Efficacy and Safety of Nutrient Supplements for Glycaemic Control and Insulin Resistance in Type 2 Diabetes: An Umbrella Review and Hierarchical Evidence Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Certainty of Evidence
3. Results
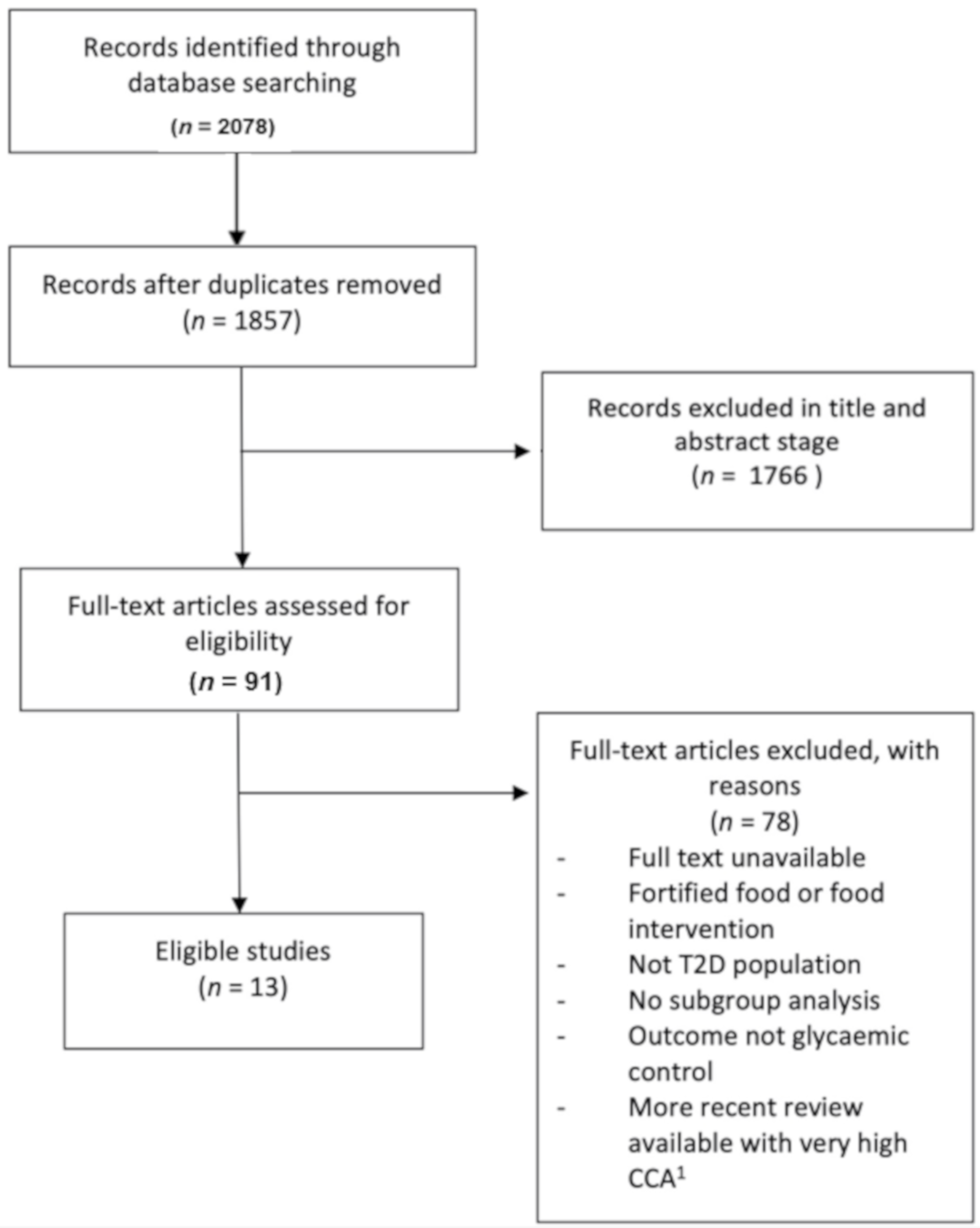
3.1. Search Results
3.2. Study Characteristics
3.3. Quality Assessment Using AMSTAR 2
3.4. Efficacy of Nutrient Supplementation in Glycaemic Control
3.4.1. Vitamin C
3.4.2. Chromium
3.4.3. Probiotics
3.4.4. Zinc
3.4.5. Magnesium
3.4.6. Polyphenols
3.4.7. Ω-3 PUFAs
3.4.8. Vitamin D
3.4.9. Folate
3.4.10. Safety of Nutrient Supplementation
3.4.11. Certainty of the Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Fact Sheet: Leading Causes of Death and Disability Worldwide; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sarwar, N.; Gao, P.; Seshasai, S.R.; Gobin, R.; Kaptoge, S.; di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S17–S38. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2018 Abridged for Primary Care Providers. Clin. Diabetes 2018, 36, 14–37. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S83–S96. [Google Scholar] [CrossRef]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2019, 42, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R. Insulin resistance and type 2 diabetes. Diabetes 2012, 61, 778–779. [Google Scholar] [CrossRef] [Green Version]
- Ferrannini, E.; Gastaldelli, A.; Iozzo, P. Pathophysiology of prediabetes. Med. Clin. N. Am. 2011, 95, 327–339. [Google Scholar] [CrossRef] [Green Version]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S60–S82. [Google Scholar] [CrossRef]
- Rhee, T.G.; Westberg, S.M.; Harris, I.M. Complementary and alternative medicine in US adults with diabetes: Reasons for use and perceived benefits. J. Diabetes 2018, 10, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.S.; Price, M.J.; Greenfield, S.M.; Paudyal, V. Global prevalence and types of complementary and alternative medicines use amongst adults with diabetes: Systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2021, 77, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Barr, S.I.; McNulty, H.; Li, D.; Blumberg, J.B. Health effects of vitamin and mineral supplements. BMJ 2020, 369, m2511. [Google Scholar] [CrossRef] [PubMed]
- Gahche, J.J.; Bailey, R.L.; Potischman, N.; Ershow, A.G.; Herrick, K.A.; Ahluwalia, N.; Dwyer, J.T. Federal Monitoring of Dietary Supplement Use in the Resident, Civilian, Noninstitutionalized US Population, National Health and Nutrition Examination Survey. J. Nutr. 2018, 148, 1436S–1444S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, S.K.; Malacova, E.; Sherriff, J.L.; Black, L.J. The Prevalence and Predictors of Dietary Supplement Use in the Australian Population. Nutrients 2017, 9, 1154. [Google Scholar] [CrossRef] [Green Version]
- Skeie, G.; Braaten, T.; Hjartåker, A.; Lentjes, M.; Amiano, P.; Jakszyn, P.; Pala, V.; Palanca, A.; Niekerk, E.M.; Verhagen, H.; et al. Use of dietary supplements in the European Prospective Investigation into Cancer and Nutrition calibration study. Eur. J. Clin. Nutr. 2009, 63, S226–S238. [Google Scholar] [CrossRef]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31 Suppl. 1, S61–S78. [Google Scholar] [CrossRef] [Green Version]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, H.; Pipingas, A.; Pase, M.P. Multivitamin-multimineral supplementation and mortality: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Mitri, J.; Pittas, A.G. Vitamin D and diabetes. Endocrinol. Metab. Clin. N. Am. 2014, 43, 205–232. [Google Scholar] [CrossRef] [Green Version]
- Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, J.M. Multivitamins in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2012, 308, 1751–1760. [Google Scholar] [CrossRef]
- Kaur, B.; Henry, J. Micronutrient status in type 2 diabetes: A review. Adv. Food Nutr. Res. 2014, 71, 55–100. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, M.; Chiefari, E.; Arcidiacono, B.; Corigliano, D.M.; Brunetti, F.S.; Maggisano, V.; Russo, D.; Foti, D.P.; Brunetti, A. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020, 12, 1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, M.; Fernandes, R.M.; Pieper, D.; Tricco, A.C.; Gates, M.; Gates, A.; Hartling, L. Preferred Reporting Items for Overviews of Reviews (PRIOR): A protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst. Rev. 2019, 8, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunny, C.; Brennan, S.E.; Reid, J.; McDonald, S.; McKenzie, J.E. Overviews of reviews incompletely report methods for handling overlapping, discordant, and problematic data. J. Clin. Epidemiol. 2020, 118, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.-L.; Mathes, T.; Neugebauer, E.A.M.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.; Fernandes, R.; Becker, L.; Pieper, D.; Hartling, L. Chapter V: Overviews of Reviews. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022); Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: London, UK, 2022; Available online: www.training.cochrane.org/handbook (accessed on 2 March 2022).
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schunemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Ashor, A.W.; Brown, R.; Keenan, P.D.; Willis, N.D.; Siervo, M.; Mathers, J.C. Limited evidence for a beneficial effect of vitamin C supplementation on biomarkers of cardiovascular diseases: An umbrella review of systematic reviews and meta-analyses. Nutr. Res. 2019, 61, 1–12. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Singh Mann, A.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef]
- Raimundo, A.F.; Félix, F.; Andrade, R.; García-Conesa, M.-T.; González-Sarrías, A.; Gilsa-Lopes, J.; do Ó, D.; Raimundo, A.; Ribeiro, R.; Rodriguez-Mateos, A.; et al. Combined effect of interventions with pure or enriched mixtures of (poly)phenols and anti-diabetic medication in type 2 diabetes management: A meta-analysis of randomized controlled human trials. Eur. J. Nutr. 2020, 59, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- Ebada, M.A.; Fayed, N.; Fayed, L.; Alkanj, S.; Abdelkarim, A.; Farwati, H.; Hanafy, A.; Negida, A.; Ebada, M.; Noser, Y. Efficacy of Alpha-lipoic Acid in The Management of Diabetes Mellitus: A Systematic Review and Meta-analysis. Iran. J. Pharm. Res. IJPR 2019, 18, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liu, Y.; Gan, Y.; Bao, W.; Peng, X.; Xing, Q.; Gao, H.; Lai, J.; Liu, L.; Wang, Z.; et al. Effects of fish oil supplementation on glucose control and lipid levels among patients with type 2 diabetes mellitus: A Meta-analysis of randomized controlled trials. Lipids Health Dis. 2020, 19, 87. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, J.A.; Sun, X.; Wang, L.; Wang, A. Efficacy of vitamin D supplementation on glycemic control in type 2 diabetes patients: A meta-analysis of interventional studies. Medicine 2019, 98, e14970. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.X.; Wong, E.Y.; Vela, M.N.; Le, Q.T. Effect of Probiotic Supplementation on Glycemic Outcomes in Patients with Abnormal Glucose Metabolism: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Nutr. Metab. 2021, 77, 251–261. [Google Scholar] [CrossRef]
- Verma, H.; Garg, R. Effect of magnesium supplementation on type 2 diabetes associated cardiovascular risk factors: A systematic review and meta-analysis. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2017, 30, 621–633. [Google Scholar] [CrossRef]
- Zhao, F.; Pan, D.; Wang, N.; Xia, H.; Zhang, H.; Wang, S.; Sun, G. Effect of Chromium Supplementation on Blood Glucose and Lipid Levels in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Biol. Trace Elem. Res. 2021, 200, 516–525. [Google Scholar] [CrossRef]
- Lind, M.V.; Lauritzen, L.; Kristensen, M.; Ross, A.B.; Eriksen, J.N. Effect of folate supplementation on insulin sensitivity and type 2 diabetes: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 29–42. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Zheng, W.; Fang, X.; Chen, L.; Rink, L.; Min, J.; Wang, F. Zinc supplementation improves glycemic control for diabetes prevention and management: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 110, 76–90. [Google Scholar] [CrossRef] [Green Version]
- Mason, S.A.; Keske, M.A.; Wadley, G.D. Effects of Vitamin C Supplementation on Glycemic Control and Cardiovascular Risk Factors in People with Type 2 Diabetes: A GRADE-Assessed Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2021, 44, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejad, S.; Mahboobi, S.; McFarland, L.V.; Taghizadeh, M.; Rahimi, F. Meta-Analysis: Effects of Zinc Supplementation Alone or with Multi-Nutrients, on Glucose Control and Lipid Levels in Patients with Type 2 Diabetes. Prev. Nutr. Food Sci. 2019, 24, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Werner, A.D.; Lara, J.; Willis, N.D.; Mathers, J.C.; Siervo, M. Effects of vitamin C supplementation on glycaemic control: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Clin. Nutr. 2017, 71, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei-Malazy, O.; Nikfar, S.; Larijani, B.; Abdollahi, M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J. Pharm. Pharm. Sci. 2014, 17, 554–582. [Google Scholar] [CrossRef] [PubMed]
- Khodaeian, M.; Tabatabaei-Malazy, O.; Qorbani, M.; Farzadfar, F.; Amini, P.; Larijani, B. Effect of vitamins C and E on insulin resistance in diabetes: A meta-analysis study. Eur. J. Clin. Investig. 2015, 45, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 103, 66–70. [Google Scholar] [CrossRef]
- Mason, S.A.; Della Gatta, P.A.; Snow, R.J.; Russell, A.P.; Wadley, G.D. Ascorbic acid supplementation improves skeletal muscle oxidative stress and insulin sensitivity in people with type 2 diabetes: Findings of a randomized controlled study. Free Radic. Biol. Med. 2016, 93, 227–238. [Google Scholar] [CrossRef]
- Sherifali, D.; Nerenberg, K.; Pullenayegum, E.; Cheng, J.E.; Gerstein, H.C. The effect of oral antidiabetic agents on A1C levels: A systematic review and meta-analysis. Diabetes Care 2010, 33, 1859–1864. [Google Scholar] [CrossRef] [Green Version]
- Doerner, P.G., 3rd; Liao, Y.H.; Ding, Z.; Wang, W.; Ivy, J.L.; Bernard, J.R. Chromium chloride increases insulin-stimulated glucose uptake in the perfused rat hindlimb. Acta Physiol. 2014, 212, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.B. Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 483–489. [Google Scholar] [CrossRef]
- Lee, J.O.; Lee, S.K.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Kim, S.J.; Park, S.H.; Kim, H.S. Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J. Biol. Chem. 2012, 287, 44121–44129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suksomboon, N.; Poolsup, N.; Yuwanakorn, A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014, 39, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, X.; Chen, K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2021, 117, 3–14. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 2014, 16, 9–21. [Google Scholar] [CrossRef]
- MacDonald, P.E.; El-kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef] [Green Version]
- Rittiphairoj, T.; Pongpirul, K.; Janchot, K.; Mueller, N.T.; Li, T. Probiotics Contribute to Glycemic Control in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 12, 722–734. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.C.; Messora, M.R. Effectiveness of Multistrain Versus Single-strain Probiotics: Current Status and Recommendations for the Future. J. Clin. Gastroenterol. 2018, 52 (Suppl. 1), S35–S40. [Google Scholar] [CrossRef]
- El-Yazigi, A.; Hannan, N.; Raines, D.A. Effect of diabetic state and related disorders on the urinary excretion of magnesium and zinc in patients. Diabetes Res. 1993, 22, 67–75. [Google Scholar]
- Kaur, B.; Henry, J. Chapter Two—Micronutrient Status in Type 2 Diabetes: A Review. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: San Diego, CA, USA, 2014; Volume 71. [Google Scholar]
- Jansen, J.; Karges, W.; Rink, L. Zinc and diabetes—clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef]
- Scott, D.A.; Fisher, A.M. The insulin and the zinc content of normal and diabetic pancreas. J. Clin. Investig. 1938, 17, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Chabosseau, P.; Rutter, G.A. Zinc and diabetes. Arch. Biochem. Biophys. 2016, 611, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellomo, E.; Massarotti, A.; Hogstrand, C.; Maret, W. Zinc ions modulate protein tyrosine phosphatase 1B activity. Metallomics 2014, 6, 1229–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butler, A.E.; Dargham, S.R.; Latif, A.; Mokhtar, H.R.; Robay, A.; Chidiac, O.M.; Jayyousi, A.; Al Suwaidi, J.; Crystal, R.G.; Abi Khalil, C.; et al. Association of vitamin D3 and its metabolites in patients with and without type 2 diabetes and their relationship to diabetes complications. Ther. Adv. Chronic Dis. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.R.; Reed, B.J.; Sweet, I.R. A highly energetic process couples calcium influx through L-type calcium channels to insulin secretion in pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E717–E727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balbi, M.E.; Tonin, F.S.; Mendes, A.M.; Borba, H.H.; Wiens, A.; Fernandez-Llimos, F.; Pontarolo, R. Antioxidant effects of vitamins in type 2 diabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Lin, N.; Shi, J.J.; Li, Y.M.; Zhang, X.Y.; Chen, Y.; Calder, P.C.; Tang, L.J. What is the impact of n-3 PUFAs on inflammation markers in Type 2 diabetic mellitus populations?: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016, 15, 133. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Pittas, A.G.; Jorde, R.; Kawahara, T.; Dawson-Hughes, B. Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D? J. Clin. Endocrinol. Metab. 2020, 105, 3721–3733. [Google Scholar] [CrossRef]
| Author and Year (Review Type) | N of Participants (Trials) Inclusion Criteria | Intervention (Control Type) | Outcomes—Glycaemic Control | Outcomes—Insulin Resistance | Certainty of Evidence for the Primary Outcome: HbA1c (GRADE) |
|---|---|---|---|---|---|
| VITAMIN C | |||||
| Ashor, A., et al. 2019 [31] (UR) Inception–Feb 2018. Date of search for most recent MA (Ashor 2017) was February 2016. | N= 6409 (10 SR/MA; 3 on T2D) P = SRs and Mas of RCTs in adults of any health status; I = vitamin C administered alone; C = placebo; O = biomarkers of CVD risk (arterial stiffness, blood pressure, endothelial function, glycaemic control, and lipid profile). | Ashor, et al. 2017: duration of 28 to 120 days, dose administered varied between 500 and 2000 mg per day [45] Tabatabaei-Malazy, et al. 2014: duration 4 weeks to 9 years, dose between 200 to 1000 mg/day [46] Khodaeian, et al. 2015: duration 4 to 16 weeks, dose between 800–1000 mg/day [47] | ↓FBG: SMD −20.59 (CI −40.77 to −0.4, 5 trials, n = NR) [46] ↓FBG: WMD −0.41 mmol/L (CI −0.78 to −0.04, 15 trials, n = 469) [45] HbA1c: NS (9 trials, n = NR) [45] | Insulin: NS except for ↓fasting insulin in Ashor, et al. 2017: WMD: −15.67 pmol/L (CI −31.61 to 0.27, 3 trials, n = NR) [45] HOMA-IR: NS (3 trials, n = 92) [47] | NA |
| Mason, S., et al. 2021 [43] (MA) Inception–September 2020 | N = 1574 (28 trials) P = RCTs in people with T2D; I = vitamin C; C = placebo; O = HbA1c, FBG, PPG, FI, HOMA-IR, clamp insulin sensitivity, lipids, BP, oxidative stress markers 10 trials published after the Ashor umbrella review. | Oral vitamin C supplementation Dose range: 200 to 3000 mg daily Duration: 2 weeks to 1 year, majority of studies <6 months duration (Placebo) | ↓FBG: MD −0.74 mmol/L (CI −1.17 to −0.31, 19 trials, n = 1305) ↓HbA1c: MD −0.54% (CI −0.90 to −0.17, 16 trials, n = 1133) ↓PPG: MD −0.95 mmol/L (CI −1.83 to −0.06, 4 trials, n = 235). No modifying effect from baseline Vitamin C concentration | Fasting insulin (9 trials, n = 436), HOMA-IR (5 trials, n = 263) and clamp insulin sensitivity (3 trials, n = 86): NS | Very low certainty * Evidence rated down for inconsistency (1 level), imprecision (1 level), and indirectness (1 level). |
| CHROMIUM | |||||
| Zhao, F., et al. 2021 [40] (MA) Inception–July 2020 | N = 509 (10 trials) P = RCTs in people with T2D with lab values FBG ≥ 140 mg/dL, HbA1c ≥ 6.9%, triglyceride ≥ 125 mg/dL; I = chromium supplementation; C = placebo; O = HbA1c, FBG, triglycerides | 5 different forms of chromium supplements: Cr- containing milk powder, Cr-enriched yeast, chromium nicotinate, brewer’s yeast and chromium picolinate. Doses of chromium ranged from 42 to 1000 μg per day. Duration of intervention ranged from 90 days to 25 weeks. (Placebo) | FBG: NS (10 trials, n = 522) ↓HbA1c: MD −0.54% (CI −0.98 to −0.09, 9 trials, n = 481) | Very low certainty Quality of the evidence was downgraded one level for “risk of bias” due to high risk of bias for blinding and unclear allocation concealment; two levels for “inconsistency” due to varying point estimates, inconsistent direction of effect, limited overlap of confidence intervals and point estimates, and high heterogenetiy; one level for “imprecision” due to small sample sizes, and one level for “publication bias” | |
| PROBIOTICS | |||||
| Cao, D., et al. 2021 [38] (MA) From inception–May 2020 | N = 1948 (31 trials, 17 in people with T2D) P = RCTs in people with prediabetes, T2D or GDM; I = probiotics or synbiotics; C = placebo; O = FBG, HbA1c, fasting insulin, HOMA-IR, HOMA-B, and QUICKI Search: May 2020 | Single-strain formulation was used in 4 studies and bacteria from Lactobacillus (including Lactobacillus sporogenes) and Bifidobacterium genera were included in probiotic formulations in all 17 and 9 of the included studies, respectively. 7 studies—synbiotics 10 studies—probiotics Dose range: 1 × 108 CFU to 1.00001 × 1012 CFU per day. Duration: 6 weeks to 6 months (Placebo) | ↓FBG: WMD −9.48 mg/dL (CI −16.24 to −2.72, n of trials NR, n = 1016) ↓HbA1c: WMD −0.43% (CI −0.69 to −0.18, n of trials NR, n = 635) | Not provided by review authors, and GRADE assessment not possible due to insufficient reporting of individual RCT risk of bias for subgroups with T2D. Most (>50%) included trials for all disease groups were at unclear or high risk of bias, publication bias could not be ruled out, and heterogeneity was low. | |
| ZINC | |||||
| Wang, X., et al. 2019 [42] (MA) From inception–February 2019 | N-1700 (32 trials, 19 in people with T2D) P = RCTs in people with T2D, GDM, obesity, prediabetes; I = Zinc supplementation; C = placebo or co-supplementation only; O = FBG, 2-h postprandial glucose (2h-PG), fasting insulin, HOMA-IR, HbA1c, or hs-CRP. | Zinc sulphate, gluconate, amino chelate, oxide, and acetate; in some cases, the anion was not specified. Dose range: 4–240 mg/d; median: 30 mg/d); mean 35 mg/d. Duration: 1 to 12 months. (Placebo or co-supplement only) | ↓FBG: WMD −20.34 mg/dL (CI −29.04 to −11.64, 12 trials, n = 752) HbA1c: NS (8 trials, n = 639) PPG: NS (5 trials, n = 256) | HOMA-IR (4 trials, n = 234), fasting insulin (5 trials, n = 292): NS | Very low certainty Quality of the evidence was downgraded one level for “risk of bias” due to limited appropriate sequence generation, blinding, and reporting of withdrawals and dropouts; two levels for “inconsistency” due to high heterogeneity, no consistent direction of effect, and varying point estimates; one level for “indirectness” due to insufficient timeframe in some trials; and one level for “imprecision” due to small sample sizes |
| MAGNESIUM | |||||
| Verma, H. and Garg, R. 2017 [39] (MA) Inception–June 2016 | N = 1694 (28 trials; 17 T2D) P = RCTs in people with T2D/high risk of T2D; I = Magnesium (organic or inorganic) for at least 1 month; C = placebo; O = T2D associated CVD risk factors (FBG, FPI, HbA1C, TC, HDL, LDL, TG, SBP and DBP) | Form of magnesium supplementation included Mg pidolate, citrate, aspartate, chloride, lactate, sulphate and oxide. Dosage ranged from 31.5 mg to 1006 mg of elemental Mg. Duration of intervention ranged from 4 to 24 weeks (Placebo) | ↓FBG: WMD −6.253 mg/dL (CI −10.602 to −1.904, 15 trials, n = 773) HbA1c: NS (7 trials, n = 505) | Fasting insulin: NS | Not provided by review authors, and GRADE assessment not possible due to insufficient reporting of individual RCT risk of bias for subgroups with T2D. All studies were at unclear risk of bias for allocation concealment, and none were at high risk of bias for sequence generation or selective reporting. Risk of bias was generally low for blinding of participants, personnel and outcome assesors. Heterogeneity was moderate, and there was no evidence of publication bias. |
| POLYPHENOLS | |||||
| Jeyaraman, M., et al. 2020 [32] (MA) For Cochrane database: Inception –December 2018 Other databases: Inception–April 2018 | N = 50 (3 trials) P = RCTs in people with T2D (for mixed studies, at least 80% of participants had to be adults with T2D); I = oral resveratrol (any regimen); C = placebo, anti-diabetic medications (OHA, insulin, herbal or nutrient supplements), diet/exercise or no treatment; O = adverse events (primary); diabetes-related or all-cause mortality, diabetes complications, HbA1c, FBG, insulin sensitivity, etc. (secondary). | Resveratrol Dose range: 10 mg, 150 mg, or 1000 mg daily. Duration: 4 to 5 weeks. (Placebo) | FBG: NS (2 trials, n = 33) HbA1c: NS (2 trials, n = 33) | HOMA-IR: NS (2 trials, n = 36) | Very low certainty * Downgraded by one level because of indirectness (surrogate outcome and insufficient time frame) and by two levels because of serious imprecision (low median sample size and small number of studies, CI ranging between benefit and harm) |
| Raimundo, A., et al. 2020 [33] (MA) Initial search: Inception–November 2016, and updated in Jane 2018 | N = 1200 (20 total,14 in people with T2D) P = RCTs in people with prediabetes or T2D; I = pure (poly)phenol or an enriched fraction of (poly)phenols (4 weeks or more for glucose, 12 weeks or more for HbA1c); C = placebo; O = FBG, HbA1c, insulin, HOMA-IR, IAPP/amylin, glucagon, and C-peptide. Included cross-over trials. | 3 trials: Polyphenol mixture (from passion fruit, grape, pine tree park, among others- doses of 125–2093 mg/day) 5 trials: Resveratrol (doses of 40–1000 mg/day) 3 trials: Isoflavones (doses of 33–100 mg/day) 2 trials: Flavanols (doses of 560–1270 mg/day) 1 trial: Anthocyanin (392 mg/day) Duration: 4 to 52 weeks. (Placebo) | ↓FBG: MD −5.86 mg/dL (CI −11.34 to −0.39, 13 trials, n = 740) Subgroup analysis: ↓FBG in those taking anti-diabetic medication: MD − 10.17 (CI −17.59 to −3.75, 6 trials, n = 378) No subgroup analysis conducted in people with T2D for HbA1c | Insulin (9 trials, n = 552) and HOMA-IR (7 trials, n = 489): NS | Not provided by review authors, and GRADE assessment not possible due to insufficient reporting of individual RCT risk of bias for subgroups with T2D. 12/14 trials were at moderate-high risk of bias. Heterogeneity was moderate and there was no evidence of publication bias for the included trials for all disease groups. |
| Ω-3 PUFAs | |||||
| Gao, C., et al. 2020 [35] (MA) Inception–May 2019 | N = 820 (12 trials) P = RCTs in people with T2D; I = Fish oil supplementation alone; C = placebo; O = TG, TC, HDL-C, LDL-C, FBG, FPI, HbA1c, and HOMA-IR. | n3-PUFAs in fish oil Dose range n-3 PUFA: 0.3 g/d to 10.08 g/d. Duration: 3 weeks to 6 months. (Placebo) | FBG: NS at any time point; ≤1 month (3 trials, n = 102); 1–3 months (6 trials, n = 451) or >3 months (4 trials, n = 381) HbA1c: NS at any time-point; ≤1 month (1 trial, n = 20), 1–3 months (5 trials, n = 329), or >3 months (4 trials, n = 309) | HOMA-IR: NS at any time-point; ≤1 month (2 trials, n = 82), 1–3 months (2 trials, n = 224) or >3 months (4 trials, n = 381) Fasting insulin: NS at any time-point; ≤1 month (2 trials, n = 61), 1–3 months (2 trials, n = 224), or >3 months (4 trials, n = 381) | Very low certainty Quality of the evidence was downgraded one level for “risk of bias” due to unclear risk of bias across random sequence generation, allocation concealment, and blinding; one level for “inconsistency” due to limited overlap of confidence intervals and point estimates and inconsistent direction of the effect; one level for “imprecision” due to small sample sizes; and one level for “publication bias” |
| O’Mahoney, L., et al. 2018 [36] (MA and MR) Inception–July 2017 | N = 1187 T2D (45 total, 31 with glycaemic outcomes) P = Parallel or cross-over RCTs in people with T2D; I = n3-PUFAs including in diet as long as dosage and duration could be determined; C = placebo; FBG, HbA1c, fasting insulin, HOMA-IR and C-peptide, lipid profile, inflammatory markers, BP. | Ω-3 PUFAs in capsule/liquid or diet (sardine-enriched) form. Origin of n3-PUFAs not reported. All trials used EPA, DHA, or a combination. Dose range: 0.40 to 18.00 g. Duration: 2 to 104 weeks (14/33 RCTs were >3 months duration) (Placebo) | FBG: NS (28 trials, n = 1702) ↓HbA1c: Effect size −0.27 (CI −0.48 to −0.06, 31 trials, n = 2021) In “leave one out” sensitivity analysis for HbA1c, removal of two RCTs attenuated the statistical significance of the results to non-significant. | HOMA-IR, fasting insulin: NS | Very low certainty Quality of the evidence was downgraded one level for “inconsistency” due to high heterogeneity, inconsistent direction of effect and limited overlap of confidence intervals and point estimates; one level due to insufficient timeframe in some trials; and one level for “imprecision” due to small sample sizes and confidence interval not consistent with benefit |
| Ebada, M., et al. 2019 [34] (MA) Inception–May 2017, and updated on April 2018 | N = 553 (10 trials, 8 T2D) P = RCTs in people with T1D or T2D; I = 1) RCTs with DM patients (both T1 and T2); alpha-lipoic acid (ALA); C = placebo; HbA1c, FBG, PPG, HDL, LDL, TG, TC, HOMA, Glutathione peroxidase, and waist circumference. | Alpha-lipoic acid oral or intravenous. Dose range: 300–600 mg/d. The follow-up duration ranged from three weeks to six months. Duration of intervention NR. (Placebo) | FBG: NS (6 trials, n = 322), HbA1c: NS (6 trials, n = 316) PPG: NS (3 trials, n = 190): | Very low certainty Quality of the evidence was downgraded by one level for “inconsistency” due to limited overlap of confidence intervals and point estimates, moderate heterogeneity and inconsistent direction of effect; one level for “indirectness” due to limited applicability and insufficient timeframe for some trials; one level for “imprecision” due to small sample sizes; and one level for “publication bias” | |
| VITAMIN D | |||||
| Hu, Z., et al. 2019 [37] (MA) From inception of database–March 2018 | N = 747 (19 trials) P = RCTs in people with T2D; I = Vitamin D; C = placebo; O = FBG, insulin, HbA1c, HOMA-IR. | Vitamin D (type NR) Dose range: 1000 IU/d to 300,000 IU single IM injection Duration: 4 wk to 12 mo. Duration <6 mo considered short-term. (Placebo) | FBG: NS (14 trials, n = 289), HbA1c: NS (19 trials, n = 747) Short-term (<6 mo): ↓ HbA1c: SMD −0.17% (CI −0.27 to −0.04, 15 trials, n = 1059) Long term: NS. | ↓ HOMA-IR: SMD −0.60 (CI −0.79 to −0.42, 9 trials, n = 425) ↓Fasting insulin: SMD −0.49 (CI −0.68 to −0.31, 9 trials, n = 436) Short-term (<6 mo): ↓ HOMA-IR: SMD −0.75 (CI −0.97 to −0.53, 8 trials, n = 405) ↓ Insulin: SMD −0.57 (CI −0.78 to −0.35, 8 trials, n = 389). Long term: NS. | Very low certainty Quality of the evidence was downgraded one level for “risk of bias” because allocation concealment was unclear; two levels for “inconsistency” because the direction of the effect was inconsistent and there was high heterogeneity; and one level for “imprecision” due to small sample sizes |
| FOLATE | |||||
| Lind, M., et al. 2019 [41] (MA) Pubmed from 1953–March 2018 Web of Science from 1900–March 2018 EMBASE from 1974–March 2018. | N = 572 (29 total, 8 T2D) P = Parallel and cross-over RCTs with no restriction on health condition; folate supplementation; C = placebo; O = glucose, insulin, HOMA-IR, or HbA1c. | Folate given as adjuvant therapy (alongside antidiabetic medication ± insulin). Two studies combined folate with B12 and B6. Dose range: 0.25 mg folate and 5 mg folic acid/d, with most studies using dosage of 5 mg. Duration: 2 weeks to 2 years, with the majority of studies lasting between 4 and 8 weeks. (Placebo) | FBG: NS (6 trials, n = 309) HbA1c: NS (7 trials, n = 482). No differences found on subgroup analysis by baseline folate concentration, however this was conducted on the pooled sample which included people without T2D. | HOMA-IR: NS (9 trials, n = 431) | Very low certainty Quality of the evidence was downgraded two levels for “risk of bias” due to unclear allocation concealment and blinding of outcome assessment, and high risk of selective reporting; one level for “inconsistency” due to varying point estimates and limited overlap of confidence intervals and point estimates; one level for “indirectness” due to insufficient timeframe and inclusion of co-interventions in some trials; and one level for “imprecision” due to small sample sizes. |
| Nutrient Supplement | Reference | Critical Flaws a | AMSTAR 2 Rating b |
|---|---|---|---|
| Vitamin C | Mason, et al. 2021 [43] | 7 | Low |
| Chromium | Zhao, et al. 2021 [40] | 2, 7, 15 | Critically low |
| Probiotics | Cao, et al. 2021 [38] | 2, 7, 13 | Critically low |
| Zinc | Wang, et al. 2019 [42] | 7, 9, 11 | Critically low |
| Magnesium | Verma, H., Garg, R. [39] | 2 | Low |
| Polyphenols | Jeyaraman, et al. 2020 [32] | None | High |
| Raimundo, et al. 2020 [33] | 7 | Low | |
| PUFAs | Gao, et al. 2020 [35] | 7, 11, 13 | Critically low |
| O’Mahoney, et al. 2018 [36] | 7 | Low | |
| Ebada, et al. 2019 [34] | 7 | Low | |
| Vitamin D | Hu, et al. 2019 [37] | 4, 7, 9, 11, 13 | Critically low |
| Folate | Lind, et al. 2019 [41] | 7 | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fong, C.; Alesi, S.; Mousa, A.; Moran, L.J.; Deed, G.; Grant, S.; Tapia, K.; Ee, C. Efficacy and Safety of Nutrient Supplements for Glycaemic Control and Insulin Resistance in Type 2 Diabetes: An Umbrella Review and Hierarchical Evidence Synthesis. Nutrients 2022, 14, 2295. https://doi.org/10.3390/nu14112295
Fong C, Alesi S, Mousa A, Moran LJ, Deed G, Grant S, Tapia K, Ee C. Efficacy and Safety of Nutrient Supplements for Glycaemic Control and Insulin Resistance in Type 2 Diabetes: An Umbrella Review and Hierarchical Evidence Synthesis. Nutrients. 2022; 14(11):2295. https://doi.org/10.3390/nu14112295
Chicago/Turabian StyleFong, Charmie, Simon Alesi, Aya Mousa, Lisa J. Moran, Gary Deed, Suzanne Grant, Kriscia Tapia, and Carolyn Ee. 2022. "Efficacy and Safety of Nutrient Supplements for Glycaemic Control and Insulin Resistance in Type 2 Diabetes: An Umbrella Review and Hierarchical Evidence Synthesis" Nutrients 14, no. 11: 2295. https://doi.org/10.3390/nu14112295
APA StyleFong, C., Alesi, S., Mousa, A., Moran, L. J., Deed, G., Grant, S., Tapia, K., & Ee, C. (2022). Efficacy and Safety of Nutrient Supplements for Glycaemic Control and Insulin Resistance in Type 2 Diabetes: An Umbrella Review and Hierarchical Evidence Synthesis. Nutrients, 14(11), 2295. https://doi.org/10.3390/nu14112295









