullin, G.E.; Limketkai, B.N.; Parian, A.M. Fish oil for inflammatory bowel disease: Panacea or placebo? Gastroenterol. Clin. N. Am. 2021, 50, 169–182. [Google Scholar] [CrossRef]
- Brint, E.K.; MacSharry, J.; Fanning, A.; Shanahan, F.; Quigley, E.M. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 329–336. [Google Scholar] [CrossRef]
- Wang, C.W.; Colas, R.A.; Dalli, J.; Arnardottir, H.H.; Nguyen, D.; Hasturk, H.; Chiang, N.; Van Dyke, T.E.; Serhan, C.N. Maresin 1 biosynthesis and proresolving anti-infective functions with human-localized aggressive periodontitis leukocytes. Infect. Immun. 2015, 84, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Albenberg, L.; Esipova, T.V.; Judge, C.P.; Bittinger, K.; Chen, J.; Laughlin, A.; Grunberg, S.; Baldassano, R.N.; Lewis, J.D.; Li, H.; et al. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 2014, 147, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Borgo, F.; Garbossa, S.; Riva, A.; Severgnini, M.; Luigiano, C.; Benetti, A.; Pontiroli, A.E.; Morace, G.; Borghi, E. Body mass index and sex affect diverse microbial niches within the gut. Front. Microbiol. 2018, 9, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avgerinos, A.; Kalantzis, N.; Rekoumis, G.; Pallikaris, G.; Arapakis, G.; Kanaghinis, T. Bowel preparation and the risk of explosion during colonoscopic polypectomy. Gut 1984, 25, 361–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levitt, M.D. Volume and composition of human intestinal gas determined by means of an intestinal washout technic. N. Engl. J. Med. 1971, 284, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.L.; Serhan, C.N.; Colgan, S.P. Antimicrobial aspects of inflammatory resolution in the mucosa: A role for proresolving mediators. J. Immunol. 2011, 187, 3475–3481. [Google Scholar] [CrossRef] [Green Version]
- Arita, M.; Clish, C.B.; Serhan, C.N. The contributions of aspirin and microbial oxygenase to the biosynthesis of anti-inflammatory resolvins: Novel oxygenase products from omega-3 polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 2005, 338, 149–157. [Google Scholar] [CrossRef]
- Rohwer, N.; Chiu, C.Y.; Huang, D.; Smyl, C.; Rothe, M.; Rund, K.M.; Helge Schebb, N.; Kuhn, H.; Weylandt, K.H. Omega-3 fatty acids protect from colitis via an Alox15-derived eicosanoid. FASEB J. 2021, 35, e21491. [Google Scholar] [CrossRef]
- Bento, A.F.; Claudino, R.F.; Dutra, R.C.; Marcon, R.; Calixto, J.B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J. Immunol. 2011, 187, 1957–1969. [Google Scholar] [CrossRef] [Green Version]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.; Calixto, J.B. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef] [Green Version]
- Irun, P.; Lanas, A.; Piazuelo, E. Omega-3 polyunsaturated fatty acids and their bioactive metabolites in gastrointestinal malignancies related to unresolved inflammation. A review. Front. Pharmacol. 2019, 10, 852. [Google Scholar] [CrossRef]






| Program | CLC Genomics Genomics Workbench 21.0.4 BLASTp |
|---|---|
| Blast version | 2.9.0+ |
| Scoring Matrix | BLOSUM62 (BLOcks SUbstitution Matrix) (Existence 11, Extension 1) |
| Minimum query coverage | 60% |
| Minimum identity | 70% |
| Word size | 3 |
| Maximum number of hits versus a database | 1500 |
| Variables | Study Population |
|---|---|
| Age (years) | 51.0 (47.7–54.3) |
| BMI (kg/m2) | 28.3 (27.1–29.5) |
| Cholesterol (mg/dL) | 209.5 (189.6–229.4) |
| LDL-cholesterol (mg/dL) | 135.1 (116.4–153.8) |
| GPT (U/L) | 27.7 (20.9–34.4) |
| GOT (U/L) | 20.8 (17.9–23.7) |
| Systolic blood pressure | 131.2 (123.2–139.2) |
| Diastolic blood pressure | 87.3 (83.4–91.3) |
| n-3 PUFA index in erythrocytes | 4.9 (4.6–5.2) |
| Variables | Baseline | 24 h | 1 Week | 2 Weeks | 4 Weeks |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| EPA [%] | 0.85 (0.71–0.99) | 0.84 (0.70–0.97) | 1.06 (0.90–1.21) p = 0.0002 | 0.98 (0.85–1.12) p = 0.0008 | 1.08 (0.93–1.22) p = 0.0006 |
| DHA [%] | 2.52 (2.30–2.73) | 2.46 (2.26–2-67) | 2.73 (2.47–2.99) p = 0.0055 | 2.81 (2.57–3.04) p = 0.0029 | 2.84 (2.57–3.11) p = 0.0006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speckmann, B.; Kleinbölting, J.; Börner, F.; Jordan, P.M.; Werz, O.; Pelzer, S.; tom Dieck, H.; Wagner, T.; Schön, C. Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators. Nutrients 2022, 14, 2265. https://doi.org/10.3390/nu14112265
Speckmann B, Kleinbölting J, Börner F, Jordan PM, Werz O, Pelzer S, tom Dieck H, Wagner T, Schön C. Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators. Nutrients. 2022; 14(11):2265. https://doi.org/10.3390/nu14112265
Chicago/Turabian StyleSpeckmann, Bodo, Jessica Kleinbölting, Friedemann Börner, Paul M. Jordan, Oliver Werz, Stefan Pelzer, Heike tom Dieck, Tanja Wagner, and Christiane Schön. 2022. "Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators" Nutrients 14, no. 11: 2265. https://doi.org/10.3390/nu14112265
APA StyleSpeckmann, B., Kleinbölting, J., Börner, F., Jordan, P. M., Werz, O., Pelzer, S., tom Dieck, H., Wagner, T., & Schön, C. (2022). Synbiotic Compositions of Bacillus megaterium and Polyunsaturated Fatty Acid Salt Enable Self-Sufficient Production of Specialized Pro-Resolving Mediators. Nutrients, 14(11), 2265. https://doi.org/10.3390/nu14112265