Associations of Vitamin B6 Intake and Plasma Pyridoxal 5′-Phosphate with Plasma Polyunsaturated Fatty Acids in US Older Adults: Findings from NHANES 2003–2004
Abstract
:1. Introduction
2. Materials and Methods
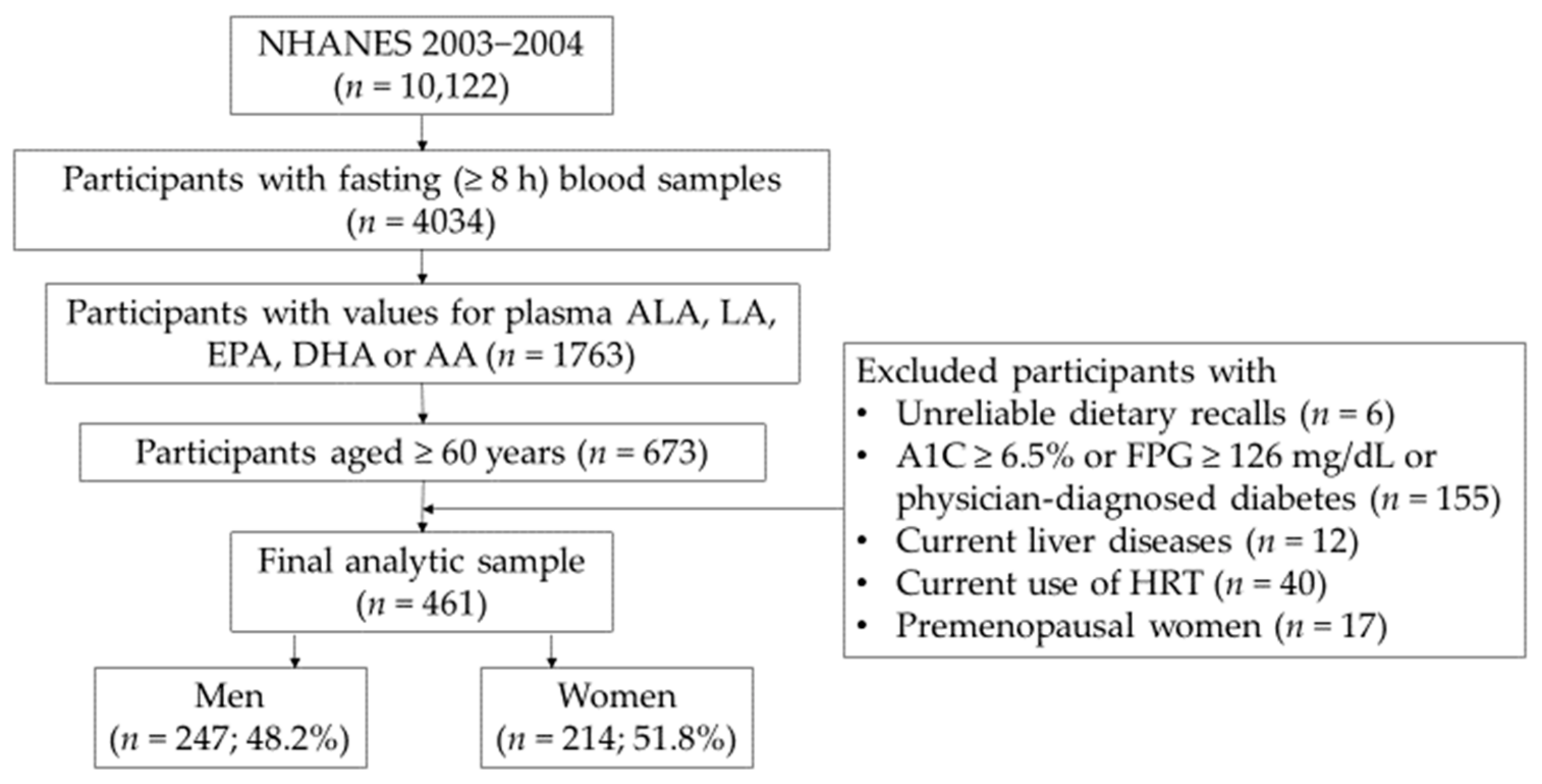
2.1. Data Source and Study Population
2.2. Assessment of Vitamin B6 and PUFA Intakes
2.3. Measurements of Plasma PLP and Plasma PUFA
Updated Plasma Fatty Acid Data in NHANES 2003–2004
2.4. Study Covariates
2.5. Terminology for Gender and Sex
2.6. Statistical Methods
2.6.1. Linear Regression Analyses
2.6.2. Logistic Regression Analyses
3. Results
3.1. Demographic, Socioeconomic, and Other Characteristics of Participants by Gender in Older Adults
3.2. Distributions of Vitamin B6 and PUFA Intakes and Plasma PUFA and PLP Concentrations by Gender in Older Adults
3.3. Associations of Vitamin B6 Intake with Plasma EPA and AA Concentrations, Stratified by Gender, in Older Adults
3.4. Associations of Plasma PLP Concentration with Plasma PUFA Concentrations and Ratios in Older Adults
3.5. Odds Ratios of High Plasma PUFA Status for Adequate Vitamin B6 Status Versus Deficient Vitamin B6 Status in Older Adults
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv Nutr 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef] [Green Version]
- Krajcovicova-Kudlackova, M.; Klvanova, J.; Dusinska, M. Polyunsaturated Fatty Acid Plasma Content in Groups of General Population with lowvitamin B6 or low iron serum levels. Ann. Nutr. Metab. 2004, 48, 118–121. [Google Scholar] [CrossRef]
- Zhao, M.; Lamers, Y.; Ralat, M.A.; Coats, B.S.; Chi, Y.Y.; Muller, K.E.; Bain, J.R.; Shankar, M.N.; Newgard, C.B.; Stacpoole, P.W.; et al. Marginal vitamin B-6 deficiency decreases plasma (n-3) and (n-6) PUFA concentrations in healthy men and women. J. Nutr. 2012, 142, 1791–1797. [Google Scholar] [CrossRef] [Green Version]
- Delorme, C.B.; Lupien, P.J. The effect of vitamin B-6 deficiency on the fatty acid composition of the major phospholipids in the rat. J. Nutr. 1976, 106, 169–180. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Manku, M.S.; Horrobin, D.F. Accumulation of linoleic and gamma-linolenic acids in tissue lipids of pyridoxine-deficient rats. J. Nutr. 1984, 114, 1754–1761. [Google Scholar] [CrossRef]
- She, Q.; Hayakawa, T.; Tsuge, H. Effect of Vitamin B6 Deficiency on Linoleic Acid Desaturation in the Arachidonic Acid Biosynthesis of Rat Liver Microsomes. Biosci. Biotechnol. Biochem 1994, 58, 459–463. [Google Scholar] [CrossRef]
- Tsuge, H.; Hotta, N.; Hayakawa, T. Effects of vitamin B-6 on (n-3) polyunsaturated fatty acid metabolism. J. Nutr. 2000, 130, 333S–334S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrini, L.; Bergami, R.; Maranesi, M.; Carloni, A.; Marchetti, M.; Tolomelli, B. Effects of short-term dietary administration of marginal levels of vitamin B(6)and fish oil on lipid composition and antioxidant defences in rat tissues. Prostaglandins Leukot. Essent. Fatty Acids 2001, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Gross, P. The Rôle of the Unsaturated Fatty Acids in the Acrodynia (Vitamin B6 Deficiency) of the Albino Rat. J. Investig. Dermatol. 1940, 3, 505–522. [Google Scholar] [CrossRef] [Green Version]
- Salmon, W.D. The effect of certain oils in alleviating localised erythematous dermatitis (acrodynia or vitamin B-6 deficiency) in rats. J. Biol. Chem. 1938, 123, 104. [Google Scholar]
- Birch, T.W.; György, P.; Harris, L.J. The vitamin B(2) complex. Differentiation of the antiblacktongue and the “P.-P.” factors from lactoflavin and vitamin B(6) (so-called “rat pellagra” factor). Parts I-VI. Biochem. J. 1935, 29, 2830–2850. [Google Scholar] [CrossRef] [PubMed]
- Birch, T.W. The relation between vitamin B6 and the unsaturated fatty acid factor. J. Clin. Investig. 1938, 17, 775–793. [Google Scholar] [CrossRef]
- Quackenbush, F.W.; Platz, B.R.; Steenbock, H. Rat Acrodynia and the Essential Fatty Acids. J. Nutr. 1939, 17, 115–126. [Google Scholar] [CrossRef]
- Fortier, M.; Tremblay-Mercier, J.; Plourde, M.; Chouinard-Watkins, R.; Vandal, M.; Pifferi, F.; Freemantle, E.; Cunnane, S.C. Higher plasma n-3 fatty acid status in the moderately healthy elderly in southern Québec: Higher fish intake or aging-related change in n-3 fatty acid metabolism? Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Bolton-Smith, C.; Woodward, M.; Tavendale, R. Evidence for age-related differences in the fatty acid composition of human adipose tissue, independent of diet. Eur. J. Clin. Nutr. 1997, 51, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Itomura, M.; Fujioka, S.; Hamazaki, K.; Kobayashi, K.; Nagasawa, T.; Sawazaki, S.; Kirihara, Y.; Hamazaki, T. Factors influencing EPA+DHA levels in red blood cells in Japan. In Vivo 2008, 22, 131–135. [Google Scholar] [PubMed]
- Crowe, F.L.; Skeaff, C.M.; Green, T.J.; Gray, A.R. Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br. J. Nutr. 2008, 99, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hrelia, S.; Bordoni, A.; Celadon, M.; Turchetto, E.; Biagi, P.L.; Rossi, C.A. Age-related changes in linoleate and alpha-linolenate desaturation by rat liver microsomes. Biochem. Biophys. Res. Commun. 1989, 163, 348–355. [Google Scholar] [CrossRef]
- Bordoni, A.; Biagi, P.L.; Turchetto, E.; Hrelia, S. Aging influence on delta-6-desaturase activity and fatty acid composition of rat liver microsomes. Biochem. Int. 1988, 17, 1001–1009. [Google Scholar] [PubMed]
- Maniongui, C.; Blond, J.P.; Ulmann, L.; Durand, G.; Poisson, J.P.; Bézard, J. Age-related changes in delta 6 and delta 5 desaturase activities in rat liver microsomes. Lipids 1993, 28, 291–297. [Google Scholar] [CrossRef]
- Bordoni, A.; Hrelia, S.; Lorenzini, A.; Bergami, R.; Cabrini, L.; Biagi, P.L.; Tolomelli, B. Dual influence of aging and vitamin B6 deficiency on delta-6-desaturation of essential fatty acids in rat liver microsomes. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 417–420. [Google Scholar] [CrossRef]
- Rose, C.S.; György, P.; Butler, M.; Andres, R.; Norris, A.H.; Shock, N.W.; Tobin, J.; Brin, M.; Spiegel, H. Age differences in vitamin B6 status of 617 men. Am. J. Clin. Nutr. 1976, 29, 847–853. [Google Scholar] [CrossRef] [Green Version]
- Bates, C.J.; Pentieva, K.D.; Prentice, A.; Mansoor, M.A.; Finch, S. Plasma pyridoxal phosphate and pyridoxic acid and their relationship to plasma homocysteine in a representative sample of British men and women aged 65 years and over. Br. J. Nutr. 1999, 81, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Bor, M.V.; Refsum, H.; Bisp, M.R.; Bleie, Ø.; Schneede, J.; Nordrehaug, J.E.; Ueland, P.M.; Nygard, O.K.; Nexø, E. Plasma vitamin B6 vitamers before and after oral vitamin B6 treatment: A randomized placebo-controlled study. Clin. Chem. 2003, 49, 155–161. [Google Scholar] [CrossRef]
- Nikkari, T.; Luukkainen, P.; Pietinen, P.; Puska, P. Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann. Med. 1995, 27, 491–498. [Google Scholar] [CrossRef]
- Bakewell, L.; Burdge, G.C.; Calder, P.C. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br. J. Nutr. 2006, 96, 93–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giltay, E.J.; Gooren, L.J.; Toorians, A.W.; Katan, M.B.; Zock, P.L. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 2004, 80, 1167–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Enrione, E.B.; Narayanan, V.; Li, T.; Campa, A. Gender Differences in the Associations of Plasma Pyridoxal 5’-Phosphate with Plasma Polyunsaturated Fatty Acids among US Young and Middle-Aged Adults: NHANES 2003–2004. Nutrients 2021, 13, 477. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2013, 161, 1–24. [Google Scholar]
- The Centers for Disease Control and Prevention MEC Interviewers Procedures Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/manuals/MECInterview.pdf (accessed on 29 April 2022).
- The Centers for Disease Control and Prevention, (CDC) National Health and Nutrition Examination Survey: NCHS Research Ethics Review Board (ERB) Approval. Available online: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed on 29 April 2022).
- The Centers for Disease Control and Prevention Laboratory Procedures Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/manuals/lab.pdf (accessed on 29 April 2022).
- Ueland, P.M.; Ulvik, A.; Rios-Avila, L.; Midttun, O.; Gregory, J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015, 35, 33–70. [Google Scholar] [CrossRef]
- Morris, M.S.; Picciano, M.F.; Jacques, P.F.; Selhub, J. Plasma pyridoxal 5’-phosphate in the US population: The National Health and Nutrition Examination Survey, 2003–2004. Am. J. Clin. Nutr. 2008, 87, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Ottosson, U.B.; Lagrelius, A.; Rosing, U.; von Schoultz, B. Relative fatty acid composition of lecithin during postmenopausal replacement therapy--a comparison between ethinyl estradiol and estradiol valerate. Gynecol. Obstet. Investig. 1984, 18, 296–302. [Google Scholar] [CrossRef]
- The Centers for Disease Control and Prevention MEC In-Person Dietary Interviewers Procedures Manual. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/manuals/DIETARY_MEC.pdf (accessed on 29 April 2022).
- USDA Food and Nutrient Database for Dietary Studies, 2.0; Agricultural Research Service, Food Surveys Research Group: Beltsville, MD, USA, 2006.
- National Center for Health Statistics National Health and Nutrition Examination Survey. Dietary Supplement Use 30-Day-File 1. 2003–2004 Data Documentation, Codebook, and Frequencies. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/DSQ1_C.htm (accessed on 29 April 2022).
- National Center for Health Statistics National Health and Nutrition Examination Survey. 1999–2018 Data Documentation, Codebook, and Frequencies. Dietary Supplement Database—Ingredient Information (DSII). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/DSII.htm (accessed on 29 April 2022).
- The Centers for Disease Control and Prevention Laboratory Procedure Manual: Pyridoxal 5′-Phosphate (PLP) NHANES 2003–2004. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l43_c_met_plp.pdf (accessed on 29 April 2022).
- The Centers for Disease Control and Prevention National Health and Nutrition Examination Survey 2003–2004 Data Documentation, Codebook, and Frequencies: Plasma Fatty Acids (Surplus) Data. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/SSFA_C.htm (accessed on 29 April 2022).
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of non-detectable value. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Murphy, R.A.; Yu, E.A.; Ciappio, E.D.; Mehta, S.; McBurney, M.I. Suboptimal Plasma Long Chain n-3 Concentrations are Common among Adults in the United States, NHANES 2003–2004. Nutrients 2015, 7, 10282–10289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schleicher, R.L.; Sternberg, M.R.; Pfeiffer, C.M. Race-ethnicity is a strong correlate of circulating fat-soluble nutrient concentrations in a representative sample of the U.S. population. J. Nutr. 2013, 143, 966S–976S. [Google Scholar] [CrossRef] [PubMed]
- de Groot, R.H.M.; Emmett, R.; Meyer, B.J. Non-dietary factors associated with n-3 long-chain PUFA levels in humans—A systematic literature review. Br. J. Nutr. 2019, 121, 793–808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisk, H.L.; Irvine, M.; Miles, E.A.; Lietz, G.; Mathers, J.C.; Packard, C.J.; Armah, C.K.; Kofler, B.M.; Curtis, P.J.; Minihane, A.M.; et al. Association of oily fish intake, sex, age, BMI and APOE genotype with plasma long-chain n-3 fatty acid composition. Br. J. Nutr. 2018, 120, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef]
- Raatz, S.K.; Bibus, D.; Thomas, W.; Kris-Etherton, P. Total fat intake modifies plasma fatty acid composition in humans. J. Nutr. 2001, 131, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Jäncke, L. Sex/gender differences in cognition, neurophysiology, and neuroanatomy. F1000Res 2018, 7. [Google Scholar] [CrossRef]
- Short, S.E.; Yang, Y.C.; Jenkins, T.M. Sex, gender, genetics, and health. Am. J. Public Health 2013, 103 (Suppl. 1), 93. [Google Scholar] [CrossRef]
- National Center for Health Statistics National Health and Nutrition Examination Survey. NHANES 2003–2004 Questionnaire Instruments. Screener Module: In-person/Telephone Instrument. Questionnaire: Screener Module #1 (2003–2004). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/questionnaires/screener_1.pdf (accessed on 22 May 2022).
- National Center for Health Statistics National Health and Nutrition Examination Survey. 2003-2004 Data Documentation, Codebook, and Frequencies. Demographic Variables & Sample Weights (DEMO_C). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/DEMO_C.htm (accessed on 29 April 2022).
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1231S. [Google Scholar] [CrossRef]
- Mujica-Coopman, M.F.; Franco-Sena, A.B.; Farias, D.R.; Vaz, J.S.; Brito, A.; Kac, G.; Lamers, Y. Vitamin B-6 Status in Unsupplemented Pregnant Women Is Associated Positively with Serum Docosahexaenoic Acid and Inversely with the n-6-to-n-3 Fatty Acid Ratio. J. Nutr. 2017, 147, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Kleinbaum, D.G.; Klein, M. Logistic Regression. Chapter 6. Modeling Strategy Guidelines; Springer: New York, NY, USA, 2010; pp. 169–173. [Google Scholar]
- Food and Nutrition Board Institute of Medicine. A Report of the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline and Subcommittee on Upper Reference Levels of Nutrients. In Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Lohner, S.; Fekete, K.; Marosvölgyi, T.; Decsi, T. Gender differences in the long-chain polyunsaturated fatty acid status: Systematic review of 51 publications. Ann. Nutr. Metab. 2013, 62, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, I.; Huybrechts, I.; Gonzalez-Gross, M.; Mouratidou, T.; Santabarbara, J.; Chajes, V.; Gonzalez-Gil, E.M.; Park, J.Y.; Bel-Serrat, S.; Cuenca-Garcia, M.; et al. Folate and vitamin B12 concentrations are associated with plasma DHA and EPA fatty acids in European adolescents: The Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br. J. Nutr. 2017, 117, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Ralat, M.A.; da Silva, V.; Garrett, T.J.; Melnyk, S.; James, S.J.; Gregory, J.F., 3rd. Vitamin B-6 restriction impairs fatty acid synthesis in cultured human hepatoma (HepG2) cells. Am. J. Physiol. Endocrinol. Metab. 2013, 304, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
- Ued, F.V.; Mathias, M.G.; Toffano, R.B.D.; Barros, T.T.; Almada, M.O.R.V.; Salomão, R.G.; Coelho-Landell, C.A.; Hillesheim, E.; Camarneiro, J.M.; Camelo-Junior, J.S.; et al. Vitamin B2 and Folate Concentrations are Associated with ARA, EPA and DHA Fatty Acids in Red Blood Cells of Brazilian Children and Adolescents. Nutrients 2019, 11, 2918. [Google Scholar] [CrossRef] [Green Version]
- Skeie, E.; Strand, E.; Pedersen, E.R.; Bjorndal, B.; Bohov, P.; Berge, R.K.; Svingen, G.F.; Seifert, R.; Ueland, P.M.; Midttun, O.; et al. Circulating B-vitamins and smoking habits are associated with serum polyunsaturated Fatty acids in patients with suspected coronary heart disease: A cross-sectional study. PLoS ONE 2015, 10, e0129049. [Google Scholar]
- Assies, J.; Mocking, R.J.; Lok, A.; Koeter, M.W.; Bockting, C.L.; Visser, I.; Pouwer, F.; Ruhé, H.G.; Schene, A.H. Erythrocyte fatty acid profiles and plasma homocysteine, folate and vitamin B6 and B12 in recurrent depression: Implications for co-morbidity with cardiovascular disease. Psychiatry Res. 2015, 229, 992–998. [Google Scholar] [CrossRef]
- Okayasu, T.; Nagao, M.; Ishibashi, T.; Imai, Y. Purification and partial characterization of linoleoyl-CoA desaturase from rat liver microsomes. Arch. Biochem. Biophys. 1981, 206, 21–28. [Google Scholar] [CrossRef]
- Cunnane, S.C.; McAdoo, K.R. Iron intake influences essential fatty acid and lipid composition of rat plasma and erythrocytes. J. Nutr. 1987, 117, 1514–1519. [Google Scholar] [CrossRef] [Green Version]
- Smuts, C.M.; Tichelaar, H.Y.; van Jaarsveld, P.J.; Badenhorst, C.J.; Kruger, M.; Laubscher, R.; Mansvelt, E.P.; Benade, A.J. The effect of iron fortification on the fatty acid composition of plasma and erythrocyte membranes in primary school children with and without iron deficiency. Prostaglandins Leukot. Essent. Fatty Acids 1995, 52, 59–67. [Google Scholar] [CrossRef]
- Aktas, M.; Elmastas, M.; Ozcicek, F.; Yilmaz, N. Erythrocyte Membrane Fatty Acid Composition in Premenopausal Patients with Iron Deficiency Anemia. J. Oleo Sci. 2016, 65, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tichelaar, H.Y.; Smuts, C.M.; Gross, R.; Jooste, P.L.; Faber, M.; Benadé, A.J. The effect of dietary iron deficiency on the fatty acid composition of plasma and erythrocyte membrane phospholipids in the rat. Prostaglandins Leukot. Essent. Fatty Acids 1997, 56, 229–233. [Google Scholar] [CrossRef]
- Stangl, G.I.; Kirchgessner, M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids 1998, 33, 889–895. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie 2009, 91, 791–795. [Google Scholar] [CrossRef]
- de Oliveira Otto, M.C.; Wu, J.H.; Baylin, A.; Vaidya, D.; Rich, S.S.; Tsai, M.Y.; Jacobs, D.R., Jr.; Mozaffarian, D. Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J. Am. Heart Assoc. 2013, 2, e000506. [Google Scholar] [CrossRef] [Green Version]
- Itakura, H.; Yokoyama, M.; Matsuzaki, M.; Saito, Y.; Origasa, H.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Kita, T.; et al. JELIS Investigators Relationships between plasma fatty acid composition and coronary artery disease. J. Atheroscler. Thromb. 2011, 18, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [Green Version]
- Superko, H.R.; Superko, A.R.; Lundberg, G.P.; Margolis, B.; Garrett, B.C.; Nasir, K.; Agatston, A.S. Omega-3 Fatty Acid Blood Levels Clinical Significance Update. Curr. Cardiovasc. Risk Rep. 2014, 8, 407. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.S.; Sakakeeny, L.; Jacques, P.F.; Picciano, M.F.; Selhub, J. Vitamin B-6 intake is inversely related to, and the requirement is affected by, inflammation status. J. Nutr. 2010, 140, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Sakakeeny, L.; Roubenoff, R.; Obin, M.; Fontes, J.D.; Benjamin, E.J.; Bujanover, Y.; Jacques, P.F.; Selhub, J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J. Nutr. 2012, 142, 1280–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosell, M.S.; Lloyd-Wright, Z.; Appleby, P.N.; Sanders, T.A.; Allen, N.E.; Key, T.J. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am. J. Clin. Nutr. 2005, 82, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl. Health. Stat. Report 2015, 79, 1–16. [Google Scholar]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use Among Adults: United States, 2017–2018. NCHS Data Brief 2021, 399, 101131. [Google Scholar]
- De Keyzer, W.; Huybrechts, I.; De Vriendt, V.; Vandevijvere, S.; Slimani, N.; Van Oyen, H.; De Henauw, S. Repeated 24-hour recalls versus dietary records for estimating nutrient intakes in a national food consumption survey. Food Nutr. Res. 2011, 55, 7307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Va, P.; Dodd, K.W.; Zhao, L.; Thompson-Paul, A.M.; Mercado, C.I.; Terry, A.L.; Jackson, S.L.; Wang, C.Y.; Loria, C.M.; Moshfegh, A.J.; et al. Evaluation of measurement error in 24-hour dietary recall for assessing sodium and potassium intake among US adults—National Health and Nutrition Examination Survey (NHANES), 2014. Am. J. Clin. Nutr. 2019, 109, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All (n = 461) | Men (n = 247) | Women (n = 214) | P1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % ± SE | n | % ± SE | n | % ± SE | |||||
| Gender | 461 | 100 ± 0 | 247 | 48.2 ± 2.7 | 214 | 51.8 ± 2.7 | 0.51 | |||
| Age (years) | 0.39 | |||||||||
| 60–69 | 195 | 49.6 ± 3.5 | 100 | 50.7 ± 5.1 | 95 | 48.5 ± 3.1 | ||||
| 70–79 | 148 | 32.2 ± 2.1 | 91 | 33.2 ± 3.5 | 57 | 31.2 ± 2.1 | ||||
| 80+ | 118 | 18.2 ± 2.1 | 56 | 16.1 ± 2.2 | 62 | 20.3 ± 2.9 | ||||
| Race/Ethnicity | 0.65 | |||||||||
| Non-Hispanic White | 292 | 85.2 ± 2.8 | 153 | 83.7 ± 3.0 | 139 | 86.7 ± 2.9 | ||||
| Non-Hispanic Black | 58 | 6.9 ± 1.8 | 34 | 7.7 ± 1.9 | 24 | 6.2 ± 2.0 | ||||
| Hispanic | 100 | 4.7 ± 1.9 | 53 | 5.0 ± 2.0 | 47 | 4.5 ± 2.0 | ||||
| Others | 11 | 3.2 ± 0.8 | 7 | 3.7 ± 0.7 | 4 | 2.7 ± 1.3 | ||||
| BMI (kg/m2) | 0.050 | |||||||||
| ≤24.9 | 141 | 31.5 ± 4.3 | 74 | 28.3 ± 3.4 | 67 | 34.6 ± 5.8 | ||||
| 25–29.9 | 191 | 42.1 ± 3.9 | 114 | 47.9 ± 3.9 | 77 | 36.8 ± 4.6 | ||||
| ≥30 | 122 | 26.3 ± 2.1 | 57 | 23.8 ± 3.0 | 65 | 28.7 ± 3.1 | ||||
| PIR | 0.036 | |||||||||
| ≤1.3 | 128 | 19.4 ± 2.4 | 61 | 14.7 ± 2.9 | 67 | 23.8 ± 3.3 | ||||
| >1.3 | 307 | 80.6 ± 2.4 | 177 | 85.3 ± 2.9 | 130 | 76.2 ± 3.3 | ||||
| Educational attainment | 0.006 | |||||||||
| ≤High school degree | 280 | 51.0 ± 3.1 | 141 | 44.1 ± 2.9 | 139 | 57.5 ± 4.3 | ||||
| >High school degree | 179 | 49.0 ± 3.1 | 105 | 55.9 ± 2.9 | 74 | 42.5 ± 4.3 | ||||
| Physical activity (MET min/week) | 0.001 | |||||||||
| <500 | 223 | 42.5 ± 3.2 | 101 | 34.6 ± 3.5 | 122 | 49.9 ± 5.1 | ||||
| 500–1000 | 61 | 14.8 ± 1.3 | 29 | 11.9 ± 1.9 | 32 | 17.6 ± 1.9 | ||||
| ≥1000 | 177 | 42.6 ± 2.3 | 117 | 53.6 ± 3.0 | 60 | 32.5 ± 3.9 | ||||
| Cigarette smoking | <0.0001 | |||||||||
| Never smoker | 207 | 42.5 ± 4.9 | 81 | 31.0 ± 5.5 | 126 | 53.2 ± 5.2 | ||||
| Former smoker | 200 | 45.7 ± 3.7 | 131 | 56.7 ± 3.9 | 69 | 35.5 ± 4.5 | ||||
| Current smoker | 54 | 11.8 ± 1.9 | 35 | 12.3 ± 2.2 | 19 | 11.3 ± 2.3 | ||||
| Alcohol consumption | 0.001 | |||||||||
| Lifetime abstainer | 72 | 15.7 ± 3.3 | 18 | 8.6 ± 3.0 | 54 | 22.1 ± 4.5 | ||||
| Former drinker | 156 | 34.2 ± 2.7 | 86 | 35.8 ± 3.4 | 70 | 32.8 ± 2.6 | ||||
| Current drinker | 224 | 50.1 ± 4.8 | 134 | 55.6 ± 4.9 | 90 | 45.1 ± 5.3 | ||||
| Vitamin B6 supplement | 0.67 | |||||||||
| No | 232 | 44.1 ± 2.7 | 129 | 42.9 ± 4.6 | 103 | 45.3 ± 3.2 | ||||
| Yes | 229 | 55.9 ± 2.7 | 118 | 57.1 ± 4.6 | 111 | 54.7 ± 3.2 | ||||
| n-3 PUFA supplement | 0.5 | |||||||||
| No | 442 | 94.8 ± 1.4 | 235 | 93.8 ± 1.3 | 207 | 95.7 ± 2.2 | ||||
| Yes | 19 | 5.2 ± 1.4 | 12 | 6.2 ± 1.3 | 7 | 4.3 ± 2.2 | ||||
| Prescription medication | 0.98 | |||||||||
| No | 90 | 17.8 ± 3.6 | 53 | 17.8 ± 3.1 | 37 | 17.9 ± 5.1 | ||||
| Yes | 371 | 82.2 ± 3.6 | 194 | 82.2 ± 3.1 | 177 | 82.1 ± 5.1 | ||||
| All (n = 461) | Men (n = 247) | Women (n = 214) | P1 | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | ||
| Nutrient intake from food | |||||||
| Total energy (kcal/d) | 424 | 1838.2 ± 47.9 | 221 | 2048.8 ± 79.7 | 203 | 1658.9 ± 37.1 | 0.002 |
| Dietary vitamin B6 (mg/d) | 424 | 1.78 ± 0.03 | 221 | 1.82 ± 0.04 | 203 | 1.74 ± 0.05 | 0.055 |
| Dietary ALA (g/d) | 424 | 1.49 ± 0.05 | 221 | 1.31 ± 0.05 | 203 | 1.41 ± 0.05 | 0.43 |
| Dietary LA (g/d) | 424 | 14.03 ± 0.35 | 221 | 13.37 ± 0.61 | 203 | 14.06 ± 0.65 | 0.3 |
| Dietary EPA (g/d) | 424 | 0.05 ± 0.01 | 221 | 0.05 ± 0.01 | 203 | 0.05 ± 0.01 | 0.25 |
| Dietary DHA (g/d) | 424 | 0.10 ± 0.02 | 221 | 0.10 ± 0.02 | 203 | 0.10 ± 0.02 | 0.13 |
| Dietary AA (g/d) | 424 | 0.13 ± 0.005 | 221 | 0.13 ± 0.01 | 203 | 0.12 ± 0.01 | 0.008 |
| Total fat (g/d) | 424 | 72.50 ± 1.18 | 221 | 71.32 ± 1.48 | 203 | 73.51 ± 1.59 | 0.47 |
| Nutrient intake from food and supplements | |||||||
| Total vitamin B6 (mg/d) | 424 | 8.82 ± 1.51 | 221 | 7.37 ± 1.37 | 203 | 10.06 ± 2.28 | 0.32 |
| Total ALA (g/d) | 424 | 1.50 ± 0.05 | 221 | 1.43 ± 0.07 | 203 | 1.56 ± 0.06 | 0.31 |
| Total EPA (g/d) | 424 | 0.06 ± 0.01 | 221 | 0.07 ± 0.02 | 203 | 0.06 ± 0.01 | 0.033 |
| Total DHA (g/d) | 424 | 0.11 ± 0.02 | 221 | 0.11 ± 0.02 | 203 | 0.10 ± 0.02 | 0.033 |
| Plasma Variables | |||||||
| ALA (μmol/L) | 456 | 67.81 ± 2.39 | 245 | 64.82 ± 2.60 | 211 | 70.71 ± 3.11 | 0.63 |
| LA (μmol/L) | 456 | 3514.9 ± 56.7 | 245 | 3346.6 ± 54.0 | 211 | 3679.0 ± 75.6 | 0.006 |
| EPA (μmol/L) | 457 | 49.09 ± 2.87 | 246 | 45.54 ± 3.05 | 211 | 52.64 ± 3.02 | 0.005 |
| DHA (μmol/L) | 457 | 140.8 ± 5.49 | 246 | 133.1 ± 6.03 | 211 | 148.4 ± 5.90 | 0.013 |
| AA (μmol/L) | 457 | 815.1 ± 9.81 | 246 | 767.1 ± 13.47 | 211 | 862.6 ± 11.93 | 0.002 |
| EPA + DHA (μmol/L) | 457 | 192.5 ± 8.18 | 246 | 181.2 ± 8.84 | 211 | 203.5 ± 8.64 | 0.01 |
| EPA/AA | 457 | 0.060 ± 0.003 | 246 | 0.059 ± 0.004 | 211 | 0.061 ± 0.003 | 0.35 |
| (EPA + DHA)/AA | 457 | 0.236 ± 0.008 | 246 | 0.236 ± 0.010 | 211 | 0.236 ± 0.009 | 0.73 |
| PLP (nmol/L) | 458 | 51.62 ± 3.89 | 246 | 58.36 ± 4.89 | 212 | 46.02 ± 3.83 | 0.031 |
| PLP category 2,3 | 0.47 | ||||||
| <20 nmol/L | 87 | 15.3 ± 2.24 | 44 | 14.3 ± 2.41 | 43 | 16.2 ± 2.78 | |
| ≥20 nmol/L | 371 | 84.7 ± 2.24 | 202 | 85.7 ± 2.41 | 169 | 83.8 ± 2.78 | |
| Men (n = 247) | Women (n = 214) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | b (95% CI) | R2 | P | β | b (95% CI) | R2 | P | P-int.1 | ||
| Plasma EPA (µmol/L) | ||||||||||
| Vitamin B6 Intake (mg/d) | 0.008 | |||||||||
| Model 0 | −0.051 | −0.001 (−0.004, 0.002) | 0.003 | 0.45 | 0.152 | 0.004 (0.001, 0.006) | 0.02 | 0.022 | ||
| Model 1 | −0.141 | −0.003 (−0.006, 0.001) | 0.12 | 0.11 | 0.150 | 0.003 (0.001, 0.006) | 0.25 | 0.017 | ||
| Model 2 | −0.151 | −0.003 (−0.005, −0.001) | 0.22 | 0.02 | 0.139 | 0.003 (0.001, 0.005) | 0.38 | 0.015 | ||
| Plasma AA (µmol/L) | ||||||||||
| Vitamin B6 Intake (mg/d) | 0.004 | |||||||||
| Model 0 | −0.080 | −0.001 (−0.002, 0.001) | 0.01 | 0.36 | 0.129 | 0.001 (−0.0001, 0.003) | 0.02 | 0.06 | ||
| Model 1 | −0.068 | −0.001 (−0.002, 0.0004) | 0.15 | 0.21 | 0.103 | 0.001 (0.0001, 0.002) | 0.04 | 0.039 | ||
| Model 2 | −0.072 | −0.001 (−0.002, 0.0004) | 0.21 | 0.23 | 0.104 | 0.001 (−0.0001, 0.002) | 0.09 | 0.07 | ||
| All (n = 461) | ||||
|---|---|---|---|---|
| β | b (95% CI) | R2 | P | |
| Plasma EPA (µmol/L) | ||||
| Plasma PLP (nmol/L) | ||||
| Model 0 | 0.215 | 0.124 (0.066, 0.183) | 0.05 | 0.0004 |
| Model 1 | 0.208 | 0.121 (0.050, 0.192) | 0.17 | 0.002 |
| Model 2 | 0.181 | 0.104 (0.045, 0.163) | 0.24 | 0.002 |
| Plasma DHA (µmol/L) | ||||
| Plasma PLP (nmol/L) | ||||
| Model 0 | 0.147 | 0.062 (0.025, 0.099) | 0.02 | 0.003 |
| Model 1 | 0.147 | 0.062 (0.028, 0.096) | 0.22 | 0.002 |
| Model 2 | 0.109 | 0.045 (0.016, 0.074) | 0.30 | 0.005 |
| Plasma AA (µmol/L) | ||||
| Plasma PLP (nmol/L) | ||||
| Model 0 | −0.022 | −0.006 (−0.043, 0.032) | 0.001 | 0.748 |
| Model 1 | 0.031 | 0.008 (−0.032, 0.048) | 0.09 | 0.674 |
| Model 2 | 0.030 | 0.008 (−0.038, 0.054) | 0.11 | 0.722 |
| Plasma EPA + DHA (µmol/L) | ||||
| Plasma PLP (nmol/L) | ||||
| Model 0 | 0.178 | 0.077 (0.038, 0.117) | 0.03 | 0.001 |
| Model 1 | 0.175 | 0.076 (0.035, 0.118) | 0.22 | 0.001 |
| Model 2 | 0.140 | 0.060 (0.026, 0.094) | 0.30 | 0.002 |
| Plasma EPA/AA ratio | ||||
| Plasma PLP (nmol/L) | ||||
| Model 0 | 0.252 | 0.130 (0.072, 0.188) | 0.06 | 0.0002 |
| Model 1 | 0.218 | 0.113 (0.047, 0.179) | 0.17 | 0.002 |
| Model 2 | 0.186 | 0.096 (0.036, 0.157) | 0.23 | 0.004 |
| Plasma (EPA + DHA)/AA ratio | ||||
| Plasma PLP (nmol/L) | ||||
| Model 0 | 0.209 | 0.083 (0.037, 0.130) | 0.04 | 0.002 |
| Model 1 | 0.171 | 0.068 (0.023, 0.114) | 0.19 | 0.006 |
| Model 2 | 0.130 | 0.052 (0.007, 0.097) | 0.25 | 0.026 |
| All (n = 461) | |||
|---|---|---|---|
| (Ref: Plasma PLP < 20 nmol/L) | OR (95% CI) | R2 | P |
| High Plasma EPA | |||
| Plasma PLP ≥ 20 nmol/L | [median (IQR), µmol/L: 49.9 (32.0–66.6)] | ||
| Model 0 | 2.47 (1.35, 4.52) | 0.02 | 0.003 |
| Model 1 | 2.70 (1.19, 6.11) | 0.14 | <0.0001 |
| Model 2 | 2.03 (1.00, 4.10) | 0.20 | 0.049 |
| High Plasma DHA | |||
| Plasma PLP ≥ 20 nmol/L | [median (IQR), µmol/L: 134.6 (107.0–187.4)] | ||
| Model 0 | 1.24 (0.81, 1.88) | 0.002 | 0.32 |
| Model 1 | 1.48 (0.94, 2.34) | 0.16 | 0.09 |
| Model 2 | 1.36 (0.87, 2.13) | 0.23 | 0.18 |
| High Plasma AA | |||
| Plasma PLP ≥ 20 nmol/L | [median (IQR), µmol/L: 846.7 (694.4–979.1)] | ||
| Model 0 | 0.80 (0.47, 1.36) | 0.002 | 0.42 |
| Model 1 | 0.73 (0.35, 1.51) | 0.09 | 0.39 |
| Model 2 | 0.65 (0.29, 1.46) | 0.10 | 0.29 |
| High Plasma EPA + DHA | |||
| Plasma PLP ≥ 20 nmol/L | [median (IQR), µmol/L: 185.9 (142.6–254.1)] | ||
| Model 0 | 1.51 (1.00, 2.29) | 0.01 | 0.053 |
| Model 1 | 1.62 (1.06, 2.49) | 0.16 | 0.027 |
| Model 2 | 1.53 (0.95, 2.47) | 0.22 | 0.08 |
| High Plasma EPA/AA | |||
| Plasma PLP ≥ 20 nmol/L | [median (IQR): 0.059 (0.043–0.078)] | ||
| Model 0 | 4.00 (2.32, 6.89) | 0.05 | <0.0001 |
| Model 1 | 4.40 (2.30, 8.41) | 0.12 | <0.0001 |
| Model 2 | 3.83 (1.97, 7.43) | 0.16 | <0.0001 |
| High Plasma (EPA + DHA)/AA | |||
| Plasma PLP ≥ 20 nmol/L | [median (IQR): 0.215 (0.180–0.292)] | ||
| Model 0 | 2.14 (1.15, 3.98) | 0.02 | 0.017 |
| Model 1 | 2.50 (1.34, 4.65) | 0.13 | 0.004 |
| Model 2 | 2.11 (0.99, 4.52) | 0.18 | 0.054 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Enrione, E.B.; Narayanan, V.; Li, T.; Campa, A. Associations of Vitamin B6 Intake and Plasma Pyridoxal 5′-Phosphate with Plasma Polyunsaturated Fatty Acids in US Older Adults: Findings from NHANES 2003–2004. Nutrients 2022, 14, 2336. https://doi.org/10.3390/nu14112336
Kim H, Enrione EB, Narayanan V, Li T, Campa A. Associations of Vitamin B6 Intake and Plasma Pyridoxal 5′-Phosphate with Plasma Polyunsaturated Fatty Acids in US Older Adults: Findings from NHANES 2003–2004. Nutrients. 2022; 14(11):2336. https://doi.org/10.3390/nu14112336
Chicago/Turabian StyleKim, Hyojung, Evelyn B. Enrione, Vijaya Narayanan, Tan Li, and Adriana Campa. 2022. "Associations of Vitamin B6 Intake and Plasma Pyridoxal 5′-Phosphate with Plasma Polyunsaturated Fatty Acids in US Older Adults: Findings from NHANES 2003–2004" Nutrients 14, no. 11: 2336. https://doi.org/10.3390/nu14112336
APA StyleKim, H., Enrione, E. B., Narayanan, V., Li, T., & Campa, A. (2022). Associations of Vitamin B6 Intake and Plasma Pyridoxal 5′-Phosphate with Plasma Polyunsaturated Fatty Acids in US Older Adults: Findings from NHANES 2003–2004. Nutrients, 14(11), 2336. https://doi.org/10.3390/nu14112336






