Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. Active Substance Content
2.2. In Vitro Free Radical Scavenging Ability of WEOR and FBOR
2.3. Cell Viability
2.4. Influence of WEOR and FBOR on Antioxidant Levels in Cells
2.5. WEOR and FBOR Can Cause Nuclear Translocation of Nrf2 in Cells
2.6. Influence of WEOR and FBOR on Antioxidant Levels in Cells
2.7. Effects of WEOR and FBOR on the Activity of MMPs in Cells Irradiated by UVA
2.8. Influence of WEOR and FBOR on Collagen and Elastin Activity
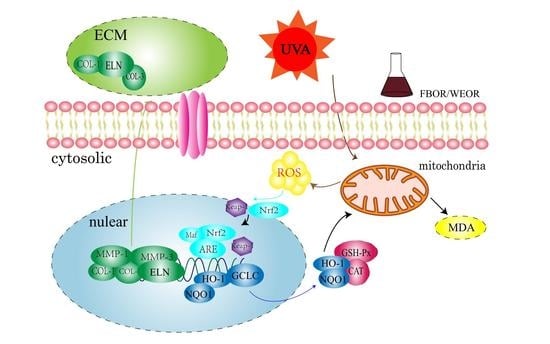
3. Discussion
4. Experimental
4.1. Materials
4.2. Lactobacillus Plantarum Fermented Rhodiola rosea
4.3. Measurement of Polysaccharides, Flavonoids, and Protein Content
4.4. Antioxidant Activity Analysis
4.5. Cell Culture
4.6. Irradiation Procedure
4.7. Assay of Cell Viability
4.8. ROS
4.9. Antioxidants and Lipid Peroxidation Levels
4.10. Enzyme-Linked Immunosorbent Assay
4.11. RT-PCR
4.12. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Pham, V.; Bui, M.; Song, L.; Wu, C.; Walia, A.; Uchio, E.; Smith-Liu, F.; Zi, X. Rhodiola rosea L.: An Herb with Anti-Stress, Anti-Aging, and Immunostimulating Properties for Cancer Chemoprevention. Curr. Pharmacol. Rep. 2017, 3, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.S.; Shao, M.L.; Ze, S.; Zheng, H.X. Salidroside from Rhodiola rosea L. attenuates diabetic nephropathy in STZ induced diabetic rats via anti-oxidative stress, anti-inflammation, and inhibiting TGF-β1/Smad pathway. J. Funct. Foods 2021, 77, 104329. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Cheng, Q.; Ding, F. Protective effect of salidroside against H2O2-induced cell apoptosis in primary culture of rat hippocampal neurons. Mol. Cell. Biochem. 2009, 332, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.N.; Kashchenko, N.I.; Akobirshoeva, A. Phenolic Compounds and Hydroxynitrile Glycosides from Roots of Rhodiola recticaulis and R. gelida. Chem. Nat. Compd. 2019, 55, 948–950. [Google Scholar] [CrossRef]
- Yousef, G.G.; Grace, M.H.; Cheng, D.M.; Belolipov, I.V.; Raskin, I.; Lila, M.A. Comparative phytochemical characterization of three Rhodiola species. Phytochemistry 2006, 67, 2380–2391. [Google Scholar] [CrossRef]
- Mishra, K.P.; Padwad, Y.S.; Jain, M.; Karan, D.; Ganju, L.; Sawhney, R.C. Aqueous extract of Rhodiola imbricata rhizome stimulates proinflammatory mediators via phosphorylated IκB and transcription factor nuclear factor-κB. Immunopharmacol. Immunotoxicol. 2006, 28, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Liou, H.C.; Chan, C.F. Tyrosinase inhibitory effect and antioxidative activities of fermented and ethanol extracts of Rhodiola rosea and lonicera japonica. Sci. World J. 2013, 2013, 612739. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.Z.; Hong, H.D.; Kim, K.I.; Choi, S.Y. Anti-fatigue effects of fermented Rhodiola rosea extract in mice. Prev. Nutr. Food Sci. 2015, 20, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.C.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef] [Green Version]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Dermatoendocrinology 2012, 4, 236–240. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Bai, D.; Qin, L.; Shao, M.; Zhang, S.; Yan, C.; Yu, G.; Hao, J. Protective effect of D-tetramannuronic acid tetrasodium salt on UVA-induced photo-aging in HaCaT cells. Biomed. Pharmacother. 2020, 126, 110094. [Google Scholar] [CrossRef] [PubMed]
- Jaszewska, E.; Soin, M.; Filipek, A.; Naruszewicz, M. UVA-induced ROS generation inhibition by Oenothera paradoxa defatted seeds extract and subsequent cell death in human dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2013, 126, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.; Tran, Q.; Cho, H.; Kim, M.; Kim, C.; Kwon, S.H.; Park, S.J.; Park, J.; Park, J. A new role for the ginsenoside RG3 in antiaging via mitochondria function in ultraviolet-irradiated human dermal fibroblasts. J. Ginseng Res. 2019, 43, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Hanselmann, C.; Gassmann, M.G.; auf dem Keller, U.; Born-Berclaz, C.; Chan, K.; Kan, Y.W.; Werner, S. Nrf2 Transcription Factor, a Novel Target of Keratinocyte Growth Factor Action Which Regulates Gene Expression and Inflammation in the Healing Skin Wound. Mol. Cell. Biol. 2002, 22, 5492–5505. [Google Scholar] [CrossRef] [Green Version]
- Goodfellow, M.J.; Borcar, A.; Proctor, J.L.; Greco, T.; Rosenthal, R.E.; Fiskum, G. Transcriptional activation of antioxidant gene expression by Nrf2 protects against mitochondrial dysfunction and neuronal death associated with acute and chronic neurodegeneration. Exp. Neurol. 2020, 328, 113247. [Google Scholar] [CrossRef]
- XiaoPing, C.; Yan, C.; ShuiBing, L.; YouGuo, C.; JianYun, L.; LanPing, L. Free radical scavenging of Ganoderma lucidum polysaccharides and its effect on antioxidant enzymes and immunity activities in cervical carcinoma rats. Carbohydr. Polym. 2009, 77, 389–393. [Google Scholar] [CrossRef]
- Getoff, N. Anti-aging and aging factors in life. The role of free radicals. Radiat. Phys. Chem. 2007, 76, 1577–1586. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Chang, C.T.; Gowrisankar, Y.V.; Chen, X.Z.; Lin, H.C.; Yen, H.R.; Yang, H.L. Zerumbone exhibits antiphotoaging and dermatoprotective properties in ultraviolet A-irradiated human skin fibroblast cells via the activation of Nrf2/ARE defensive pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4098674. [Google Scholar] [CrossRef]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Fan, Y.; Wang, S.; Chen, Q.; Si, L.; Li, B. Protective effect of gelatin peptides from pacific cod skin against photoaging by inhibiting the expression of MMPs via MAPK signaling pathway. J. Photochem. Photobiol. B Biol. 2016, 165, 34–41. [Google Scholar] [CrossRef]
- Tri Nuryana, C.; Haryana, S.M.; Wirohadidjojo, Y.W.; Arfian, N. Achatina fulica mucous improves cell viability and increases collagen deposition in uvb-irradiated human fibroblast culture. J. Stem Cells Regen. Med. 2020, 16, 26–31. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, D.; Kim, H.J.; Jang, A. Protection effect of donkey hide gelatin hydrolysates on UVB-induced photoaging of human skin fibroblasts. Process Biochem. 2018, 67, 118–126. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Jin, J.S. Fermentation of traditional medicine: Present and future. Orient. Pharm. Exp. Med. 2012, 12, 163–165. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Dordević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Bose, S.; Jeon, S.; Eom, T.; Song, M.Y.; Kim, H. Evaluation of the in vitro and in vivo protective effects of unfermented and fermented Rhizoma coptidis formulations against lipopolysaccharide insult. Food Chem. 2012, 135, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Mishra, A.; Chattopadhyay, P. Herbal cosmeceuticals for photoprotection from ultraviolet B radiation: A review. Trop. J. Pharm. Res. 2011, 10, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.; Hou, K.; Mu, G.; Ma, C.; Tuo, Y. Antioxidative effect of soybean milk fermented by Lactobacillus plantarum Y16 on 2,2–azobis (2-methylpropionamidine) dihydrochloride (ABAP)-damaged HepG2 cells. Food Biosci. 2021, 44, 101120. [Google Scholar] [CrossRef]
- Hsu, M.F.; Chiang, B.H. Effect of Bacillus subtilis natto-fermented Radix astragali on collagen production in human skin fibroblasts. Process Biochem. 2009, 44, 83–90. [Google Scholar] [CrossRef]
- Liu, J.G.; Hou, C.W.; Lee, S.Y.; Chuang, Y.; Lin, C.C. Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Process Biochem. 2011, 46, 1405–1410. [Google Scholar] [CrossRef]
- Jamioł, M.; Wawrzykowski, J.; Dec, M.; Wilk, A.; Czelej, M. Comparison of Various Techniques for the Extraction, Analysis of Compounds and Determination of Antioxidant Activities of Rhodiola spp.—A Review. Food Rev. Int. 2021, 1–21. [Google Scholar] [CrossRef]
- Marracino, L.; Punzo, A.; Severi, P.; Tchoutang, R.N.; Vargas-de-la-cruz, C.; Fortini, F.; Vieceli, F.; Sega, D.; Silla, A.; Porru, E.; et al. Fermentation of Vaccinium floribundum Berries with Lactiplantibacillus plantarum Reduces Oxidative Stress in Endothelial Cells and Modulates Macrophages Function. Nutrients 2022, 14, 1560. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.; Chan, H.F.; Leung, H.H.; Galano, J.M.; Oger, C.; Durand, T.; Lee, J.C.Y. Short-time UVA exposure to human keratinocytes instigated polyunsaturated fatty acid without inducing lipid peroxidation. Free Radic. Res. 2017, 51, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–22. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Suzuki, T.; Yamamoto, M. Stress-sensing mechanisms and the physiological roles of the Keap1–Nrf2 system during cellular stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [Green Version]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yamamoto, M. Molecular basis of the Keap1-Nrf2 system. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zheng, W.; Feng, X.; Yang, F.; Qin, H.; Wu, S.; Hou, D.X.; Chen, J. Nrf2–ARE signaling acts as master pathway for the cellular antioxidant activity of fisetin. Molecules 2019, 24, 708. [Google Scholar] [CrossRef] [Green Version]
- Prasanth, M.I.; Gayathri, S.; Bhaskar, J.P.; Krishnan, V.; Balamurugan, K. Understanding the role of p38 and JNK mediated MAPK pathway in response to UV-A induced photoaging in Caenorhabditis elegans. J. Photochem. Photobiol. B Biol. 2020, 205, 111844. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.H.; Kang, S.; Chung, J.H.; Wang, Z.Q.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Investig. Dermatol. 2000, 114, 480–486. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.A.A.; Jung, M.; Lee, S.M.; Lee, T.H.; Kim, J. Protective effect of Disporum sessile D.Don extract against UVB-induced photoaging via suppressing MMP-1 expression and collagen degradation in human skin cells. J. Photochem. Photobiol. B Biol. 2014, 133, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, Y.T.; Kuo, H.C.; Chiou, W.F.; Lee, M.H. Compounds isolated from Eriobotrya deflexa leaves protect against ultraviolet radiation B-induced photoaging in human fibroblasts. J. Photochem. Photobiol. B Biol. 2017, 175, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.P.; Wang, X.; Pratheeshkumar, P.; Son, Y.O.; Roy, R.V.; Kim, D.; Dai, J.; Hitron, J.A.; Wang, L.; Asha, P.; et al. Blackberry extract inhibits UVB-induced oxidative damage and inflammation through MAP kinases and NF-κB signaling pathways in SKH-1 mice skin. Toxicol. Appl. Pharmacol. 2015, 284, 92–99. [Google Scholar] [CrossRef] [Green Version]







| Concentration (mg/mL) | WEOR | FBOR |
|---|---|---|
| Polysaccharides | 6.03 ± 0.05 a | 7.58 ± 0.25 b |
| Proteins | 2.92 ± 0.12 c | 4.11 ± 0.02 d |
| Total phenols | 0.17 ± 0.01 e | 0.25 ± 0.03 f |
| Flavonoids | 0.006 ± 0.0001 g | 0.007 ± 0.0003 g |
| Primer Name | Primer Sequences (5′–3′) | |
|---|---|---|
| Keap1 | F | GGAGGCGGAGCCCGA |
| R | GATGCCCTCAATGGACACCA | |
| Nrf2 | F | CAACTCAGCACCTTGTATC |
| R | TTCTTAGTATCTGGCTTCTT | |
| HO1 | F | CAAGCGCTATGTTCAGCGAC |
| R | GCTTGAACTTGGTGGCACTG | |
| NQO1 | F | CAGCCAATCAGCGTTCGGTA |
| R | CTTCATGGCGTAGTTGAATGATGTC | |
| CAT | F | CCTTCGACCCAAGCAA |
| R | CGATGGCGGTGAGTGT | |
| SOD | F | TGGAGATAATACAGCAGGCT |
| R | AGTCACATTGCCCAAGTCTC | |
| GSH-Px | F | AGAAGTGCGAGGTGAACGGT |
| R | CCCACCAGGAACTTCTCAAA | |
| MMP-1 | F | GCA TATCGATGCTGCTCTTTC |
| R | GATAACCTGGATCCATAGATCGTT | |
| MMP-3 | F | CAA AACATATTTCTTTGTAGAGGACAA |
| R | TTCAGCTATTTGCTTGGGAAA | |
| COL I | F | GTGCTAAAGGTGCCAATGGT |
| R | GTGGGGAATGGCAAGCAAAA | |
| COL III | F | CCAGGAGCTAACGGTCTCAG |
| R | CAGGGTTTCCATCTCTTCCA | |
| β-actin | F | TGGCACCCAGCACAATGAA |
| R | CTAAGTCATAGTCCGCCTAGAAGC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, H.; Zhang, Y.; An, Q.; Wang, D.; You, S.; Zhao, D.; Zhang, J.; Wang, C.; Li, M. Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts. Nutrients 2022, 14, 2324. https://doi.org/10.3390/nu14112324
Fu H, Zhang Y, An Q, Wang D, You S, Zhao D, Zhang J, Wang C, Li M. Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts. Nutrients. 2022; 14(11):2324. https://doi.org/10.3390/nu14112324
Chicago/Turabian StyleFu, Hao, Yuzhi Zhang, Quan An, Dongdong Wang, Shiquan You, Dan Zhao, Jiachan Zhang, Changtao Wang, and Meng Li. 2022. "Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts" Nutrients 14, no. 11: 2324. https://doi.org/10.3390/nu14112324
APA StyleFu, H., Zhang, Y., An, Q., Wang, D., You, S., Zhao, D., Zhang, J., Wang, C., & Li, M. (2022). Anti-Photoaging Effect of Rhodiola rosea Fermented by Lactobacillus plantarum on UVA-Damaged Fibroblasts. Nutrients, 14(11), 2324. https://doi.org/10.3390/nu14112324