Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases
Abstract
:1. Introduction
2. Chemical Compounds Present in Bee Products and Their Cardioprotective Potential
2.1. Propolis
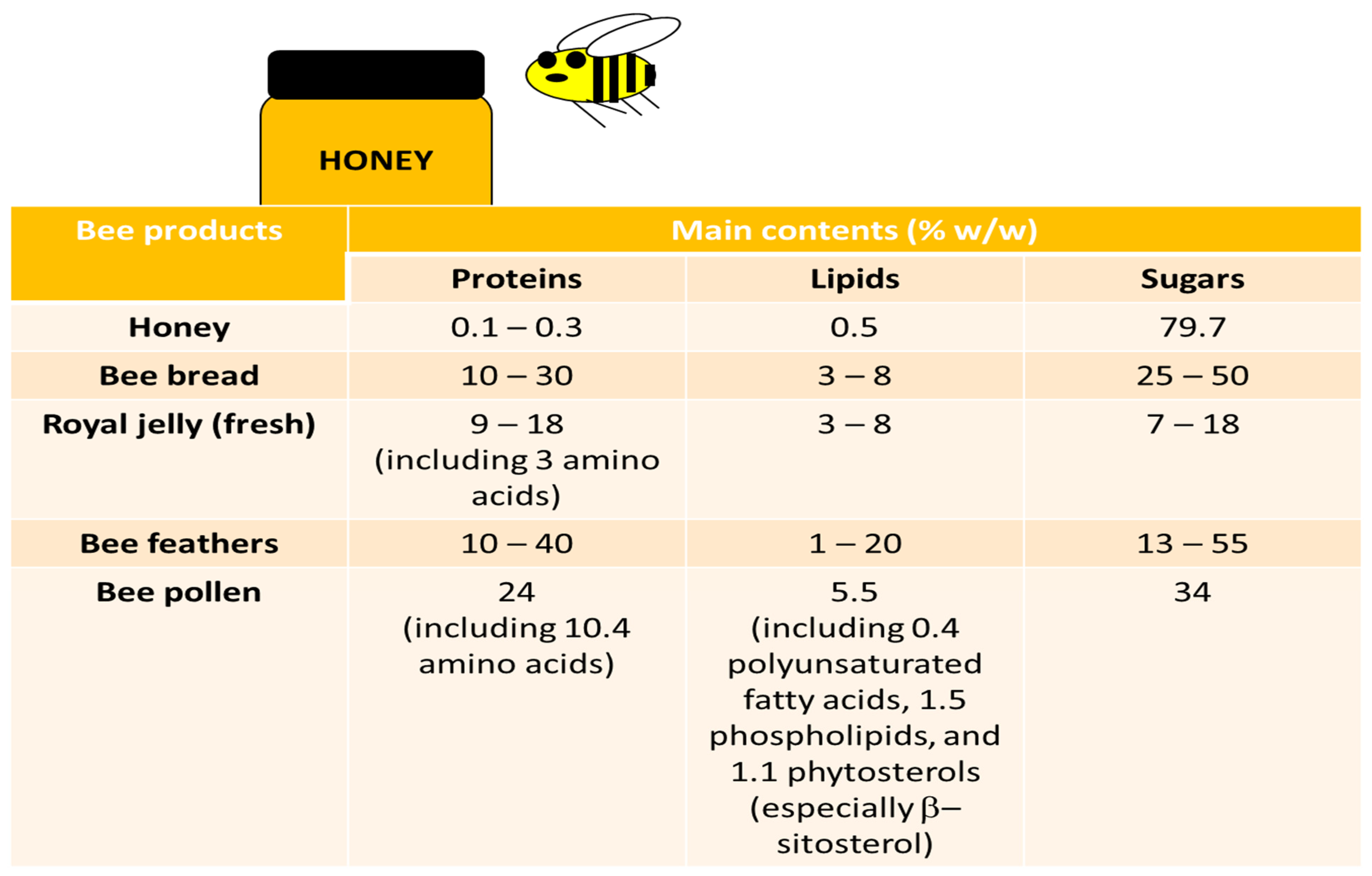
2.2. Bee Pollen
2.3. Royal Jelly
2.4. Bee Venom
2.5. Bee Bread
3. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Fratellone, P.M.; Tsimis, F.; Fratellone, G. Apitherapy Products for Medicinal Use. J. Altern. Complement. Med. 2016, 22, 1020–1022. [Google Scholar] [CrossRef]
- Khalil, M.; Sulaiman, S.A. The potential role of honey and its polyphenols in preventing heart disease: A review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Battino, M. Honey as a source of dietary antioxidants: Structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013, 20, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and modern uses of natural honey in human diseases: A review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar] [PubMed]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [Green Version]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, S.; Jain, M.K.; Nayak, L. Thrombosis. In Concise Guide to Hematology; Lazarys, H.M., Schmaier, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 149–161. [Google Scholar]
- Shen, Z.; Geng, Q.; Huang, H.; Yao, H.; Du, T.; Chen, L.; Wu, Z.; Miao, X.; Shi, P. Antioxidative and cardioprotective effects of Schisandra chinensis bee pollen extract on isoprenaline-induced myocardial infarction in rats. Molecules 2019, 24, 1090. [Google Scholar] [CrossRef] [Green Version]
- Flora, G.D.; Nayak, M.K. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr. Pharm. Des. 2019, 25, 4063–4084. [Google Scholar] [CrossRef]
- Shibata, K.; Hashimoto, T.; Miyazaki, T.; Miyazaki, A.; Nobe, K. Thrombolytic Therapy for Acute Ischemic Stroke: Past and Future. Curr. Pharm. Des. 2019, 25, 242–250. [Google Scholar] [CrossRef]
- Zampelas, A.; Magriplis, E. Dietary patterns and risk of cardiovascular diseases: A review of the evidence. Proc. Nutr. Soc. 2019, 79, 68–75. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.L.; Panyoyai, N.; Kasapis, S.; Pang, E.; Mantri, N. Honey and its role in relieving multiple facets of atherosclerosis. Nutrients 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohn, C.H.; Kim, W.; Ahn, S.; Oh, B.J.; Kim, W.Y.; Lim, K.S. Three cases of mad-honey poisoning presenting with cardiovascular emergencies. J. Korean Soc. Emerg. Med. 2005, 1, 322–325. [Google Scholar]
- Ko, Y.G.; Kim, K.H.; Kim, A.J.; Shin, D.W.; Park, J.S.; Roh, J.Y.; Ahn, J.Y. Two cases of mad-honey poisoning with cardiovascular symptom. J. Korean Soc. Clin. Toxic. 2006, 4, 78–81. [Google Scholar]
- Shrestha, T.M.; Nepal, G.; Shing, Y.K.; Shrestha, L. Cardiovascular, psychiatric, and neurological phenomena seen in mad honey disease: A clinical case report. Clin. Case Rep. 2018, 6, 2355–2357. [Google Scholar] [CrossRef] [Green Version]
- Setareh-Shenas, S.; Kaplin, S.; Bania, T.C.; Kornberg, R.A. Rare case of mad honey disease: A reversible cause of complete heart block. JACC Case Rep. 2019, 1, 579–582. [Google Scholar] [CrossRef]
- Tolba, M.F.; Omar, H.; Azab, S.S.; Khalifa, A.; Abdel-Naim, A.B.; Abdel-Rahman, S.Z. Caffeic Acid Phenethyl Ester: A Review of Its Antioxidant Activity, Protective Effects against Ischemia-reperfusion Injury and Drug Adverse Reactions. Crit. Rev. Food Sci. Nutr. 2014, 56, 2183–2190. [Google Scholar] [CrossRef]
- Bojic, M.; Antolic, A.; Tomicic, M.; Debeljak, Z.; Males, Z. Propolis ethanolic extracts reduce adenosine diphospate induced platelet aggregation determined on whole blood. Nutr. J. 2018, 17, 52. [Google Scholar] [CrossRef] [Green Version]
- Majiene, D.; Trumbeckaite, S.; Savickas, A.; Toleikis, A. Influence of propolis water solution on heart mitochondrial function. J. Pharm. Pharmacol. 2006, 58, 709–713. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, T.T.; Zhang, W.F.; Wang, J.F.; Wu, Y.P. Inhibitory effect of propolis on platelet aggregation in vitro. J. Healthc. Eng. 2017, 10, 3050895. [Google Scholar] [CrossRef]
- Bojic, M.; Debeljak, Z.; Medic-Saric, M.; Tomicic, M. Interference of selected flavonoid aglycons in platelet aggregation assays. Clin. Chem. Lab. Med. 2012, 50, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, G.P.; Wolfram, S.; Lovegrove, J.A.; Gibbins, J.M. Ingestion of queretin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J. Thromb. Haemost. 2004, 2, 2138–2145. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- He, T.; Sui, X.; Sun, W.; Yang, P.; Sui, D.; Cui, H.; Wang, W.; Wang, X.; Liu, Y.; Sun, G. The protective effects and mechanism of total flavonoids of propolis on pathological cardiac hypertrophy and heart failure in mice. J. Am. Coll. Cardiol. 2018, 72, C161. [Google Scholar] [CrossRef]
- Chao, J.; Yongming, P.; Songtao, X.; Chen, Y.; Jian, J.; Minli, C.; Fuliang, H. Propolis ameliorates restenosis in hypercholesterolemia rabbits with carotid ballon injury by inhibiting lipid accumulation, oxidative stress, and TLR4/NF-κB pathway. J. Food Biochem. 2021, 45, e13577. [Google Scholar]
- Wang, H.H.; Zeng, J.; Wang, H.Z.; Jiang, Y.X.; Wang, J.; Zhou, P.P. Effects of total flavonoids of propolis on apoptosis of myocardial cells of chronic heart failure and its possible mechanism in rats. Chin. J. Appl. Physiol. 2015, 31, 201–206. [Google Scholar]
- Kedzia, B. Chemical composition and adoptogenic activity of honey bee-collected pollen. Part I. Chemical Composition. Postepy Fitoter. 2008, 1, 47–58. [Google Scholar]
- Rzepecka-Stojko, A.; Stojko, J.; Jasik, K.; Buszman, E. Anti-atherogenic activity of polyphenol-rich extract from bee pollen. Nutrients 2017, 9, 1369. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.; Geng, Q.; Chen, L.; Du, T.; Lin, Y.; Lai, R.; Meng, F.; Wu, Z.; Miao, X.; Yao, H. Schisandra chinenis bee pollen’s chemical profiles and protective effect against H2O2-induced apoptosis in H9c2 cardiomyocytes. BMC Compl. Med. Therap. 2020, 20, 274. [Google Scholar]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef]
- Tokunaga, K.H.; Yoshida, C.; Suzuki, K.M.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004, 27, 189–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholestrolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New insights into the biological and pharmaceutical properties of royal jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Dong, J.; Ren, X.; Qin, J. Antioxidant activities of ethanol extracts from ten kinds of bee pollens. Food Sci. 2008, 29, 75–79. [Google Scholar]
- Zhang, H.C.; Wang, X.; Wang, K.; Li, C.Y. Antioxidant and tyrosine inhibitory properties of aqueous ethanol extracts from monofloral bee pollen. J. Apic. Sci. 2015, 59, 119–128. [Google Scholar]
- Kasianenko, V.I.; Komisarenko, I.A.; Dubtsova, E.A. Correction of atherogenic dyslipidemia with honey, pollen and bee bread in patients with different body mass. Ter. Arkhiv 2011, 83, 58–62. [Google Scholar]
- Gulhan, M.F. Therapeutic potentials of propolis and pollen on biochemical changes in reproductive function of L-NAME induced hypertensive male rats. Clin. Exp. Hypertens. 2018, 41, 292–298. [Google Scholar] [CrossRef]
- Fan, P.; Hn, B.; Feng, M.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and proteomic investigations reveal major royal jelly protein 1 associated with anti-hypertension activity in mouse vascular smooth muscle cells. Sci. Rep. 2016, 6, 30230. [Google Scholar] [CrossRef] [Green Version]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, propolis, and royal jelly: A comprehensive review of their biological actions and health benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemistry and Bioactivities of Royal Jelly. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 261–290. [Google Scholar]
- Vittek, J. Effect of royal jelly on serum lipids in experimental animals and humans with atherosclerosis. Experientia 1995, 51, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Mack, W.J.; Krauss, R.M.; Hodis, H.N. Lipoprotein subclasses in the Monitored Atheroscerosis Regression Study (MARS): Treatment effects and relation to coronary angiographic progression. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol 2007, 53, 345–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, H.F.; Chen, B.K.; Lu, Y.Y.; Han, Y.C.; Shen, Y.C.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Hypocholesterolemic efficacy of royal jelly in healthy mild hypercholesterolemic adults. Pharm. Biol. 2017, 55, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S. Greek-origin royal yelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef]
- Hadi, A.; Najafgholizadeh, A.; Aydenlu, E.S.; Shafiei, Z.; Pirivand, F.; Golpour, S.; Pourmasoumi, M. Royal jelly is an effective and relatively safe alternative approach to blood lipid modulation: A meta-analysis. J. Funct. Foods 2018, 41, 202–209. [Google Scholar] [CrossRef]
- Aslan, A.; Beyaz, S.; Gok, O.; Can, M.I.; Parlak, G.; Ozercan, I.H.; Gundogdu, R. Royal jelly abrogates fluoride-induced oxidative damage in rat heart tissue by activating of the nrf-2/NF-κB and bcl-2/bax pathway. Toxic. Mech. Meth. 2021, 31, 644–654. [Google Scholar] [CrossRef]
- Georgiev, D.; Goudev, A.R.; Manassiev, N.; Metka, M.; Huber, J.C. Effects of an herbal medication containing bee products on menopausal symptoms and cardiovascular risk markers: Results of a pilot open-uncontrolled trial. MedGenMed Medscape Gen. Med. 2004, 6, 46. [Google Scholar]
- Ali, M.A.A.S.M. Studies on bee venom and its medical uses. Int. J. Adv. Technol. 2012, 1, 69–83. [Google Scholar]
- Moreno, M.; Giralt, E. Three valuable peptides from bee and wasp venoms for therapeutic and biotechnological use: Melittin, apamin and mastoparan. Toxins 2015, 7, 1126–1150. [Google Scholar] [CrossRef] [Green Version]
- Yook, T.H.; Yu, J.S.; Jung, H.S. Effects of sweet bee venom and bee venom on the heart rate variability. J. Pharmacopunct. 2008, 11, 41–54. [Google Scholar] [CrossRef]
- Guimaraes, J.V.; Costa, R.S.; Machado, B.H.; dos Reis, M.A. Cardiovascular profile after intravenous injection of Africanized bee venom in awake rats. Rev. Inst. Med. 2004, 46, 55–58. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xiao, A.; Liu, W.; Shang, Y.; An, J. Melittin ameliorates CVB3-induced myocarditis via activation of the HDAC2-mediated GSH-3β/Nrf2/ARE signaling pathway. Biochem. Biohys. Res. Comm. 2016, 480, 126–131. [Google Scholar] [CrossRef]
- Nagai, T.; Nagashima, T.; Suzuki, N.; Inoue, R. Antioxidant activity and angiotensin I-converting enzyme inhibition by enzymatic hydrolasates from bee bread. Z. Naturforsch. C J. Biosci. 2005, 60, 133–138. [Google Scholar] [CrossRef]

| Bee Product | Investigated Roles | References |
|---|---|---|
| Propolis | ||
| Water extract (CAPE was the highest in this extract, followed by galangin, ferulic acid, quercetin, kaempferol, and apigenin): 25 and 300 mg/L | Anti-aggregatory potential (in vitro; three healthy volunteers) | [22] |
| Ethanol extract (content of total phenolic compounds: 136.14 mg/g, and content of total flavonoids: 19.28 mg/g): 5–10.4 mM | Anti-aggregatory potential (in vitro; ten healthy volunteers) | [20] |
| Propolis (3-O-acetyl pinobanksin, chrysin, pinocembrin, pinobanksin, and CAPE—the five most abundant components): 125 and 250 mg/kg/day | Reducing body weight and the level of blood lipids (in vivo; hypercholesterolemic rabbits (n = 24)) | [26] |
| Total flavonoids of propolis: 25 and 50 mg/kg/day | Attenuating adverse cardiac dysfunction and hypertrophy (in vivo, mice) | [27] |
| Total flavonoids of propolis | Inhibitory action on apoptosis of myocardial cells of chronic heart failure (in vivo; rats (n = 6)) | [28] |
| Proplis water solution (total phenolics: 188 mg/mL): 9, 33, 63 and 125 µg/mL | Inhibitory effect on mitochondrial respiration (in vitro; heart mitochondria) | [21] |
| Bee pollen | ||
| Bee pollen of S. chinensis (Turcz.) extract (two carbohydrates, three nucleotides, and nine quinic-acid-containing derivatives were identified): 12.5, 25 and 50 µg/mL | Antioxidant effect (in vitro; H9c2 cardiomyocytes) | [31] |
| Bee pollen of S. chinensis (Turcz.) extract (one major compound was identified as uridine): 270, 600, 1200 and 1800 mg/kg/day | Antioxidant and cardioprotective effect (in vivo, rats with myocardial infarction induced by isoprenaline, (n = 36)) | [8] |
| Polyphenol-rich extract from bee pollen (chemical content: undefined): 0.1 and 1 g/kg BM | Antioxidant and anti-atherogenic effect (in vivo, Apo-knockout mice with atherosclerosis induced by a high-fat diet, (n = 60)) | [30] |
| Royal jelly | ||
| Major royal jelly protein 1 (chemical content: undefined) | Anti-hypertension effect (in vitro, mouse vascular muscle cells (n = 3)) | [40] |
| Royal jelly (chemical content: the total protein—142.8 ± 0.35 mg/g; MRJP1 and 2—two major proteins), used doses: 350 mg/capsule, 3 months | Anti-hypercholesterolemic effect (in vivo, healthy mildly hypercholesterolemic adults (n = 40)) | [47] |
| Royal jelly, used doses: 150 mg/day for three months | Anti-hypercholesterolemic effect (in vivo, postmenopausal healthy women (n = 36)) | [48] |
| Royal jelly, used doses: 100 mg/kg five times a week for 8 weeks | Antioxidant action (in vivo, rats (n = 42)) | [50] |
| MRJP1from royal jelly, used doses: 600 mg/kg/day, for 7 days | Anti-hypercholesterolemic effect (in vivo, rats (n = 10)) | [34] |
| Different bee products (together) | ||
| Honey, pollen, and bee bread (chemical content and used doses: undefined) | Hypolipidemic effect for two tested groups: (1) patients taking bee pollen and honey; (2) patients taking bee bread (in vivo, patients with atherogenic dyslipidemia (n = 157)) | [38] |
| Bee pollen and propolis as ethanolic extracts (chemical content and used doses: undefined) | Anti-hypertension effect (in vivo, rats with hypertension induced by L-NAME (n = 28)) | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B. Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases. Nutrients 2022, 14, 2267. https://doi.org/10.3390/nu14112267
Olas B. Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases. Nutrients. 2022; 14(11):2267. https://doi.org/10.3390/nu14112267
Chicago/Turabian StyleOlas, Beata. 2022. "Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases" Nutrients 14, no. 11: 2267. https://doi.org/10.3390/nu14112267
APA StyleOlas, B. (2022). Bee Products as Interesting Natural Agents for the Prevention and Treatment of Common Cardiovascular Diseases. Nutrients, 14(11), 2267. https://doi.org/10.3390/nu14112267






