Oral Cholecalciferol Supplementation in Sahara Black People with Chronic Kidney Disease Modulates Cytokine Storm, Oxidative Stress Damage and Athero-Thromboembolic Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Informed Consent Statement
2.2. Participants and Study Design
- -
- Group D2B: 2000 IU/day/24 weeks (n = 78);
- -
- Group D60B: 60,000 IU /month/36 weeks (n = 72);
- -
- Group D2W: 2000 IU/day/24 weeks (n = 77);
- -
- Group D60W: 60,000 IU /month/36 weeks (n = 79).
2.3. Clinical Vitamin D3 Supplementation Protocol
2.4. Chronic Kidney Disease Screening
2.5. Metabolic Syndrome Clusters Screening
2.6. Blood Samples Analysis Methods
2.7. Serum Oxidative Stress Screening
2.8. Serum 25-OH Vitamin D and 1, 25(OH) 2D Assessment
2.9. Statistical Analysis
3. Results
3.1. Clinical Cohort Characterization
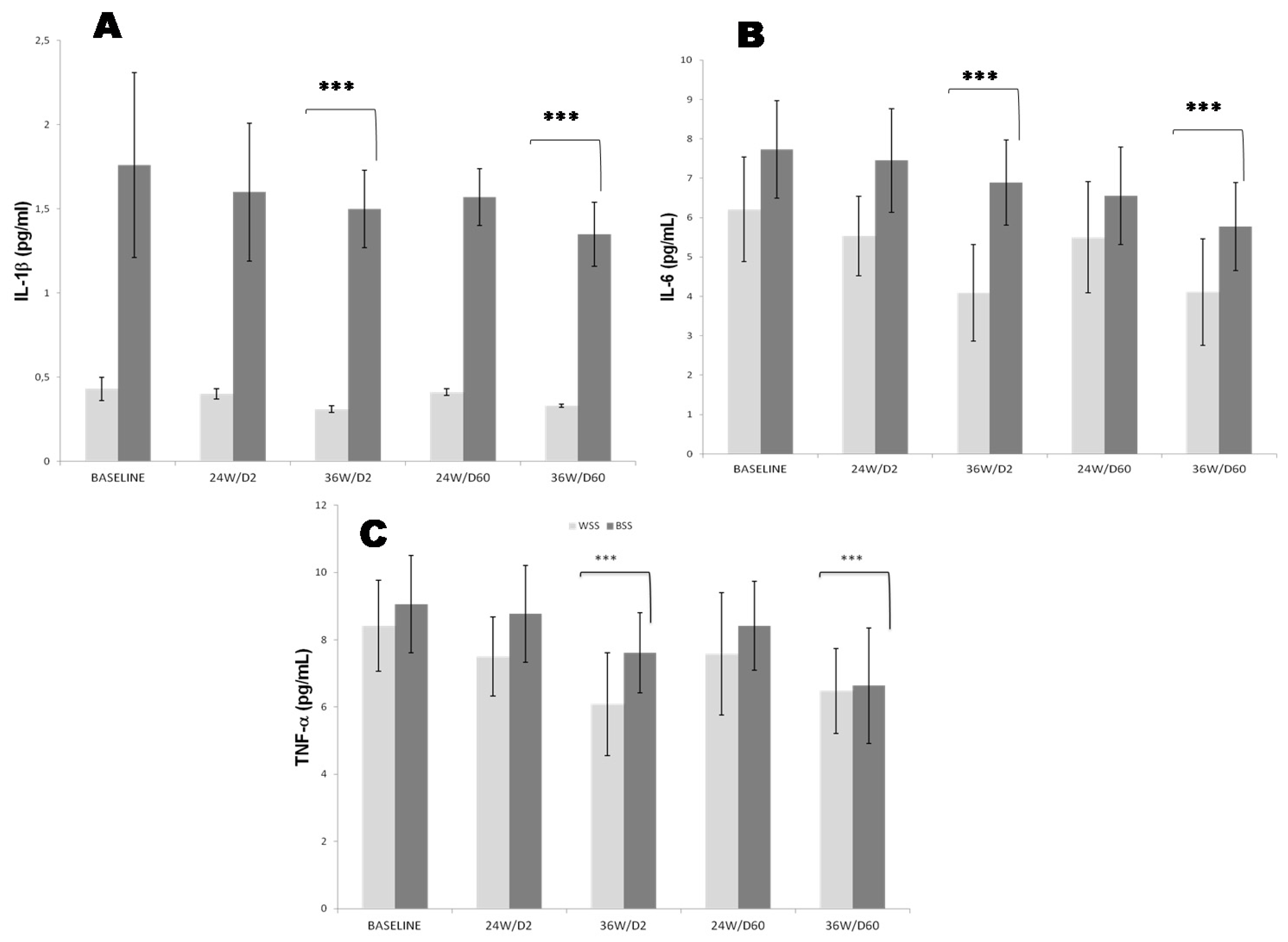
3.2. Effects of 25OHD-S on the Inflammation Status
3.2.1. Systemic Inflammation
3.2.2. Pro Inflammatory Cytokines Profile
3.3. Effects of 25OHD-S on Oxidative Stress Imbalance Status
3.3.1. Total Antioxidant Status (TAS), Malondialdehyde (MDA) Levels and Antioxidant Enzyme Activities Profile
3.3.2. Antioxidant Trace Elements Profile
3.4. Effects of 25OHD-S on MetS Components
3.5. Effects of 25OHD-S on CKD Clusters
3.6. Effects of 25OHD-S on Atherothromboembolic Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, L.C. Color in Northen Africa. Daedalus, Color and Race. Am. Acad. Arts Sci. 1967, 96, 464–482. [Google Scholar]
- Temmar, M.; Labat, C.; Benkhedda, S.; Charifi, M.; Thomas, F.; Bouafia, M.T.; Bean, K.; Darne, B.; Safar, M.E.; Benetos, A. Prevalence and determinants of hypertension in the Algerian Sahara. J. Hypertens. 2007, 25, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Levey, A.S.; Titan, S.M.; Powe, N.R.; Coresh, J.; Inker, L.A. Kidney Disease, Race, and GFR Estimation. Clin. J. Am. Soc. Nephrol. 2020, 15, 1203–1212. [Google Scholar] [CrossRef]
- Cheung, A.K.; Chang, T.I.; Cushman, W.C.; Furth, S.L.; Hou, F.F.; Ix, J.H.; Knoll, G.A.; Muntner, P.; Pecoits-Filho, R.; Sarnak, M.J.; et al. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Juppner, H.; et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef]
- Fukagawa, M.; Nii-Kono, T.; Kazama, J.J. Role of fibroblast growth factor 23 in health and in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2005, 14, 325–329. [Google Scholar] [CrossRef]
- Gutierrez, O.M.; Isakova, T.; Andress, D.L.; Levin, A.; Wolf, M. Prevalence and severity of disordered mineral metabolism in Blacks with chronic kidney disease. Kidney Int. 2008, 73, 956–962. [Google Scholar] [CrossRef]
- Isakova, T. Racial differences in parathyroid hormone levels in CKD. Nephrol Dial. Transpl. 2012, 27, 2616–2617. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. CRIC Study Investigators: Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef]
- Petreski, T.; Pikon, N.; Ekart, R.; Hojs, R.; Bevc, S. Review on Inflammation Markers in Chronic Kidney Disease. Biomedicines 2021, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Tbahriti, H.F.; Meknassi, D.; Moussaoui, R.; Messaoudi, A.; Zemour, L.; Kaddous, A.; Bouchenak, M.; Mekki, K. Inflammatory status in chronic renal failure: The role of homocysteinemia and pro-inflammatory cytokines. World J. Nephrol. 2013, 2, 231–237. [Google Scholar] [CrossRef]
- Ugovšek, S.; Šebeštjen, M. Lipoprotein(a)—The Crossroads of Atherosclerosis, Atherothrombosis and Inflammation. Biomolecules 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.P.; Dounousi, E. Chronic Kidney Disease and Disproportionally Increased Cardiovascular Damage: Does Oxidative Stress Explain the Burden? Oxid. Med. Cell Longev. 2017, 2017, 9036450. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant Mechanisms in Renal Injury and Disease. Antioxid Redox. Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Mal’tseva, V.N.; Goltyaev, M.V.; Turovsky, E.A.; Varlamova, E.G. Immunomodulatory and Anti-Inflammatory Properties of Selenium-Containing Agents: Their Role in the Regulation of Defense Mechanisms against COVID-19. Int. J. Mol. Sci. 2022, 23, 2360. [Google Scholar] [CrossRef]
- Liu, W.C.; Zheng, C.M.; Lu, C.L.; Lin, Y.F.; Shyu, J.F.; Wu, C.C.; Lu, K.C. Vitamin D and immune function in chronic kidney disease. Clin. Chim. Acta 2015, 450, 135–144. [Google Scholar] [CrossRef]
- Norris, K.C.; Olabisi, O.; Barnett, M.E.; Meng, Y.X.; Martins, D.; Obialo, C.; Lee, J.E.; Nicholas, S.B. The Role of Vitamin D and Oxidative Stress in Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2018, 15, 2701. [Google Scholar] [CrossRef]
- Arora, J.; Wang, J.; Weaver, V.; Zhang, Y.; Cantorna, M.T. Novel insight into the role of the vitamin D receptor in the development and function of the immune system. J. Steroid Biochem. Mol. Biol. 2022, 219, 106084. [Google Scholar] [CrossRef]
- Norris, K.C.; Williams, S.F. Race/Ethnicity, Serum 25-Hydroxyvitamin D, and Heart Disease. JAMA 2013, 310, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Rahme, M.; Chamoun, N.; Fuleihan, G.E.H. Vitamin D in the Middle East and North Africa. Bone Rep. 2018, 8, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dusso, A.S.; Bauerle, K.T.; Bernal-Mizrachi, C. Non-classical Vitamin D Actions for Renal Protection. Front. Med. 2021, 8, 790513. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Chou, C.L.; Ou, S.H.; Fang, T.C.; Chen, J.S. Systematic Review of Nutrition Supplements in Chronic Kidney Diseases: A GRADE Approach. Nutrients 2021, 13, 469. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Berns, J.S.; Choi, M.J.; Martin, K.; Rocco, M.V. 25-Hydroxyvitamin D Testing and Supplementation in CKD: An NKF-KDOQI Controversies Report. Am. J. Kidney Dis. 2014, 64, 499–509. [Google Scholar] [CrossRef]
- Belfki, H.; Ben Ali, S.; Aounallah-Skhiri, H.; Traissac, P.; Bougatef, S.; Maire, B.; Delpeuch, F.; Achour, N.; Ben Romdhane, H. Prevalence and determinants of the metabolic syndrome among Tunisian adults: Results of the Transition and Health Impact in North Africa (TAHINA) project. Public Health Nutr. 2013, 16, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Favier, J.C.; Ireland-Ripert, J.; Toque, C.; Findberg, M. Répertoire Général des Aliments, Table de Composition; Tec&Doc, INRA, CNEVA CIQUAL: Paris, France, 1995. [Google Scholar]
- Hagströmer, M.; Oja, P.; Sjöström, M. The international physical activity questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2005, 9, 755–762. [Google Scholar] [CrossRef]
- Melamed, M.L.; Chonchol, M.; Gutiérrez, O.M.; Kalantar-Zadeh, K.; Kendrick, J.; Norris, K.; Scialla, J.J.; Thadhani, R. The Role of Vitamin D in CKD Stages 3 to 4: Report of a Scientific Workshop Sponsored by the National Kidney Foundation. Am. J. Kidney Dis. 2018, 72, 834–845. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Cormier, C.; Cavalier, E.; Breuil, V.; Debiais, F.; Fardellone, P.; Guggenbuhl, P.; Javier, R.M.; Legrand, E.; Lespessailles, E.; et al. Vitamin D Supplementation in France in patients with or at risk for osteoporosis: Recent data and new practices. Joint. Bone Spine 2020, 87, 25–29. [Google Scholar] [CrossRef]
- Kimball, S.M.; Holick, M.F. Official recommendations for vitamin D through the life stages in developed countries. Eur. J. Clin. Nutr. 2020, 74, 1514–1518. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Bao, L.; Jiang, X.; Ye, Z.; Bing, J.; Dong, Y.; Gao, D.; Ji, X.; Jiang, T.; Li, J.; et al. The association of metabolic syndrome components and chronic kidney disease in patients with hypertension. Lipids Health Dis. 2019, 18, 229. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Deurenberg, P.; Westrate, J.A.; Seidell, J.C. Body mass index as a measure of body fatness: Age- and sex-specific prediction formulas. Br. J. Nutr. 1991, 65, 105–111. [Google Scholar] [CrossRef]
- O’Brien, E.; Mee, F.; Atkins, N.; Thomas, M. Evaluation of three devices for self measurement of blood pressure according to the revised British Hypertension Society Protocol: The Omron HEM-705CP, Philips HP5332, and Nissei DS-175. Blood Press. Monit. 1996, 1, 55–61. [Google Scholar]
- Burtis, C.; Ashwood, E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th ed.; ch 26; Elsevier Health Sciences: London, UK, 2012; pp. 719–720. [Google Scholar]
- Roeschlau, P.; Bernt, E.; Gruber, W. Enzymatic determination of total cholesterol in serum. Z. Klin. Chem. Klin. Biochem. 1974, 12, 226. [Google Scholar]
- Siedel, J.; Schmuck, R.; Staepels, J.; Town, M.H. Long term stable, liquid ready to use monoreagent for the enzymatic assay of serum or plasma triglycerides (GPO-PAP method). AACC Meeting Abstract 34. Clin. Chem. 1993, 39, 1127. [Google Scholar]
- Sugiuchi, H.; Uji, Y.; Okabe, H.; Irie, T.; Uekama, K.; Kayahara, N.; Miyauchi, K. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated α-cyclodextrin. Clin. Chem. 1995, 41, 717–723. [Google Scholar] [CrossRef]
- Knopfholz, J.; Disserol, C.C.; Pierin, A.J.; Schirr, F.L.; Streisky, L.; Takito, L.L.; Massucheto Ledesma, P.; Faria-Neto, J.R.; Olandoski, M.; da Cunha, C.L.; et al. Validation of the Friedewald formula in patients with metabolic syndrome. Cholesterol 2014, 2014, 261878. [Google Scholar] [CrossRef]
- Patel, V.I.; Patel, K.P.; Makadia, M.G.; Shah, A.D.; Chaudhari, K.S.; Nilayangode, H.N. Levels of Apolipoprotein A1, B100 and Lipoprotein (a) in Controlled and Uncontrolled Diabetic Patients and in Non-Diabetic Healthy People. J. Clin. Diagn. Res. 2017, 11, BC01–BC05. [Google Scholar] [CrossRef] [PubMed]
- Busby, D.E.; Bakris, G.L. Comparison of Commonly Used Assays for the Detection of Microalbuminuria. J. Clin. Hypertens. 2004, 6 (11 Suppl. 3), 8–12. [Google Scholar] [CrossRef]
- Bassuk, S.S.; Rifai, N.; Ridker, P.M. High-sensitivity C-reactive protein: Clinical importance. Curr. Probl. Cardiol. 2004, 29, 439–493. [Google Scholar] [PubMed]
- Aykan, A.C.; Gökdeniz, T.; Gündüz, S.; Astarcioğlu, M.A.; Gürsoy, O.M.; Ertürk, E.; Oğuz, A.E.; Bayram, Z.; Karakoyun, S.; Kalçik, M.; et al. Value of serum fibrinogen levels in the assessment of mechanical prosthetic valve thrombosis. J. Heart. Valve Dis. 2014, 23, 222–227. [Google Scholar] [PubMed]
- Wang, C.; Liu, Y.; Zhang, M.; Yang, F.; Xu, F.; Shi, S.; Zeng, C.; Chen, X.; Miao, Y.; Liu, Z.; et al. Assessment of the diagnostic value of serum ceruloplasmin for Wilson’s disease in children. Clin. J. Am. Soc. Nephrol. 2022, 18. [Google Scholar]
- Clarke, R.; Stansbie, D. Assessment of homocysteine as a cardiovascular risk factor in clinical practice. Ann. Clin. Biochem. 2001, 38 (Pt 6), 624–632. [Google Scholar] [CrossRef]
- Dalpé-Scott, M.; Heick, H.M.; Bégin-Heick, N. An improved double antibody radioimmunoassay for the determination of insulin in serum, plasma, and tissue incubation media. Can. J. Biochem. 1982, 60, 962–966. [Google Scholar] [CrossRef]
- Valaperti, A.; Li, Z.; Vonow-Eisenring, M.; Probst-Müller, E. Diagnostic methods for the measurement of human TNF-alpha in clinical laboratory. J. Pharm. Biomed. Anal. 2020, 179, 113010. [Google Scholar] [CrossRef]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Huang, D.; Ying, H.; Jiang, D.; Liu, F.; Tian, Y.; Du, C.; Zhang, L.; Pu, X. Rapid and sensitive detection of interleukin-6 in serum via time-resolved lateral flow immunoassay. Anal. Biochem. 2020, 588, 113468. [Google Scholar] [CrossRef]
- Wilkinson, V.L.; Warrier, R.R.; Truitt, T.P.; Nunes, P.; Gately, M.K.; Presky, D.H. Characterization of anti-mouse IL-12 monoclonal antibodies and measurement of mouse IL-12 by ELISA. J. Immunol. Methods 1996, 189, 15–24. [Google Scholar] [CrossRef]
- Colafrancesco, S.; Priori, R.; Alessandri, C.; Perricone, C.; Pendolino, M.; Picarelli, G.; Valesini, G. IL-18 Serum Level in Adult Onset Still’s Disease: A Marker of Disease Activity. Int. J. Inflam. 2012, 2012, 156890. [Google Scholar] [CrossRef] [PubMed]
- Shajarian, M.; Alsahebfosoul, F.; Etemadifar, M.; Sedaghat, N.; Shahbazi, M.; Firouzabadi, F.P.; Dezashibi, H.M. IL-23 plasma level measurement in relapsing remitting multiple sclerosis (RRMS) patients compared to healthy subjects. Immunol. Investig. 2015, 44, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, J.; Bellanger, J.; Bienvenu, F.; Chappuis, P.; Favier, A. Recommanded method for assaying serum zinc with flame atomic absorption. Ann. Biol. Clin. 1986, 44, 77–87. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase; an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 2015, 244, 6049–6055. [Google Scholar] [CrossRef]
- Scott, E.M. Purification and properties of glutathione reductase of human erythrocytes. J. Biol. Chem. 1963, 238, 3928–3933. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutatione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food. Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Bunch, D.R.; Miller, A.Y.; Wang, S. Development and validation of a liquid chromatography-tandem mass spectrometry assay for serum 25-hydroxyvitamin D2/D3 using a turbulent flow online extraction technology. Clin. Chem. Lab. Med. 2009, 12, 1565–1572. [Google Scholar] [CrossRef][Green Version]
- Reinhardt, T.A.; Horst, R.L.; Orf, J.W.; Hollis, B.W. A microassay for 1,25-Dihydroxy-vitamin D not requiring high performance liquid chromatography: Application to clinical studies. J. Clin. Endocrinol. Metab. 1984, 58, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lunyera, J.; Davenport, C.A.; Pendergast, J.; Musani, S.K.; Bhavsar, N.A.; Sims, M.; Mwasongwe, S.; Wolf, M.; Diamantidis, C.J.; Boulware, L.E.; et al. Modifiers of Plasma 25-Hydroxyvitamin D and Chronic Kidney Disease Outcomes in Black Americans: The Jackson Heart Study. J. Clin. Endocrinol. Metab. 2019, 104, 2267–2276. [Google Scholar] [CrossRef] [PubMed]
- Musemwa, N.; Gadegbeku, C.A. Hypertension in African Americans. Curr. Cardiol. Rep. 2017, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D and Pigmented Skin. Nutrients 2022, 14, 325. [Google Scholar] [CrossRef]
- Luxwolda, M.; Kuipers, R.; Kema, I.; Dijck-Brouwer, D.; Muskiet, F. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/L. Br. J. Nutr. 2012, 108, 1557–1561. [Google Scholar] [CrossRef]
- Jorgetti, V.; dos Reis, L.M.; Ott, S.M. Ethnic differences in bone and mineral metabolism in healthy people and patients with CKD. Kidney Int. 2014, 85, 1283–1289. [Google Scholar] [CrossRef]
- Dawson-Hughes, B. Racial/ethnic considerations in making recommendations for vitamin D for adult and elderly men and women. Am. J. Clin. Nutr. 2004, 80, 1763S–1766S. [Google Scholar] [CrossRef]
- Grabulosa, C.C.; Manfredi, S.R.; Canziani, M.E.; Quinto, B.M.R.; Barbosa, R.B.; Rebello, J.F.; Batista, M.C.; Cendoroglo, M.; Dalboni, M.A. Chronic kidney disease induces inflammation by increasing Toll-like receptor-4, cytokine and cathelicidin expression in neutrophils and monocytes. Exp. Cell. Res. 2018, 365, 157–162. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.C. NF-κB in inflammation and renal diseases. Cell Biosci. 2015, 5, 63. [Google Scholar] [CrossRef]
- Berzal, S.; González-Guerrero, C.; Rayego-Mateos, S.; Ucero, Á.; Ocaña-Salceda, C.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M.; Ramos, A.M. TNF-related weak inducer of apoptosis (TWEAK) regulates junctional proteins in tubular epithelial cells via canonical NF-nB pathway and ERK activation. J. Cell. Physiol. 2015, 230, 1580–1593. [Google Scholar] [CrossRef]
- Kendrick, J.; Targher, G.; Smits, G.; Chonchol, M. 25-Hydroxyvitamin D Deficiency and Inflammation and Their Association with Hemoglobin Levels in Chronic Kidney Disease. Am. J. Nephrol. 2009, 30, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Yuan, J.; Zhao, Y.; Zha, Y. Urine interleukin-18 in prediction of acute kidney injury: A systemic review and meta-analysis. J. Nephrol. 2015, 28, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lin, C.Y. Interleukin-12 and -18 levels in peritoneal dialysate effluent correlate with the outcome of peritonitis in patients undergoing peritoneal dialysis: Implications for the Type I/Type II T-cell immune response. Am. J. Kidney Dis. 2005, 46, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsokos, M.G.; Bhargava, R.; Adamopoulos, I.E.; Menn-Josephy, H.; Stillman, I.E.; Rosenstiel, P.; Jordan, J.; Tsokos, G.C. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J. Clin. Investig. 2021, 131, e142428. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.F.; Turner, J.E.; Riedel, J.H.; Panzer, U. Tissue-specific therapy in immune-mediated kidney diseases: New ARGuments for targeting the IL-23/IL-17 axis. J. Clin. Investig. 2021, 131, e150588. [Google Scholar] [CrossRef] [PubMed]
- Bonnemaison, M.L.; Marks, E.S.; Boesen, E.I. Interleukin-1beta as a driver of renal NGAL production. Cytokine 2017, 91, 38–43. [Google Scholar] [CrossRef]
- Lech, M.; Grobmayr, R.; Ryu, M.; Lorenz, G.; Hartter, I.; Mulay, S.R.; Susanti, H.E.; Kobayashi, K.S.; Flavell, R.A.; Anders, H.J. Macrophage phenotype controls long-term AKI outcomes-kidney regeneration versus atrophy. J. Am. Soc. Nephrol. 2014, 25, 292–304. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Goulart, R.A.; Gasparini, R.G. Associations between inflammatory bowel diseases and vitamin D. Crit. Rev. Food Sci. Nutr. 2019, 59, 1347–1356. [Google Scholar] [CrossRef]
- Bendix-Struve, M.; Bartels, L.E.; Agnholt, J.; Dige, A.; Jorgensen, S.P.; Dahlerup, J.F. Vitamin D3 treatment of Crohn’s disease patient’s increases stimulated T cell IL-6 production and proliferation. Aliment. Pharmacol. Ther. 2010, 32, 1364–1372. [Google Scholar] [CrossRef]
- O’Connor, W., Jr.; Kamanaka, M.; Booth, C.J.; Town, T.; Nakae, S.; Iwakura, Y.; Kolls, J.K.; Flavell, R.A. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 2009, 10, 603–609. [Google Scholar] [CrossRef]
- Lang, C.L.; Wang, M.H.; Chiang, C.K.; Lu, K.C. Vitamin D and the Immune System from the Nephrologist’s Viewpoint. ISRN Endocrinol. 2014, 2014, 105456. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.B.O.; Rebello, J.F.; Grabulosa, C.C.; Pinto, W.; Morales, A., Jr.; Elias, R.M.; Moyses, R.M.A.; Dalboni, M.A. 25-vitamin D reduces inflammation in uremic environment. Sci. Rep. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80 (Suppl. 6), 1717S–1720S. [Google Scholar] [CrossRef] [PubMed]
- Agharazii, M.; St-Louis, R.; Gautier-Bastien, A.; Ung, R.V.; Mokas, S.; Larivière, R.; Richard, D.E. Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 2015, 28, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Zachara, B.A.; Gromadzińska, J.; Wasowicz, W.; Zbróg, Z. Red blood cell and plasma glutathione peroxidase activities and selenium concentration in patients with chronic kidney disease: A review. Acta Biochim. Pol. 2006, 53, 663–677. [Google Scholar] [CrossRef]
- Schweizer, U.; Streckfuss, F.; Pelt, P.; Carlson, B.A.; Hatfield, D.L.; Kohrle, J.; Schomburg, L. Hepatically derived selenoprotein P is a key factor for kidney but not for brain selenium supply. Biochem. J. 2005, 386, 221–226. [Google Scholar] [CrossRef]
- Aziz, M.A.; Majeed, G.H.; Diab, K.S.; Al-Tamimi, R.J. The association of oxidant–antioxidant status in patients with chronic renal failure. Ren. Fail. 2016, 38, 20–26. [Google Scholar] [CrossRef]
- Montazerifar, F.; Hashemi, M.; Karajibani, M.; Sanadgol, H.; Dikshit, M. Evaluation of lipid peroxidation and erythrocyte glutathione peroxidase and superoxide dismutase in hemodialysis patients. Saudi J. Kidney Dis. Transpl. 2012, 23, 274–279. [Google Scholar]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Vunta, H.; Belda, B.J.; Arner, R.J.; Channa Reddy, C.; Vanden Heuvel, J.P.; Sandeep Prabhu, K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol. Nutr. Food Res. 2008, 52, 1316–1323. [Google Scholar] [CrossRef]
- Xin, L.; Che, B.; Zhai, B.; Luo, Q.; Zhang, C.; Wang, J.; Wang, S.; Fan, G.; Liu, Z.; Feng, J.; et al. 1,25-Dihydroxy Vitamin D 3 Attenuates the Oxidative Stress-Mediated Inflammation Induced by PM 2.5 via the p38/NF-κB/NLRP3 Pathway. Inflammation 2019, 42, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Effects of zinc deficiency on Th1 and Th2 cytokine shifts. J. Infect. Dis. 2000, 182 (Suppl. 1), S62–S68. [Google Scholar] [CrossRef] [PubMed]
- de Romana, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of copper in light of new insights of copper homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, R.; Xu, Q.; Xu, S.; Zuo, S.; Qiu, J.; Zhong, X.; Tan, R.; Liu, Y. High Blood Cu/Zn Ratio is Associated with Nutritional Risk in Patients Undergoing Maintenance Hemodialysis. Biol. Trace Elem. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Dashti, N.; Lamei, N.; Abdi, K.; Nazari, F.; Abbasian, S.; Gerayeshnejad, S. Evaluation of plasma concentrations of homocysteine, IL-6, TNF-alpha, hs-CRP, and total antioxidant capacity in patients with end-stage renal failure. Acta Med. Iran. 2014, 52, 893–898. [Google Scholar]
- Verdoia, M.; Nardin, M.; Gioscia, R.; Saghir Afifeh, A.M.; Viglione, F.; Negro, F.; Marcolongo, M.; De Luca, G. Novara Atherosclerosis Study Group (NAS). Association between vitamin D deficiency and serum Homocysteine levels and its relationship with coronary artery disease. J. Thromb. Thrombolysis. 2021, 52, 523–531. [Google Scholar] [CrossRef]
- Hargreaves, M.K.; Liu, J.; Buchowski, M.S.; Patel, K.A.; Larson, C.O.; Schlundt, D.G.; Kenerson, D.M.; Hill, K.E.; Burk, R.F.; Blot, W.J. Plasma selenium biomarkers in low income black and white americans from the south eastern United States. PLoS ONE 2014, 9, e84972. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Q.; Zhang, K.; Sun, W.; Jia, X.; Yang, Y.; Yin, J.; Tang, C.; Zhang, J. Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metallomics 2020, 12, 1576–1584. [Google Scholar] [CrossRef]
- Hopewell, J.C.; Haynes, R.; Baigent, C. The role of lipoprotein (a) in chronic kidney disease. J. Lipid. Res. 2018, 59, 577–585. [Google Scholar] [CrossRef]
- Antonicelli, R.; Testa, R.; Bonfigli, A.R.; Sirolla, C.; Pieri, C.; Marra, M.; Marcovina, S.M. Relationship between lipoprotein (a) levels, oxidative stress, and blood pressure levels in patients with essential hypertension. Clin. Exp. Med. 2001, 1, 145–150. [Google Scholar] [CrossRef]
- Hassan, M.O.; Dix-Peek, T.; Duarte, R.; Dickens, C.; Naidoo, S.; Vachiat, A.; Grinter, S.; Manga, P.; Naicker, S. Association of chronic inflammation and accelerated atherosclerosis among an indigenous black population with chronic kidney disease. PLoS ONE 2020, 15, e0232741. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Umemoto, T. Atorvastatin decreases lipoprotein (a): A meta-analysis of randomized trials. Int. J. Cardiol. 2012, 154, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Heravifard, S.; Neyestani, T.R.; Nikooyeh, B.; Alavi-Majd, H.; Houshiarrad, A.; Kalayi, A.; Shariatzadeh, N.; Zahedirad, M.; Tayebinejad, N.; Salekzamani, S.; et al. Regular consumption of both vitamin D- and calcium- and vitamin D-fortified yogurt drink is equally accompanied by lowered blood lipoprotein (a) and elevated apoprotein A1 in subjects with type 2 diabetes: A randomized clinical trial. J. Am. Coll. Nutr. 2013, 32, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Jaimungal, S.; Wehmeier, K.; Mooradian, A.D.; Haas, M.J. The emerging evidence for vitamin D-mediated regulation of apolipoprotein A-I synthesis. Nutr. Res. 2011, 31, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Murhadi, L.L.; Kurpad, A.V.; Chan She Ping-Delfos, W.L.; Piers, L.S. Mechanistic roles for calcium and vitamin D in the regulation of body weight. Obes. Rev. 2012, 13, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, L.J.; Watson, P.; Zhang, Q.; Lappe, J.M. The effect of calcium and vitamin D supplementation on obesity in postmenopausal women: Secondary analysis for a large-scale, placebo controlled, double-blind, 4-year longitudinal clinical trial. Nutr. Metab. 2010, 7, 62. [Google Scholar] [CrossRef]
- Caan, B.; Neuhouser, M.; Aragaki, A.; Lewis, C.B.; Jackson, R.; LeBoff, M.S.; Margolis, K.L.; Powell, L.; Uwaifo, G.; Whitlock, E. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch. Int. Med. 2007, 167, 893–902. [Google Scholar] [CrossRef]
- Brewer, L.C.; Miller, E.R.; Appel, L.J.; Anderson, C.A. Do African American Women Require Fewer Calories to Maintain Weight? Results from a Controlled Feeding Trial. Nutr. Clin. Pract. 2012, 27, 561–567. [Google Scholar] [CrossRef]
- Weyer, C.; Snitker, S.; Bogardus, C.; Ravussin, E. Energy metabolism in African Americans: Potential risk factors for obesity. Am. J. Clin. Nutr. 1999, 70, 13–20. [Google Scholar] [CrossRef]
- Alzaman, N.S.; Dawson-Hughes, B.; Nelson, J.; D’Alessio, D.; Pittas, A.G. Vitamin D status of black and white Americans and changes in vitamin D metabolites after varied doses of vitamin D supplementation. Am. J. Clin. Nutr. 2016, 104, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, L.; Rydzewski, A.; Fiderkiewicz, B.; Wasińska-Krawczyk, A.; Grzechnik, A.; Rosołowska-Huszcz, D. Adiponectin, resistin and leptin response to dietary intervention in diabetic nephropathy. J. Ren. Nutr. 2010, 20, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Naini, A.E.; Vahdat, S.; Hedaiati, Z.P.; Shahzeidi, S.; Pezeshki, A.H.; Nasri, H. The effect of vitamin D administration on serum leptin and adiponectin levels in end-stage renal disease patients on hemodialysis with vitamin D deficiency: A placebo-controlled double-blind clinical trial. J. Res. Med. Sci. 2016, 21, 1. [Google Scholar] [PubMed]
- Bell, N.H.; Shaw, S.; Turner, R.T. Evidence that 1,25-dihydroxyvitamin D3 inhibits the hepatic production of 25-hydroxyvitamin D in man. J. Clin. Investig. 1984, 74, 1540–1544. [Google Scholar] [CrossRef]
- Deo, R.; Yang, W.; Khan, A.M.; Bansal, N.; Zhang, X.; Leonard, M.B.; Keane, M.G.; Soliman, E.Z.; Steigerwalt, S.; Townsend, R.R.; et al. CRIC Study Investigators. Serum aldosterone and death, end-stage renal disease, and cardiovascular events in blacks and whites: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Hypertension 2014, 64, 103–110. [Google Scholar] [CrossRef]
- Nguyen, T.; Joe, D.; Shah, A.D. Forget the phosphorus: A case of hypervitaminosis D-induced symptomatic hypercalcemia. Clin. Nephrol. Case Stud. 2021, 9, 1–3. [Google Scholar] [CrossRef]




| Parameters | Whites (N = 79) | Blacks (N = 72) | p Value-Baseline |
|---|---|---|---|
| Skin colour (%) | 46.3 | 53.7 | <0.01 |
| Age (year) | 52 ± 3 | 49 ± 1 | 0.072 |
| Gender (%) | 48/33 (F/M) | 53/41 (F/M) | 0.083 |
| CKD3 (%) | 37 | 49 | <0.001 |
| Comorbidities (%) | |||
| Hypertension | 60.5 | 74.9 | <0.001 |
| Anemia | 66.6 | 57.2 | <0.01 |
| Coronary artery disease | 45 | 32 | <0.01 |
| Congestive heart failure | 19 | 25 | <0.01 |
| Cerebral vascular accident | 15 | 9 | <0.01 |
| Peripheral vascular disease | 19 | 12 | <0.01 |
| Obesity | 60 | 86 | <0.01 |
| Dyslipidemia | 75 | 28 | <0.01 |
| Hypocalcaemia | 42.8 | 60.6 | <0.01 |
| Hyperuricemia | 22.5 | 36.5 | <0.01 |
| Smoking current (%) | 20 | 18 | 0.091 |
| Alcohol use (%) | 2 | 1 | 0.073 |
| Current drug use (%) | |||
| Calcium channel blocker | 45 | 52 | 0.144 |
| β-Blockers | 45 | 46 | 0.061 |
| Thiazide | 7 | 10 | 0.205 |
| Diuretic | 63 | 76 | 0.331 |
| Aspirin | 56 | 58 | 0.069 |
| Statins | 28 | 39 | 0.177 |
| Vitamin D intake (µg/day) | 69 ± 4 | 97 ± 3 | <0.001 |
| Vitamin D intake (IU/day) | 2760 ± 160 | 3880 ± 120 | <0.001 |
| Calcium intake (mg/day) | 977 ± 11 | 691 ± 22 | <0.01 |
| Caloric intake (kcal/kg/day) | 35 ± 5 | 62 ± 9 | <0.001 |
| Physical activity (min/week) | 60 ± 5 | 40 ± 3 | <0.01 |
| Baseline | 24 Weeks | 24 Weeks | 36 Weeks | p Value | |||
|---|---|---|---|---|---|---|---|
| Parameters | WSS (N = 79) | BSS (N = 72) | WSS (N = 74) | BSS (N = 70) | WSS (N = 71) | BSS (N = 68) | |
| Body Weight (Kg) | 81.2 ± 12.3 | 93.3 ± 3.61 | 78.6 ± 6.42 | 91.9 ± 7.60 | 76.9 ± 3.30 | 87.3 ± 5.80 | <0.01 |
| BMI (kg/m2) | 28.1 ± 4.25 | 32.3 ± 1.26 | 27.2 ± 2.22 | 31.8 ± 2.63 | 26.6 ± 1.15 | 30.2 ± 1.99 | <0.01 |
| WC (cm) | 84.9 ± 3.98 | 107 ± 6.90 | 83.9 ± 5.55 | 100 ± 3.89 | 82.5 ± 4.11 | 101 ± 2.11 | <0.001 |
| WC/WHratio | 1.09 ± 0.02 | 1.13 ± 0.02 | 1.05 ± 0.01 | 1.11 ± 0.04 | 1.03 ± 0.03 | 1.09 ± 0.05 | <0.01 |
| BF (%) | 46.6 ± 2.17 | 55.1 ± 8.12 | 45.9 ± 3.32 | 54.5 ± 4.11 | 39.3 ± 7.71 | 53.9 ± 2.41 | <0.001 |
| Glycemia (mmol/L) | 6.70 ± 1.49 | 5.91 ± 1.97 | 6.38 ± 1.09 | 5.66 ± 1.83 | 5.67 ± 1.11 | 5.49 ± 1.33 | 0.223 |
| Insulinemia (pmol/mL) | 81 ± 7.15 | 114 ± 2.91 | 77.7 ± 3.55 | 105 ± 5.62 | 72.9 ± 5.76 | 95.6 ± 4.79 | <0.001 |
| HOMA-IR | 3.50 ± 0.93 | 5.01 ± 0.67 | 3.17 ± 0.11 | 4.50 ± 0.55 | 2.64 ± 0.34 | 4.09 ± 0.26 | <0.001 |
| Triglycerides (mmol/L) | 2.19 ± 0.28 | 1.45 ± 0.26 | 1.84 ± 0.71 | 1.32 ± 0.31 | 1.65 ± 0.05 | 1.15 ± 0.01 | <0.001 |
| Total Cholesterol (mmol/L) | 5.84 ± 0.68 | 4.75 ± 0.32 | 5.39 ± 0.88 | 4.26 ± 0.43 | 5.12 ± 0.33 | 3.82 ± 0.81 | <0.001 |
| HDL-C (mmol/L) | 0.85 ± 0.08 (M) | 0.95 ± 0.18 (M) | 1.14 ± 0.3 (M) | 1.09 ± 0.17 (M) | 1.24 ± 0.04 (M) | 1.25 ± 0.04 (M) | <0.01 |
| 1.11 ± 0.15 (F) | 1.24 ± 0.13 (F) | 1.21 ± 0.8 (F) | 1.27 ± 0.05 (F) | 1.26 ± 0.03 (F) | 1.28 ± 0.02 (F) | <0.01 | |
| LDL-C (mmol/L) | 4.52 ± 0.81 | 3.17 ± 0.79 | 4.29 ± 0.27 | 3.02 ± 0.44 | 4.06 ± 0.16 | 2.66 ± 0.23 | <0.001 |
| Hs-CRP (mg/L) | 5.90 ± 1.11 | 8.18 ± 1.33 | 5.07 ± 1.44 | 8.06 ± 1.01 | 4.45 ± 1.09 | 7.81 ± 1.68 | <0.01 |
| Fibrinogen (g/L) | 4.28 ± 1.15 | 4.44 ± 1.27 | 4.31 ± 1.22 | 4.52 ± 1.13 | 4.25 ± 1.09 | 4.49 ± 1.11 | 0.457 |
| Ceruloplasmin (µmol/L) | 1.04 ± 0.32 | 1.68 ± 0.57 | 1.90 ± 0.99 | 1.79 ± 0.33 | 2.44 ± 0.16 | 1.91 ± 0.33 | <0.001 |
| SBP (mmHg) | 141 ± 3 | 159 ± 7 | 135 ± 1 | 157 ± 2 | 130 ± 5 | 155 ± 6 | <0.01 |
| DBP (mmHg) | 72 ± 3 | 90 ± 2 | 70 ± 5 | 87 ± 1 | 68 ± 3 | 85 ± 2 | 0.145 |
| S-Phosphorus (mmol/L) | 1.20 ± 0.19 | 1.19 ± 0.11 | 1.15 ± 0.81 | 1.16 ± 0.44 | 1.12 ± 0.73 | 1.14 ± 0.55 | 0.117 |
| U-Phosphorus (mmol/24 h) | 302 ± 25 | 221 ± 17 | 272 ± 51 | 211 ± 33 | 254 ± 13 | 209 ± 47 | <0.01 |
| Baseline | 24 Weeks | 36 Weeks | p Value | ||||
|---|---|---|---|---|---|---|---|
| Parameters | WSS (N = 79) | BSS (N = 72) | WSS (N = 74) | BSS (N = 70) | WSS (N = 71) | BSS (N = 68) | |
| Body Weight (Kg) | 81.2 ± 12.3 | 93.3 ± 3.61 | 78.6 ± 9.11 | 89.9 ± 4.16 | 77.2 ± 7.31 | 86.1 ± 6.11 | <0.01 |
| BMI (kg/m2) | 28.1 ± 4.25 | 32.3 ± 1.26 | 27.2 ± 3.11 | 31.1 ± 1.37 | 26.7 ± 2.51 | 30.1 ± 2.08 | <0.01 |
| WC (cm) | 84.9 ± 3.98 | 107 ± 6.90 | 83.4 ± 3.74 | 103 ± 2.98 | 82.5 ± 3.21 | 99.1 ± 3.77 | <0.001 |
| WC/WH ratio | 1.09 ± 0.02 | 1.13 ± 0.02 | 1.05 ± 0.02 | 1.11 ± 0.03 | 1.02 ± 0.01 | 1.01 ± 0.02 | <0.01 |
| BF (%) | 46.6 ± 2.17 | 55.1 ± 8.12 | 45.7 ± 2.23 | 53.7 ± 3.21 | 40.1 ± 5.18 | 52.1 ± 3.14 | <0.001 |
| Glycemia (mmol/L) | 6.70 ± 1.49 | 5.91 ± 1.97 | 6.55 ± 1.17 | 5.46 ± 1.37 | 5.69 ± 1.71 | 5.38 ± 1.24 | 0.251 |
| Insulinemia (pmol/mL) | 81 ± 7.15 | 114 ± 2.91 | 73.5 ± 2.81 | 92.1 ± 4.26 | 71.5 ± 3.67 | 80.9 ± 3.92 | <0.001 |
| HOMA-IR | 3.50 ± 0.93 | 5.01 ± 0.67 | 3.10 ± 0.14 | 3.80 ± 0.27 | 2.60 ± 0.43 | 3.30 ± 0.63 | <0.001 |
| Triglycerides (mmol/L) | 2.19 ± 0.28 | 1.45 ± 0.26 | 1.80 ± 0.77 | 1.21 ± 0.13 | 1.68 ± 0.08 | 1.09 ± 0.02 | <0.01 |
| Total Cholesterol (mmol/L) | 5.84 ± 0.68 | 4.75 ± 0.32 | 4.94 ± 0.77 | 4.31 ± 0.35 | 4.40 ± 0.22 | 3.91 ± 0.17 | <0.001 |
| HDL-C (mmol/L) | 0.85 ± 0.08 (M) | 0.95 ± 0.18 (M) | 1.12 ± 0.01 (M) | 1.18 ± 0.11 (M) | 1.24 ± 0.04 (M) | 1.27 ± 0.05 (M) | <0.01 |
| 1.11 ± 0.15 (F) | 1.24 ± 0.13 (F) | 1.25 ± 0.05 (F) | 1.28 ± 0.05 (F) | 1.26 ± 0.03 (F) | 1.30 ± 0.04 (F) | <0.01 | |
| LDL-C (mmol/L) | 4.52 ± 0.81 | 3.17 ± 0.79 | 4.01 ± 0.73 | 3.08 ± 0.31 | 3.61 ± 0.74 | 2.63 ± 0.33 | <0.001 |
| Hs-CRP (mg/L) | 5.90 ± 1.11 | 8.18 ± 1.33 | 5.01 ± 1.54 | 6.45 ± 1.23 | 4.24 ± 1.12 | 5.33 ± 1.21 | <0.01 |
| Fibrinogen (g/L) | 4.28 ± 1.15 | 4.44 ± 1.27 | 4.25 ± 1.07 | 4.39 ± 1.19 | 4.31 ± 1.13 | 4.47 ± 1.33 | 0.327 |
| Ceruloplasmin (µmol/L) | 1.04 ± 0.32 | 1.68 ± 0.57 | 1.92 ± 0.77 | 1.92 ± 0.22 | 2.39 ± 0.63 | 2.66 ± 0.12 | <0.001 |
| SBP (mmHg) | 141 ± 3 | 159 ± 7 | 136 ± 3 | 150 ± 4 | 131 ± 4 | 147 ± 5 | <0.01 |
| DBP (mmHg) | 72 ± 3 | 90 ± 2 | 69 ± 2 | 85 ± 2 | 67 ± 1 | 83 ± 2 | 0.178 |
| S-Phosphorus (mmol/L) | 1.20 ± 0.19 | 1.19 ± 0.11 | 1.14 ± 0.21 | 1.15 ± 0.16 | 1.11 ± 0.83 | 1.12 ± 0.71 | 0.256 |
| U-Phosphorus (mmol/24 h) | 302 ± 25 | 221 ± 17 | 248 ± 17 | 207 ± 21 | 222 ± 18 | 205 ± 55 | <0.01 |
| Baseline | 24 Weeks | 36 Weeks | p Value | ||||
|---|---|---|---|---|---|---|---|
| Parameters | WSS (N = 79) | BSS (N = 72) | WSS (N = 74) | BSS (N = 70) | WSS (N = 71) | BSS (N = 68) | |
| 25(OH)D (ng/mL) | 19.8 ± 4.33 | 6.98 ± 1.91 | 30.9 ± 5.18 | 19.1 ± 6.15 | 48.1 ± 4.22 | 26.8 ± 3.54 | <0.001 |
| 1,25(OH)2D (pg/mL) | 29.9 ± 7.51 | 44.3 ± 3.55 | 40.4 ± 1.66 | 72.6 ± 2.89 | 55.3 ± 3.11 | 87.9 ± 1.98 | <0.001 |
| eGFR (mL/minper1.73 m2) | 48.2 ± 2.31 | 45.4 ± 2.29 | 52.2 ± 9.09 | 49.6 ± 7.11 | 61.6 ± 4.22 | 55.8 ± 3.11 | <0.001 |
| S-Creatinine (µmol/L) | 168 ± 19 | 221 ± 27 | 146 ± 63 | 199 ± 18 | 130 ± 13 | 161 ± 22 | <0.001 |
| U-Creatinine (mmol/24 h) | 10.9 ± 2.11 | 14.8 ± 2.17 | 13.7 ± 1.99 | 18.5 ± 3.34 | 17.6 ± 1.09 | 22.8 ± 1.77 | <0.001 |
| S-Creatinine-BMIratio | 5.97 ± 1.22 | 6.84 ± 1.31 | 5.52 ± 1.11 | 6.25 ± 1.43 | 5.01 ± 1.31 | 5.99 ± 1.18 | <0.001 |
| S-Uricacid (µmol/L) | 404 ± 36 | 441 ± 22 | 359 ± 17 | 410 ± 20 | 287 ± 32 | 366 ± 17 | <0.001 |
| U-Uricacid (mmol/24 h) | 2.45 ± 0.66 | 2.71 ± 0.45 | 2.67 ± 0.19 | 2.97 ± 0.31 | 2.91 ± 0.24 | 3.21 ± 0.55 | <0.001 |
| S-Ca (mmol/L) | 2.32 ± 0.62 | 2.20 ± 0.33 | 2.39 ± 0.61 | 2.44 ± 0.39 | 2.51 ± 0.16 | 2.65 ± 0.71 | <0.02 |
| U-Ca (mmol/24 h) | 41.4 ± 3.41 | 25.2 ± 4.33 | 47.9 ± 6.11 | 29.7 ± 2.41 | 52.4 ± 1.99 | 32.4 ± 3.11 | <0.02 |
| iCa (mmol/24 h) | 1.17 ± 0.15 | 1.30 ± 0.25 | 1.20 ± 0.19 | 1.41 ± 0.55 | 1.38 ± 0.11 | 1.49 ± 0.31 | <0.02 |
| S-Alb (µmol/L) | 595 ± 44 | 580 ± 33 | 665 ± 71 | 609 ± 55 | 744 ± 24 | 720 ± 19 | <0.001 |
| U-Alb (mg/24 h) | 40.5 ± 7.11 | 54.8 ± 3.27 | 31.9 ± 2.08 | 42.5 ± 1.44 | 29.7 ± 3.11 | 34.9 ± 2.71 | <0.001 |
| ApoproteinA1 (µmol/L) | 42.4 ± 2.17 | 41.1 ± 3.66 | 44.1 ± 2.09 | 43.7 ± 3.11 | 46.9 ± 7.88 | 45.3 ± 3.77 | <0.02 |
| ApoproteinB100 (µmol/L) | 35.9 ± 5.83 | 38.3 ± 7.15 | 33.4 ± 1.77 | 36.4 ± 4.51 | 32.9 ± 2.24 | 33.1 ± 1.91 | <0.01 |
| ApoB100-ApoA1ratio | 0.846 ± 0.06 | 0.931 ± 0.07 | 0.757 ± 0.03 | 0.832 ± 0.05 | 0.702 ± 0.07 | 0.717 ± 0.04 | <0.01 |
| Lp(a) (nmol/L) | 78.5 ± 4.10 | 94.5 ± 3.27 | 76.9 ± 6.11 | 92.8 ± 1.72 | 72.1 ± 2.66 | 91.2 ± 3.27 | 0.772 |
| tHcy (µmol/L) | 17.2 ± 2.52 | 21.1 ± 3.31 | 16.5 ± 2.15 | 19.8 ± 2.81 | 16.9 ± 3.55 | 20.7 ± 1.88 | 0.568 |
| Baseline | 24 Weeks | 36 Weeks | p Value | ||||
|---|---|---|---|---|---|---|---|
| Parameters | WSS (N = 79) | BSS (N = 72) | WSS (N = 74) | BSS (N = 70) | WSS (N = 71) | BSS (N = 68) | |
| 25OHD (ng/mL) | 19.8 ± 4.33 | 6.98 ± 1.91 | 31.5 ± 4.11 | 22.7 ± 5.09 | 46.3 ± 3.42 | 44.9 ± 2.45 | <0.001 |
| 1,25(OH)2D (pg/mL) | 29.9 ± 7.51 | 44.3 ± 3.55 | 41.2 ± 2.58 | 82.6 ± 3.77 | 56.2 ± 5.08 | 95.8 ± 2.09 | <0.001 |
| eGFR (mL/minper1.73 m2) | 48.2 ± 2.31 | 45.4 ± 2.29 | 49.7 ± 5.32 | 55.1 ± 5.22 | 58.7 ± 4.11 | 61.9 ± 2.72 | <0.001 |
| S-Creatinine (µmol/L) | 168 ± 19 | 221 ± 27 | 154 ± 44 | 178 ± 22 | 137 ± 25 | 144 ± 13 | <0.001 |
| U-Creatinine (mmol/L) | 10.9 ± 2.11 | 14.8 ± 2.17 | 13.1 ± 2.08 | 20.5 ± 1.88 | 16.7 ± 3.11 | 25.1 ± 2.09 | <0.001 |
| S–Creatinine-BMIratio | 5.97 ± 1.22 | 6.84 ± 1.71 | 5.25 ± 2.33 | 5.57 ± 2.07 | 4.76 ± 2.45 | 5.34 ± 1.87 | <0.001 |
| S-Uricacid (µmol/L) | 404 ± 36 | 441 ± 22 | 360 ± 22 | 374 ± 31 | 289 ± 24 | 311 ± 55 | <0.001 |
| U-Uricacid (mmol/24 h) | 2.45 ± 0.66 | 2.71 ± 0.45 | 2.62 ± 0.88 | 3.05 ± 0.14 | 2.95 ± 0.43 | 3.49 ± 0.27 | <0.001 |
| S-Ca (mmol/L) | 2.32 ± 0.62 | 2.20 ± 0.33 | 2.40 ± 0.17 | 2.66 ± 0.88 | 2.55 ± 0.27 | 2.77 ± 0.19 | <0.02 |
| U-Ca (mmol/24 h) | 41.4 ± 3.41 | 25.2 ± 4.33 | 48.7 ± 5.09 | 33.5 ± 1.18 | 53.5 ± 2.23 | 38.9 ± 3.51 | <0.02 |
| iCa (mmol/L) | 1.17 ± 0.15 | 1.30 ± 0.25 | 1.19 ± 0.22 | 1.43 ± 0.19 | 1.39 ± 0.31 | 1.52 ± 0.15 | <0.02 |
| S-Alb (µmol/L) | 595 ± 44 | 580 ± 33 | 632 ± 55 | 649 ± 23 | 747 ± 31 | 785 ± 27 | <0.001 |
| U-Alb(mg/24 h) | 40.5 ± 7.11 | 54.8 ± 3.27 | 31.2 ± 1.31 | 37.8 ± 2.09 | 28.3 ± 4.07 | 31.4 ± 3.18 | <0.001 |
| ApoproteinA1 (µmol/L) | 42.4 ± 2.17 | 41.1 ± 3.66 | 43.8 ± 1.98 | 44.9 ± 2.72 | 45.1 ± 5.09 | 47.2 ± 3.54 | <0.001 |
| ApoproteinB100 (µmol/L) | 35.9 ± 5.83 | 38.3 ± 7.15 | 32.7 ± 1.33 | 34.3 ± 2.17 | 31.7 ± 1.89 | 30.1 ± 1.74 | <0.02 |
| ApoB100-ApoA1ratio | 0.846 ± 0.06 | 0.931 ± 0.07 | 0.746 ± 0.02 | 0.763 ± 0.05 | 0.744 ± 0.05 | 0.637 ± 0.02 | <0.01 |
| Lp(a) (nmol/L) | 78.5 ± 4.10 | 94.5 ± 3.27 | 77.5 ± 3.71 | 91.1 ± 1.22 | 76.9 ± 1.87 | 90.7 ± 2.37 | 0.401 |
| tHcy (µmol/L) | 17.2 ± 2.52 | 21.1 ± 3.31 | 16.1 ± 1.54 | 18.8 ± 1.27 | 15.8 ± 2.32 | 18.3 ± 1.09 | 0.711 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoubiri, H.; Tahar, A.; AitAbderrhmane, S.; Saidani, M.; Koceir, E.-A. Oral Cholecalciferol Supplementation in Sahara Black People with Chronic Kidney Disease Modulates Cytokine Storm, Oxidative Stress Damage and Athero-Thromboembolic Risk. Nutrients 2022, 14, 2285. https://doi.org/10.3390/nu14112285
Zoubiri H, Tahar A, AitAbderrhmane S, Saidani M, Koceir E-A. Oral Cholecalciferol Supplementation in Sahara Black People with Chronic Kidney Disease Modulates Cytokine Storm, Oxidative Stress Damage and Athero-Thromboembolic Risk. Nutrients. 2022; 14(11):2285. https://doi.org/10.3390/nu14112285
Chicago/Turabian StyleZoubiri, Houda, Amina Tahar, Samir AitAbderrhmane, Messaoud Saidani, and Elhadj-Ahmed Koceir. 2022. "Oral Cholecalciferol Supplementation in Sahara Black People with Chronic Kidney Disease Modulates Cytokine Storm, Oxidative Stress Damage and Athero-Thromboembolic Risk" Nutrients 14, no. 11: 2285. https://doi.org/10.3390/nu14112285
APA StyleZoubiri, H., Tahar, A., AitAbderrhmane, S., Saidani, M., & Koceir, E.-A. (2022). Oral Cholecalciferol Supplementation in Sahara Black People with Chronic Kidney Disease Modulates Cytokine Storm, Oxidative Stress Damage and Athero-Thromboembolic Risk. Nutrients, 14(11), 2285. https://doi.org/10.3390/nu14112285







