The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus
Abstract
:1. Introduction
2. Roles and Mechanisms of Trace Elements in Viral Infections
2.1. Roles and Mechanism of Trace Elements in Chronic Hepatitis
2.1.1. Zinc (Zn)
2.1.2. Selenium (Se)
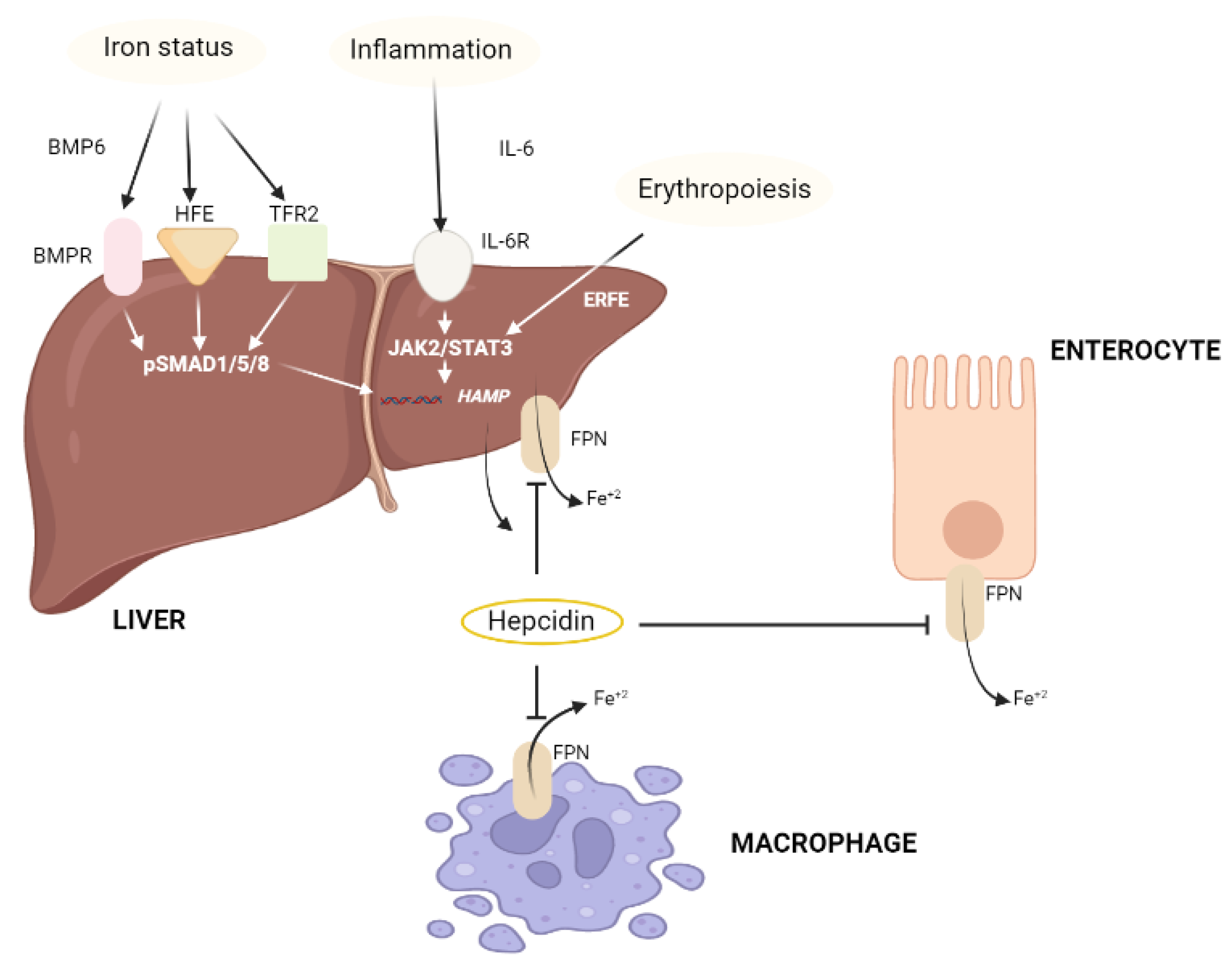
2.1.3. Iron (Fe)
2.1.4. Copper (Cu)
2.2. The Roles and Mechanism of Trace Elements in COVID-19
2.2.1. Zinc (Zn)
2.2.2. Selenium (Se)
2.2.3. Copper (Cu)
2.2.4. Iron (Fe)
3. Roles and Mechanism of Micronutrients in Diabetes Mellitus
3.1. Zinc (Zn)
3.2. Selenium (Se)
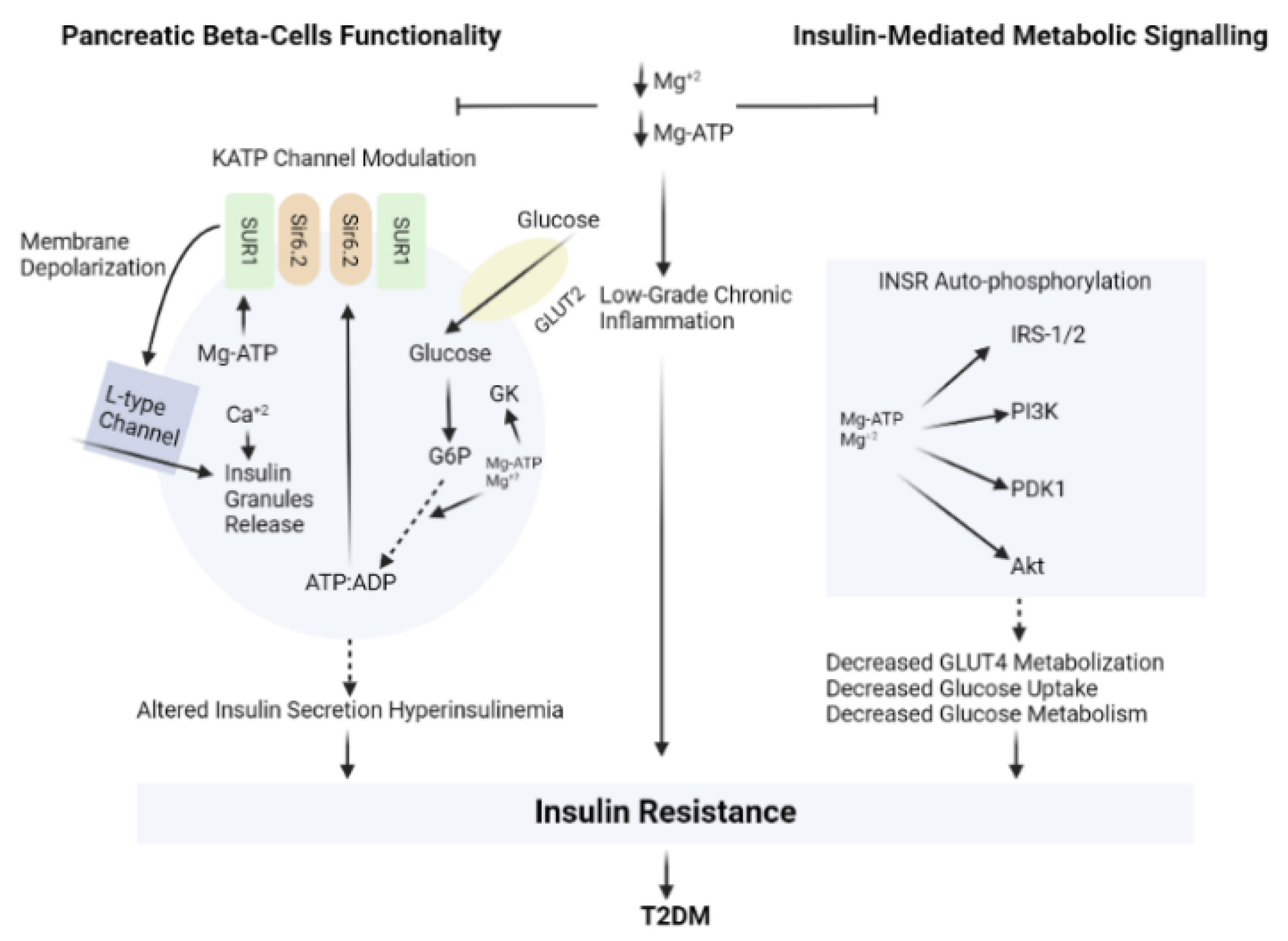
3.3. Magnesium (Mg)
4. Current Status and Clinical Trials
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHC | Chronic hepatitis C |
| CO-NPs | Coprous oxide nanoparticles |
| COVID | Coronavirus disease |
| Cu | Copper |
| DAA | Direct acting antiviral |
| DPP | Diabetes prevention program |
| Fe | Iron |
| GPX1 | Glutathione peroxidase-1 |
| H1N1 | Human influenza |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| HDIVZn | High dose intravenous zinc |
| HEV | Hepatitis E Virus |
| Huh | Human hepatoma |
| hZAP | Human zinc finger antiviral protein |
| ICU | Intensive care unit |
| IFN | Interferon |
| IL | Interleukin |
| Mg | Magnesium |
| mRNA | Messenger ribonucleic acid |
| MT | Metallothionein |
| NK cell | Natural killer cell |
| pgRNA | Pregenomic ribonucleic acid |
| RCT | Randomized controlled trial |
| RdRp | RNA-dependent RNA polymerase |
| ROS | Reactive oxygen species |
| Se | Selenium |
| SELENOP | Selenoproteins |
| T2DM | Type-2 diabetes mellitus |
| TEs | Trace elements |
| TH cell | T helper cell |
| TXNRD | Thioredoxin reductase |
| Zn | Zinc |
References
- Bhattacharya, P.T.; Misra, S.R.; Hussain, M. Nutritional Aspects of Essential Trace Elements in Oral Health and Disease: An Extensive Review. Scientifica 2016, 2016, 5464373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attar, T. A Mini-Review on Importance and Role of Trace Elements in the Human Organism. Chem. Rev. Lett. 2020, 3, 117–130. [Google Scholar]
- Şahin, M.; Karayakar, F.; Erdogan, K.E.; Bas, F.; Colak, T. Liver Tissue Trace Element Levels in HepB Patients and the Relationship of These Elements with Histological Injury in the Liver and with Clinical Parameters. J. Trace Elem. Med. Biol. 2018, 45, 70–77. [Google Scholar] [CrossRef]
- Gupta, S.; Read, S.A.; Shackel, N.A.; Hebbard, L.; George, J.; Ahlenstiel, G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells 2019, 8, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral Activity of Cuprous Oxide Nanoparticles against Hepatitis C Virus in Vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Ko, W.S. Status of Essential Trace Minerals and Oxidative Stress in Viral Hepatitis C Patients with Nonalcoholic Fatty Liver Disease. Int. J. Med. Sci. 2013, 10, 730–737. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, N.; Subramani, C.; Anang, S.; Muthumohan, R.; Shalimar Nayak, B.; Ranjith-Kumar, C.T.; Surjit, M. Crossm Zinc Salts Block Hepatitis E Virus. J. Virol. 2017, 91, e00754-17. [Google Scholar]
- Kumar, A.; Kubota, Y.; Chernov, M.; Kasuya, H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses 2020, 144, 109848. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Georgiopoulou, A.K.; Perlepes, S.P.; Bjørklund, G.; Peana, M. A SARS-CoV-2 -human metalloproteome interaction map. J. Inorg. Biochem. 2021, 219, 111423. [Google Scholar] [CrossRef]
- Andreou, A.; Trantza, S.; Filippou, D.; Filippou, D.; Sipsas, N.; Tsiodras, S. COVID-19: The Potential Role of Copper and N-Acetylcysteine (NAC) in a Combination of Candidate Antiviral Treatments against SARS-CoV-2. In Vivo (Brooklyn) 2020, 34, 1567–1588. [Google Scholar] [CrossRef]
- Gattermann, N.; Muckenthaler, M.U.; Kulozik, A.E.; Metzgeroth, G.; Hastka, J. Investigation of Iron Deficiency and Iron Overload. Dtsch. Arztebl. Int. 2021, 118, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, I.D.; Pal, A.; Simonelli, I.; Atanasova, B.; Ventriglia, M.; Rongioletti, M.; Squitti, R. Evaluation of Zinc, Copper, and Cu:Zn Ratio in Serum, and Their Implications in the Course of COVID-19. J. Trace Elem. Med. Biol. 2022, 71, 126944. [Google Scholar] [CrossRef] [PubMed]
- Hackler, J.; Heller, R.A.; Sun, Q.; Schwarzer, M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relation of Serum Copper Status to Survival in COVID-19. Nutrients 2021, 13, 1898. [Google Scholar] [CrossRef] [PubMed]
- Asprouli, E.; Kalafati, I.P.; Sakellari, A.; Karavoltsos, S.; Vlachogiannakos, J.; Revenas, K.; Kokkinos, A.; Dassenakis, M.; Dedoussis, G.V.; Kalogeropoulos, N. Evaluation of Plasma Trace Elements in Different Stages of Nonalcoholic Fatty Liver Disease. Biol. Trace Elem. Res. 2019, 188, 326–333. [Google Scholar] [CrossRef]
- Parlakgül, G.; Arruda, A.P.; Pang, S.; Cagampan, E.; Min, N.; Güney, E.; Lee, G.Y.; Inouye, K.; Hess, H.F.; Xu, C.S.; et al. Regulation of Liver Subcellular Architecture Controls Metabolic Homeostasis. Nature 2022, 603, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Kozeniecki, M.; Ludke, R.; Kerner, J.; Patterson, B. Micronutrients in Liver Disease: Roles, Risk Factors for Deficiency, and Recommendations for Supplementation. Nutr. Clin. Pract. 2020, 35, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.C.; Chen, X.J.; Kong, X.; Cai, Y.D. Investigation of the Roles of Trace Elements during Hepatitis C Virus Infection Using Protein-Protein Interactions and a Shortest Path Algorithm. Biochim. Biophys. Acta—Gen. Subj. 2016, 1860, 2756–2768. [Google Scholar] [CrossRef]
- Pincemail, J.; Cavalier, E.; Charlier, C.; Cheramy–bien, J.P.; Brevers, E.; Courtois, A.; Fadeur, M.; Meziane, S.; Goff, C.L.; Misset, B.; et al. Oxidative Stress Status in COVID-19 Patients Hospitalized in Intensive Care Unit for Severe Pneumonia. A Pilot Study. Antioxidants 2021, 10, 257. [Google Scholar] [CrossRef]
- Nedić, O.; Šunderić, M.; Robajac, D.; Miljuš, G.; Četić, D.; Penezić, A. Major Trace Elements and Their Binding Proteins in the Early Phase of COVID-19 Infection. J. Biol. Inorg. Chem. 2022, 27, 261–269. [Google Scholar] [CrossRef]
- Zeng, H.L.; Yang, Q.; Yuan, P.; Wang, X.; Cheng, L. Associations of Essential and Toxic Metals/Metalloids in Whole Blood with Both Disease Severity and Mortality in Patients with COVID-19. FASEB J. 2021, 35, 1–12. [Google Scholar] [CrossRef]
- De Jesus, J.R.; De Araújo Andrade, T. Understanding the Relationship between Viral Infections and Trace Elements from a Metallomics Perspective: Implications for COVID-19. Metallomics 2020, 12, 1912–1930. [Google Scholar] [CrossRef] [PubMed]
- Fooladi, S.; Matin, S.; Mahmoodpoor, A. Copper as a Potential Adjunct Therapy for Critically Ill COVID-19 Patients. Clin. Nutr. ESPEN 2020, 40, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Can Trace Element Supplementations (Cu, Se, and Zn) Enhance Human Immunity against COVID-19 and Its New Variants? Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Bahrami, A.; Habibi, P.; Nouri, F. A Review on the Serum Electrolytes and Trace Elements Role in the Pathophysiology of COVID-19. Biol. Trace Elem. Res. 2021, 199, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Reply to “Comment on: Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181”. Nutrients 2020, 12, 2326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Pérez, C.; Gómez-Peña, C.; Pérez-Carrascosa, F.M.; Vrhovnik, P.; Echeverría, R.; Salcedo-Bellido, I.; Mustieles, V.; Željka, F.; Arrebola, J.P. Trace Elements Concentration in Adipose Tissue and the Risk of Incident Type 2 Diabetes in a Prospective Adult Cohort. Environ. Pollut. 2021, 286, 117496. [Google Scholar] [CrossRef]
- Himoto, T.; Masaki, T. Current Trends of Essential Trace Elements in Patients with Chronic Liver Diseases. Nutrients 2020, 12, 2084. [Google Scholar] [CrossRef]
- Siddiqui, K.; Bawazeer, N.; Scaria Joy, S. Variation in Macro and Trace Elements in Progression of Type 2 Diabetes. Sci. World J. 2014, 2014, 461591. [Google Scholar] [CrossRef] [Green Version]
- The National Diabetes Prevention Program (NDPP). The Diabetes Prevention Program (DPP). Diabetes Care 2002, 25, 2165–2171. [Google Scholar] [CrossRef] [Green Version]
- Kant, R.; Verma, V.; Patel, S.; Chandra, R.; Chaudhary, R.; Shuldiner, A.R.; Munir, K.M. Effect of Serum Zinc and Copper Levels on Insulin Secretion, Insulin Resistance and Pancreatic β Cell Dysfunction in US Adults: Findings from the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Diabetes Res. Clin. Pract. 2021, 172, 108627. [Google Scholar] [CrossRef]
- Yousaf, T.; Sun, Y.; Naz, W.; Liu, Y.; Xu, J.; Yuan, S.; Wu, K.; Wang, M.; Wang, J.; Guo, M.; et al. Multiomics Analysis of Endocytosis upon HBV Infection and Identification of SCAMP1 as a Novel Host Restriction Factor against HBV Replication. Int. J. Mol. Sci. 2022, 23, 2211. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Nie, H.; Cai, D.; Zhang, J.; Liu, H.; Yan, R.; Cuconati, A.; Block, T.M.; Guo, J.T.; Guo, H. Inhibition of Hepatitis B Virus Replication by the Host Zinc Finger Antiviral Protein. PLoS Pathog. 2013, 9, e1003494. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, A.; Yada, M.; Nagasawa, S.; Tanaka, K.; Morita, Y.; Masumoto, A.; Motomura, K. Serum α-Fetoprotein Level at Treatment Completion Is a Useful Predictor of Hepatocellular Carcinoma Occurrence More than One Year after Hepatitis C Virus Eradication by Direct-Acting Antiviral Treatment. J. Viral Hepat. 2022, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, L.; Li, L.; Huang, Z.; Ye, L. Metallothionein 1: A New Spotlight on Inflammatory Diseases. Front. Immunol. 2021, 12, 13–21. [Google Scholar] [CrossRef]
- Xq, W.; Cm, L.; Chen, L.; Ck, C.; Zf, Y.; Yx, C. Selenium Levels in Patients with Hepatitis C Virus-Related Chronic Hepatitis, Liver Cirrhosis, and Hepatocellular Carcinoma: A Pilot Study. Hepatology 2013, 57, 2543–2544. [Google Scholar] [CrossRef]
- Petrović, S.; Maletić, M.; Lakić, N.; Aleksić, N.; Maletić, J.; Ristanić, M.; Stanimirović, Z. The Effects of Antioxidants Provided with Feed on Certain Quality Parameters of Bull Semen under Heat Stress Conditions. Acta Vet. 2021, 70, 453–470. [Google Scholar] [CrossRef]
- Wei, Y.; Ye, W.; Zhao, W. Serum Iron Levels Decreased in Patients with HBV-Related Hepatocellular Carcinoma, as a Risk Factor for the Prognosis of HBV-Related HCC. Front. Physiol. 2018, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.H.; Wang, J.Y.; Liu, P.Y.; Sun, J.; Wang, X.M.; Wu, R.H.; He, X.T.; Tu, Z.K.; Wang, C.G.; Xu, H.Q.; et al. Iron Metabolism Disorders in Patients with Hepatitis B-Related Liver Diseases. World J. Clin. Cases 2018, 6, 600–610. [Google Scholar] [CrossRef]
- Yang, Y.M.; Cho, Y.E.; Hwang, S. Crosstalk between Oxidative Stress and Inflammatory Liver Injury in the Pathogenesis of Alcoholic Liver Disease. Int. J. Mol. Sci. 2022, 23, 774. [Google Scholar] [CrossRef]
- Cao, X.L.; Zhao, M.F.; Li, D.G.; Xing, Y.; Zhang, Y.C.; Chen, J.; He, X.Y.; Cui, R.; Meng, J.X.; Xiao, X.; et al. Establishment of Macrophage Model of Iron Overload in Vitro and the Injury Induced by Oxidative Stress on Macrophage with Iron Overload. Zhonghua Yi Xue Za Zhi 2016, 96, 129–133. [Google Scholar]
- Tao, T.Y.; Gitlin, J.D. Hepatic Copper Metabolism: Insights from Genetic Disease. Hepatology 2003, 37, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Read, S.A.; Obeid, S.; Ahlenstiel, C.; Ahlenstiel, G. The Role of Zinc in Antiviral Immunity. Adv Nutr. 2019, 10, 696–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, E.; Nikfarjam, M.; Jackett, L.; Bolton, D.M.; Ischia, J.; Patel, O. The Protective Effect of Zinc Against Liver Ischaemia Reperfusion Injury in a Rat Model of Global Ischaemia. J. Clin. Exp. Hepatol. 2020, 10, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Rahimian, J.O.; Yaghi, S.; Liu, M.; Lewis, A.; De Havenon, A.; Mainali, S.; Huang, J.; Scher, E.; Wisniewski, T.; et al. Treatment with Zinc is Associated with Reduced In-Hospital Mortality Among COVID-19 Patients: A Multi-Center Cohort Study. Res. Sq. 2020, rs-3. [Google Scholar] [CrossRef]
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between Regional Selenium Status and Reported Outcome of COVID-19 Cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299. [Google Scholar] [CrossRef]
- Hiffler, L.; Rakotoambinina, B. Selenium and RNA Virus Interactions: Potential Implications for SARS-CoV-2 Infection (COVID-19). Front. Nutr. 2020, 7, 164. [Google Scholar] [CrossRef]
- Ermakov, V.V.; Jovanović, L.N. Biological Role of Trace Elements and Viral Pathologies. Geochem. Int. 2022, 60, 137–153. [Google Scholar] [CrossRef]
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098. [Google Scholar] [CrossRef]
- Anuk, A.T.; Polat, N.; Akdas, S.; Erol, S.A.; Tanacan, A.; Biriken, D.; Keskin, H.L.; Moraloglu Tekin, O.; Yazihan, N.; Sahin, D. The Relation Between Trace Element Status (Zinc, Copper, Magnesium) and Clinical Outcomes in COVID-19 Infection During Pregnancy. Biol. Trace Elem. Res. 2021, 199, 3608–3617. [Google Scholar] [CrossRef]
- Lee, Y.H.; Bang, E.S.; Lee, J.H.; Lee, J.D.; Kang, D.R.; Hong, J.; Lee, J.M. Serum Concentrations of Trace Elements Zinc, Copper, Selenium, and Manganese in Critically Ill Patients. Biol. Trace Elem. Res. 2019, 188, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Raha, S.; Mallick, R.; Basak, S.; Duttaroy, A.K. Is Copper Beneficial for COVID-19 Patients? Med. Hypotheses 2020, 142, 109814. [Google Scholar] [CrossRef] [PubMed]
- Yary, T.; Virtanen, J.K.; Ruusunen, A.; Tuomainen, T.P.; Voutilainen, S. Serum Zinc and Risk of Type 2 Diabetes Incidence in Men: The Kuopio Ischaemic Heart Disease Risk Factor Study. J. Trace Elem. Med. Biol. 2016, 33, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Vashum, K.P.; McEvoy, M.; Milton, A.H.; Islam, M.R.; Hancock, S.; Attia, J. Is Serum Zinc Associated with Pancreatic Beta Cell Function and Insulin Sensitivity in Pre-Diabetic and Normal Individuals? Findings from the Hunter Community Study. PLoS ONE 2014, 9, e83944. [Google Scholar] [CrossRef] [Green Version]
- Fukunaka, A.; Fujitani, Y. Role of Zinc Homeostasis in the Pathogenesis of Diabetes and Obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Z.; Bao, W.; Zhang, Y.; Rong, Y.; Wang, X.; Jin, Y.; Song, Y.; Yao, P.; Sun, C.; Hu, F.B.; et al. Interactions between Zinc Transporter-8 Gene (SLC30A8) and Plasma Zinc Concentrations for Impaired Glucose Regulation and Type 2 Diabetes. Diabetes 2014, 63, 1796–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbrenner, H. Interference of Selenium and Selenoproteins with the Insulin-Regulated Carbohydrate and Lipid Metabolism. Free Radic. Biol. Med. 2013, 65, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P.; Stranges, S. Epidemiology of Selenium and Type 2 Diabetes: Can We Make Sense of It? Free Radic. Biol. Med. 2013, 65, 1557–1564. [Google Scholar] [CrossRef]
- De Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in Man: Implications for Health and Disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Von Ehrlich, B.; Barbagallo, M.; Classen, H.G.; Guerrero-Romero, F.; Mooren, F.C.; Rodriguez-Moran, M.; Vierling, W.; Vormann, J.; Kisters, K. Significance of Magnesium in Insulin Resistance, Metabolic Syndrome, and Diabetes—Recommendations of the Association of Magnesium Research e.V. Trace Elem. Electrolytes 2017, 34, 124–129. [Google Scholar] [CrossRef]
- Bertinato, J.; Wang, K.C.; Hayward, S. Serum Magnesium Concentrations in the Canadian Population and Associations with Diabetes, Glycemic Regulation, and Insulin Resistance. Nutrients 2017, 9, 296. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic Tissue Inflammation and Metabolic Disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Grubaugh, N.D.; Ladner, J.T.; Lemey, P.; Pybus, O.G.; Rambaut, A.; Holmes, E.C.; Andersen, K.G. Tracking Virus Outbreaks in the Twenty-First Century. Nat. Microbiol. 2019, 4, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, S.; Nekhai, S.; Liu, S. Depriving Iron Supply to the Virus Represents a Promising Adjuvant Therapeutic Against Viral Survival. Curr. Clin. Microbiol. Rep. 2020, 7, 13–19. [Google Scholar] [CrossRef]
- Misumi, I.; Mitchell, J.E.; Lund, M.M.; Cullen, J.M.; Lemon, S.M.; Whitmire, J.K. T Cells Protect against Hepatitis A Virus Infection and Limit Infection-Induced Liver Injury. J. Hepatol. 2021, 75, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Sahin, M.; Karayakar, F.; Koksal, A.R.; Yetim, A.; İyisoy, M.S.; Şen, İ.; Alkım, H.; Alkım, C.; Colak, T. Changes in Liver Tissue Trace Element Concentrations During Hepatitis B Viral Infection Treatment. Biol. Trace Elem. Res. 2019, 188, 245–250. [Google Scholar] [CrossRef]
- Nangliya, V.; Sharma, A.; Yadav, D.; Sunder, S.; Nijhawan, S.; Mishra, S. Study of Trace Elements in Liver Cirrhosis Patients and Their Role in Prognosis of Disease. Biol. Trace Elem. Res. 2015, 1, 35–40. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Al-Thubaiti, E.H.; Safa, H.; Qahl, R.A.A.-E.; Reham, Z.H. Synthesis and Spectroscopic Characterization of Dapagliflozin/Zn (II), Cr (III) and Se (IV) Novel Complexes That Ameliorate Hepatic Damage, Hyperglycemia and Oxidative Injury Induced by Streptozotocin-Induced Diabetic Male Rats and Their Antibacterial Act. Crystals 2022, 12, 304. [Google Scholar] [CrossRef]
- Lin, Y.; He, F.; Lian, S.; Xie, B.; Liu, T.; He, J.; Liu, C. Selenium Status in Patients with Chronic Liver Disease: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 952. [Google Scholar] [CrossRef]
- Diglio, D.C.; Fernandes, S.A.; Stein, J.; Azeredo-da-Silva, A.; De Mattos, A.A.; Tovo, C.V. Role of Zinc Supplementation in the Management of Chronic Liver Diseases: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2020, 19, 190–196. [Google Scholar] [CrossRef]
- Coni, P.; Pichiri, G.; Lachowicz, J.I.; Ravarino, A.; Ledda, F.; Fanni, D.; Gerosa, C.; Piras, M.; Coghe, F.; Gibo, Y.; et al. Zinc as a Drug for Wilson’s Disease, Non-Alcoholic Liver Disease and COVID-19-Related Liver Injury. Molecules 2021, 26, 6614. [Google Scholar] [CrossRef]
- Miwa, T.; Hanai, T.; Toshihide, M.; Ogiso, Y.; Imai, K.; Suetsugu, A.; Takai, K.; Shiraki, M.; Katsumura, N.; Shimizu, M. Zinc Deficiency Predicts Overt Hepatic Encephalopathy and Mortality in Liver Cirrhosis Patients with Minimal Hepatic Encephalopathy. Hepatol. Res. 2021, 51, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.A.; Vermeulen-Serpa, K.M.; Grilo, E.C.; Leite-Lais, L.; Brandão-Neto, J.; Vale, S.H.L. Association between Zinc and Body Composition: An Integrative Review. J. Trace Elem. Med. Biol. 2022, 71, 126940. [Google Scholar] [CrossRef] [PubMed]
- Barbara, M.; Mindikoglu, A.L. The Role of Zinc in the Prevention and Treatment of Nonalcoholic Fatty Liver Disease. Metab. Open 2021, 11, 100105. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The Role of Zinc in Liver Cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef]
- Girirajan, S.; Campbell, C.; Eichler, E. EASL Clinical Practice Guidelines on Nutrition in Chronic Liver Disease. Physiol. Behav. 2011, 176, 139–148. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, D.; Ma, D.; Chang, J.; Dougherty, A.M.; Cuconati, A.; Block, T.M.; Guo, J.-T. Activation of Pattern Recognition Receptor-Mediated Innate Immunity Inhibits the Replication of Hepatitis B Virus in Human Hepatocyte-Derived Cells. J. Virol. 2009, 83, 847–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uprichard, S.L.; Wieland, S.F.; Althage, A.; Chisari, F.V. Transcriptional and Posttranscriptional Control of Hepatitis B Virus Gene Expression. Proc. Natl. Acad. Sci. USA 2003, 100, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Kmiec, D.; Lista-Brotos, M.-J.; Ficarelli, M.; Swanson, C.M.; Neil, S.J. The C-Terminal PARP Domain of the Long ZAP Isoform Contributes Essential Effector Functions for CpG-Directed Antiviral Activity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Ficarelli, M.; Neil, S.J.D.; Swanson, C.M. Targeted Restriction of Viral Gene Expression and Replication by the ZAP Antiviral System. Annu. Rev. Virol. 2021, 8, 265–283. [Google Scholar] [CrossRef]
- Palomo ig Palomo, I.G.; Jaramillo, J.C.; Alarcon, M.L.; Gutierrez, C.L.; Moore-Carrasco, R.; Segovia, F.M.; Leiva, E.M.; Mujica, V.E.; Icaza, G.; Diaz, N.S. Increased Concentrations of Soluble Vascular Cell Adhesion Molecule-1 and Soluble CD40L in Subjects with Metabolic Syndrome. Molecular. Mol. Med. Rep. 2008, 1, 667–671. [Google Scholar] [CrossRef]
- Shen, J.; Qi, W.; Dai, J.; Leng, S.; Jiang, K.; Zhang, Y.; Ran, S.; Li, C.; Wen, T. Tenofovir vs. Entecavir on Recurrence of Hepatitis B Virus-Related Hepatocellular Carcinoma beyond Milan Criteria after Hepatectomy. Chin. Med. J. 2022, 135, 301–308. [Google Scholar] [CrossRef]
- Read, S.A.; O’Connor, K.S.; Suppiah, V.; Ahlenstiel, C.L.E.; Obeid, S.; Cook, K.M.; Cunningham, A.; Douglas, M.W.; Hogg, P.J.; Booth, D.; et al. Zinc Is a Potent and Specific Inhibitor of IFN-Λ3 Signalling. Nat. Commun. 2017, 8, 15245. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Anang, S.; Ganti, K.P.; Surjit, M. Zinc: A Potential Antiviral Against Hepatitis e Virus Infection? DNA Cell Biol. 2018, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Li, S.; Zhang, K.; Li, J.; Han, Y.; Zhan, T.; Zhao, Q.; Guo, X.; Zhang, J. Selenium Deficiency-Induced Redox Imbalance Leads to Metabolic Reprogramming and Inflammation in the Liver. Redox Biol. 2020, 36, 101519. [Google Scholar] [CrossRef]
- Regina, B.F.; Gladyshev, V.N.; Arnér, E.S.; Berry, M.J.; Bruford, E.A.; Burk, R.F.; Carlson, B.A.; Castellano, S.; Chavatte, L.; Conrad, M.; et al. Selenoprotein Gene Nomenclature. J. Biol. Chem. 2016, 291, 24036–24040. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Chen, W.; Dai, G.; Huang, Y. Cordycepin Suppresses the Migration and Invasion of Human Liver Cancer Cells by Downregulating the Expression of CXCR4. Int. J. Mol. Med. 2020, 45, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Reja, M.; Makar, M.; Visaria, A.; Marino, D.; Rustgi, V. Increased Serum Selenium Levels Are Associated with Reduced Risk of Advanced Liver Fibrosis and All-Cause Mortality in NAFLD Patients: National Health and Nutrition Examination Survey (NHANES) III. Ann. Hepatol. 2020, 19, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Kim, J.S.; Ju, S.; Seo, H.W.; Chang, Y.; Kang, J.A.; Park, S.G.; Jung, G. Oxidatively Modified Protein-Disulfide Isomerase–Associated 3 Promotes Dyskerin Pseudouridine Synthase 1–Mediated Malignancy and Survival of Hepatocellular Carcinoma Cells. Hepatology 2018, 68, 1851–1864. [Google Scholar] [CrossRef] [Green Version]
- Lesnichaya, M.; Karpova, E.; Sukhov, B. Effect of High Dose of Selenium Nanoparticles on Antioxidant System and Biochemical Profile of Rats in Correction of Carbon Tetrachloride-Induced Toxic Damage of Liver. Colloids Surf. B Biointerfaces 2021, 197, 111381. [Google Scholar] [CrossRef]
- Morbitzer, M.; Herget, T. Expression of Gastrointestinal Glutathione Peroxidase Is Inversely Correlated to the Presence of Hepatitis C Virus Subgenomic RNA in Human Liver Cells. J. Biol. Chem. 2005, 280, 8831–8841. [Google Scholar] [CrossRef] [Green Version]
- Demircan, K.; Bengtsson, Y.; Sun, Q.; Brange, A.; Vallon-Christersson, J.; Rijntjes, E.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; et al. Serum Selenium, Selenoprotein P and Glutathione Peroxidase 3 as Predictors of Mortality and Recurrence Following Breast Cancer Diagnosis: A Multicentre Cohort Study. Redox Biol. 2021, 47, 102145. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K. Selenium and Viral Infection: Are There Lessons for COVID-19. Br. J. Nutr. 2021, 125, 618–627. [Google Scholar] [CrossRef]
- John, R.; Giudicessi, B.A.; Michael, J.; Pantalone, D.W.; Schneider, K.L.; Valentine, S.E.; Simoni, J.M.; Liu-Smith, F.; Pantalone, D.W.; Rood, B.A.; et al. Iron Levels in Hepatocytes and Portal Tract Cells Predict Progression and Outcome of Patients with Advanced Chronic Hepatitis C. AIDS Behav. 2012, 23, 1031–1043. [Google Scholar] [CrossRef]
- Milic, S.; Mikolasevic, I.; Orlic, L.; Devcic, E.; Starcevic-Cizmarevic, N.; Stimac, D.; Kapovic, M.; Ristic, S. The Role of Iron and Iron Overload in Chronic Liver Disease. Med. Sci. Monit. 2016, 22, 2144–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostoker, G.; Vaziri, N.D. Impact of Iatrogenic Iron Overload on the Course of Hepatitis C in the Dialysis Population: A Plea for Caution. Hemodial. Int. 2017, 21, S68–S77. [Google Scholar] [CrossRef] [Green Version]
- De Campos, W.N.; Massaro, J.D.; Cançado, E.L.R.; Wiezel, C.E.V.; Simões, A.L.; Teixeira, A.C.; De Souza, F.F.; Mendes-Junior, C.T.; Martinelli, A.D.L.C.; Donadi, E.A. Comprehensive Analysis of HFE Gene in Hereditary Hemochromatosis and in Diseases Associated with Acquired Iron Overload. World J. Hepatol. 2019, 11, 186–193. [Google Scholar] [CrossRef]
- Morris, B.J.; Willcox, D.C.; Donlon, T.A.; Willcox, B.J. 2012 FOXO3: A major gene for human longevity-a mini-review. Gerontology 2015, 61, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Chhabra, R.; Saha, A.; Chamani, A.; Schneider, N.; Nanjundan, M.; Shah, R. Iron Pathways and Iron Chelation Approaches in Viral, Microbial, and Fungal Infections. Pharmaceuticals 2020, 13, 275. [Google Scholar] [CrossRef]
- Brem, H.; Stojadinovic, O.; Diegelmann, R.F.; Entero, H.; Lee, B.; Pastar, I.; Golinko, M.; Rosenberg, H.; Tomic-Canic, M. Cholinergic Anti-Inflammatory Pathway Activity and High High Mobility Group Box-1 (HMGB1) Serum Levels in Patients with Rheumatoid Arthritis. Mol. Med. 2007, 13, 30–39. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; An, D.; Diao, H.; Xu, W.; He, X.; Sun, R.; Wei, L.; Li, L. Regulation of Hepatitis C Virus Translation Initiation by Iron: Role of EIF3 and La Protein. Virus Res. 2012, 167, 302–309. [Google Scholar] [CrossRef]
- Vela, D. Low Hepcidin in Liver Fibrosis and Cirrhosis; A Tale of Progressive Disorder and a Case for a New Biochemical Marker. Mol. Med. 2018, 24, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Gaou, I.; Fromenty, B.; Berson, A.; Letteron, P.; Degott, C.; Erlinger, S.; Pessayre, D. “Premature MtDNA Deletions in Wilson’s Livers” Premature Oxidative Aging of Hepatic Mitochondrial DNA in Wilson’s Disease. Gastroenterology 1997, 113, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Liou, I.W.; Biggins, S.W.; Yeh, M.; Jalikis, F.; Chan, L.N.; Burkhead, J. Copper Deficiency in Liver Diseases: A Case Series and Pathophysiological Considerations. Hepatol. Commun. 2019, 3, 1159–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pauff, S.M.; Miller, S.C. High Fructose Feeding Induces Copper Deficiency in SpragueDawley Rats: A Novel Mechanism for Obesity Related Fatty Liver. Bone 2012, 78, 711–716. [Google Scholar] [CrossRef]
- Jorquera, F.; Monte, M.J.; Guerra, J.; Sanchez-Campos, S.; Merayo, J.A.; Olcóz, J.L.; González-Gallego, J.; Marin, J.J.G. Usefulness of Combined Measurement of Serum Bile Acids and Ferritin as Additional Prognostic Markers to Predict Failure to Reach Sustained Response to Antiviral Treatment in Chronic Hepatitis C. J. Gastroenterol. Hepatol. 2005, 20, 547–554. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and Copper/Zn Ratio in a Series of Children with Chronic Diseases: A Cross-Sectional Study. Nutrients 2021, 13, 3578. [Google Scholar] [CrossRef]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly Efficient Antiviral and Antibacterial Activities of Solid-State Cuprous Compounds. J. Hazard. Mater. 2012, 235, 265–270. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced Graphene Oxide Conjugated Cu2O Nanowire Mesocrystals for High-Performance NO2 Gas Sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef]
- Singh, J.; Srivastava, M.; Roychoudhury, A.; Lee, D.W.; Lee, S.H.; Malhotra, B.D. Bienzyme-Functionalized Monodispersed Biocompatible Cuprous Oxide/Chitosan Nanocomposite Platform for Biomedical Application. J. Phys. Chem. B 2013, 117, 141–152. [Google Scholar] [CrossRef]
- Joshi, S.; Joshi, M.; Degani, M.S. Tackling SARS-CoV-2: Proposed Targets and Repurposed Drugs. Future Med. Chem. 2020, 12, 1579–1601. [Google Scholar] [CrossRef]
- Basu, S. Non-Communicable Disease Management in Vulnerable Patients during COVID-19. Indian J. Med. Ethics 2020, 2, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieliszek, M.; Lipinski, B. Selenium supplementation in the prevention of coronavirus infections (COVID-19). Med. Hypotheses 2020, 143, 109878. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, J.; Varona, S.; Cañes, L.; Galán, M.; Briones, A.M.; Cachofeiro, V.; Rodríguez, C. Emerging Roles of Lysyl Oxidases in the Cardiovascular System: New Concepts and Therapeutic Challenges. Biomolecules 2019, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Cherukuri, S.; Potla, R.; Sarkar, J.; Nurko, S.; Harris, Z.L.; Fox, P.L. Unexpected Role of Ceruloplasmin in Intestinal Iron Absorption. Cell Metab. 2005, 2, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Cassat, J.E.; Skaar, E.P. Iron in Infection and Immunity. Cell Host Microbe 2013, 13, 509–519. [Google Scholar] [CrossRef] [Green Version]
- Tarifeño-Saldivia, E.; Aguilar, A.; Contreras, D.; Mercado, L.; Morales-Lange, B.; Márquez, K.; Henríquez, A.; Riquelme-Vidal, C.; Boltana, S. Iron Overload Is Associated with Oxidative Stress and Nutritional Immunity during Viral Infection in Fish. Front. Immunol. 2018, 9, 1296. [Google Scholar] [CrossRef] [Green Version]
- Bastin, A.; Shiri, H.; Zanganeh, S.; Fooladi, S.; Momeni Moghaddam, M.A.; Mehrabani, M.; Nematollahi, M.H. Iron Chelator or Iron Supplement Consumption in COVID-19? The Role of Iron with Severity Infection. Biol. Trace Elem. Res. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Woodby, B.; Arnold, M.M.; Valacchi, G. SARS-CoV-2 Infection, COVID-19 Pathogenesis, and Exposure to Air Pollution: What Is the Connection? Ann. N. Y. Acad. Sci. 2021, 1486, 15–38. [Google Scholar] [CrossRef]
- Shahid, Z.; Kalayanamitra, R.; McClafferty, B.; Kepko, D.; Ramgobin, D.; Patel, R.; Aggarwal, C.S.; Vunnam, R.; Sahu, N.; Bhatt, D.; et al. COVID-19 and Older Adults: What We Know. J. Am. Geriatr. Soc. 2020, 68, 926–929. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Kim, H.W.; Kang, J.M.; Kim, D.H.; Cho, E.Y. Epidemiology and Clinical Features of Coronavirus Disease 2019 in Children. Korean J. Pediatr. 2020, 63, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Kernan, K.F.; Carcillo, J.A. Hyperferritinemia and Inflammation. Int. Immunol. 2017, 29, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Edeas, M.; Saleh, J.; Peyssonnaux, C. Iron: Innocent Bystander or Vicious Culprit in COVID-19 Pathogenesis? Int. J. Infect. Dis. 2020, 97, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Leermakers, P.A.; Remels, A.H.V.; Zonneveld, M.I.; Rouschop, K.M.A.; Schols, A.M.W.J.; Gosker, H.R. Iron Deficiency-Induced Loss of Skeletal Muscle Mitochondrial Proteins and Respiratory Capacity; the Role of Mitophagy and Secretion of Mitochondria-Containing Vesicles. FASEB J. 2020, 34, 6703–6717. [Google Scholar] [CrossRef] [Green Version]
- Ersöz, A.; Yılmaz, T.E. The Association between Micronutrient and Hemogram Values and Prognostic Factors in COVID-19 Patients: A Single-Center Experience from Turkey. Int. J. Clin. Pract. 2021, 75, 1–9. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron Sequestration and Anemia of Inflammation. Semin. Hematol. 2009, 46, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Augustine, L.F.; Mullapudi, V.; Subramanian, S.; Kulkarni, B. Infection-Iron Interaction during COVID-19 Pandemic: Time to Re-Design Iron Supplementation Programs. Med. Hypotheses 2020, 143, 110173. [Google Scholar] [CrossRef]
- Habib, H.M.; Ibrahim, S.; Zaim, A.; Ibrahim, W.H. The Role of Iron in the Pathogenesis of COVID-19 and Possible Treatment with Lactoferrin and Other Iron Chelators. Biomed. Pharmacother. 2020, 136, 111228. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Ajsuvakova, O.P.; Shehtman, A.M.; Boev, V.M.; Nikonorov, A.A. Influence of Iron and Copper Consumption on Weight Gain and Oxidative Stress in Adipose Tissue of Wistar Rats. Interdiscip. Toxicol. 2012, 5, 127–132. [Google Scholar] [CrossRef]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Cruz, K.J.C.; De Oliveira, A.R.S.; Morais, J.B.S.; Severo, J.S.; Mendes, P.M.V.; Melo, S.R.d.; De Sousa, G.S. Zinc and Insulin Resistance: Biochemical and Molecular Aspects. Biol. Trace Elem. Res. 2018, 186, 407–412. [Google Scholar] [CrossRef]
- Tamaki, M.; Fujitani, Y.; Hara, A.; Uchida, T.; Tamura, Y.; Takeno, K.; Kawaguchi, M.; Watanabe, T.; Ogihara, T.; Fukunaka, A.; et al. The Diabetes-Susceptible Gene SLC30A8/ZnT8 Regulates Hepatic Insulin Clearance. J. Clin. Investig. 2013, 123, 4513–4524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranasinghe, P.; Wathurapatha, W.S.; Galappatthy, P.; Katulanda, P.; Jayawardena, R.; Constantine, G.R. Zinc Supplementation in Prediabetes: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Diabetes 2018, 10, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Van Dam, R.M.; Willett, W.C.; Hu, F.B. Prospective Study of Zinc Intake and Risk of Type 2 Diabetes in Women. Diabetes Care 2009, 32, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19—A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Roden, M.; Shulman, G.I. The Integrative Biology of Type 2 Diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Steinbrenner, H.; Duntas, L.H.; Rayman, M.P. The Role of Selenium in Type-2 Diabetes Mellitus and Its Metabolic Comorbidities. Redox Biol. 2022, 50, 102236. [Google Scholar] [CrossRef]
- Kim, J.; Chung, H.S.; Choi, M.K.; Roh, Y.K.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Yu, J.M.; Moon, S. Association between Serum Selenium Level and the Presence of Diabetes Mellitus: A Meta-Analysis of Observational Studies. Diabetes Metab. J. 2019, 43, 447–460. [Google Scholar] [CrossRef]
- Stranges, S.; Galletti, F.; Farinaro, E.; D’Elia, L.; Russo, O.; Iacone, R.; Capasso, C.; Carginale, V.; De Luca, V.; Della Valle, E.; et al. Associations of Selenium Status with Cardiometabolic Risk Factors: An 8-Year Follow-up Analysis of the Olivetti Heart Study. Atherosclerosis 2011, 217, 274–278. [Google Scholar] [CrossRef]
- Alghobashy, A.A.; Alkholy, U.M.; Talat, M.A.; Abdalmonem, N.; Zaki, A.; Ahmed, I.A.; Mohamed, R.H. Trace Elements and Oxidative Stress in Children with Type 1 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2018, 11, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinceti, M.; Filippini, T.; Wise, L.A.; Rothman, K.J. A Systematic Review and Dose-Response Meta-Analysis of Exposure to Environmental Selenium and the Risk of Type 2 Diabetes in Nonexperimental Studies. Environ. Res. 2021, 197, 111210. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.L.; Wang, Z.H.; Liang, X.N.; Liang, J.; Wei, X.B.; Wang, S.H.; Guo, W.X. The Association of Circulating Selenium Concentrations with Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 4755–4761. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Young, J.L.; Cheng, N.; Yang, C.; Papandonatos, G.D.; Kelsey, K.T.; Wise, J.P.; Shi, K.; Zheng, T.; et al. Long-Term Association of Serum Selenium Levels and the Diabetes Risk: Findings from a Case-Control Study Nested in the Prospective Jinchang Cohort. Sci. Total Environ. 2022, 818, 151848. [Google Scholar] [CrossRef] [PubMed]
- Panel, E.; Nda, A. Scientific Opinion on Dietary Reference Values for Magnesium. EFSA J. 2015, 13, 1–63. [Google Scholar] [CrossRef] [Green Version]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.M.R. Type 2 Diabetes. Nurs. Made Incred. Easy 2012, 10, 30–31. [Google Scholar] [CrossRef]
- Fang, X.; Han, H.; Li, M.; Liang, C.; Fan, Z.; Aaseth, J.; He, J.; Montgomery, S.; Cao, Y. Dose-Response Relationship between Dietary Magnesium Intake and Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Regression Analysis of Prospective Cohort Studies. Nutrients 2016, 8, 739. [Google Scholar] [CrossRef]
- Kostov, K. Effects of Magnesium Deficiency on Mechanisms of Insulin Resistance in Type 2 Diabetes: Focusing on the Processes of Insulin Secretion and Signaling. Int. J. Mol. Sci. 2019, 20, 1351. [Google Scholar] [CrossRef] [Green Version]
- Apell, H.J.; Hitzler, T.; Schreiber, G. Modulation of the Na,K-ATPase by Magnesium Ions. Biochemistry 2017, 56, 1005–1016. [Google Scholar] [CrossRef]
- Zhao, B.; Zeng, L.; Zhao, J.; Wu, Q.; Dong, Y.; Zou, F.; Gan, L.; Wei, Y.; Zhang, W. Association of Magnesium Intake with Type 2 Diabetes and Total Stroke: An Updated Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e032240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawardena, R.; Ranasinghe, P.; Galappatthy, P.; Malkanthi, R.L.; Constantine, G.R.; Katulanda, P. Effects of Zinc Supplementation on Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2012, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05320510?term=selenium&cond=diabetes+type+2&draw=2&rank=1 (accessed on 11 May 2022).
- Saeed, H.; Haj, S.; Qasim, B. Estimation of Magnesium Level in Type 2 Diabetes Mellitus and Its Correlation with HbA1c Level. Endocrinol. Diabetes Metab. 2019, 19, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03002545?term=magnesium&cond=type+2+diabetes&draw=2&rank=3 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT05033054?term=magnesium&cond=type+2+diabetes&draw=2&rank=6 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=selenium&cntry=&state=&city=&dist= (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04877509?term=selenium&cond=COVID-19&draw=2&rank=2 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04941703?term=magnesium&cond=covid19&draw=2&rank=1 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04716985?term=magnesium&cond=covid19&draw=2&rank=3 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04641195?term=zinc&cond=COVID-19&draw=2&rank=1 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04370782?term=zinc&cond=COVID-19&draw=2&rank=2 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04558424?term=zinc&cond=COVID-19&draw=2&rank=3 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04542993?term=zinc&cond=COVID-19&draw=2&rank=6 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT04072822?term=zinc&cond=hepatitis&draw=2&rank=1 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01809132?term=zinc&cond=hepatitis&draw=2&rank=2 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01355107?term=selenium&cond=hepatitis&draw=2&rank=1 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03349008?term=magnesium&cond=hepatitis&draw=2&rank=1 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03166280?term=NCT03166280&draw=2&rank=1 (accessed on 11 May 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02744560?term=iron&cond=hepatitis&draw=2&rank=4 (accessed on 11 May 2022).
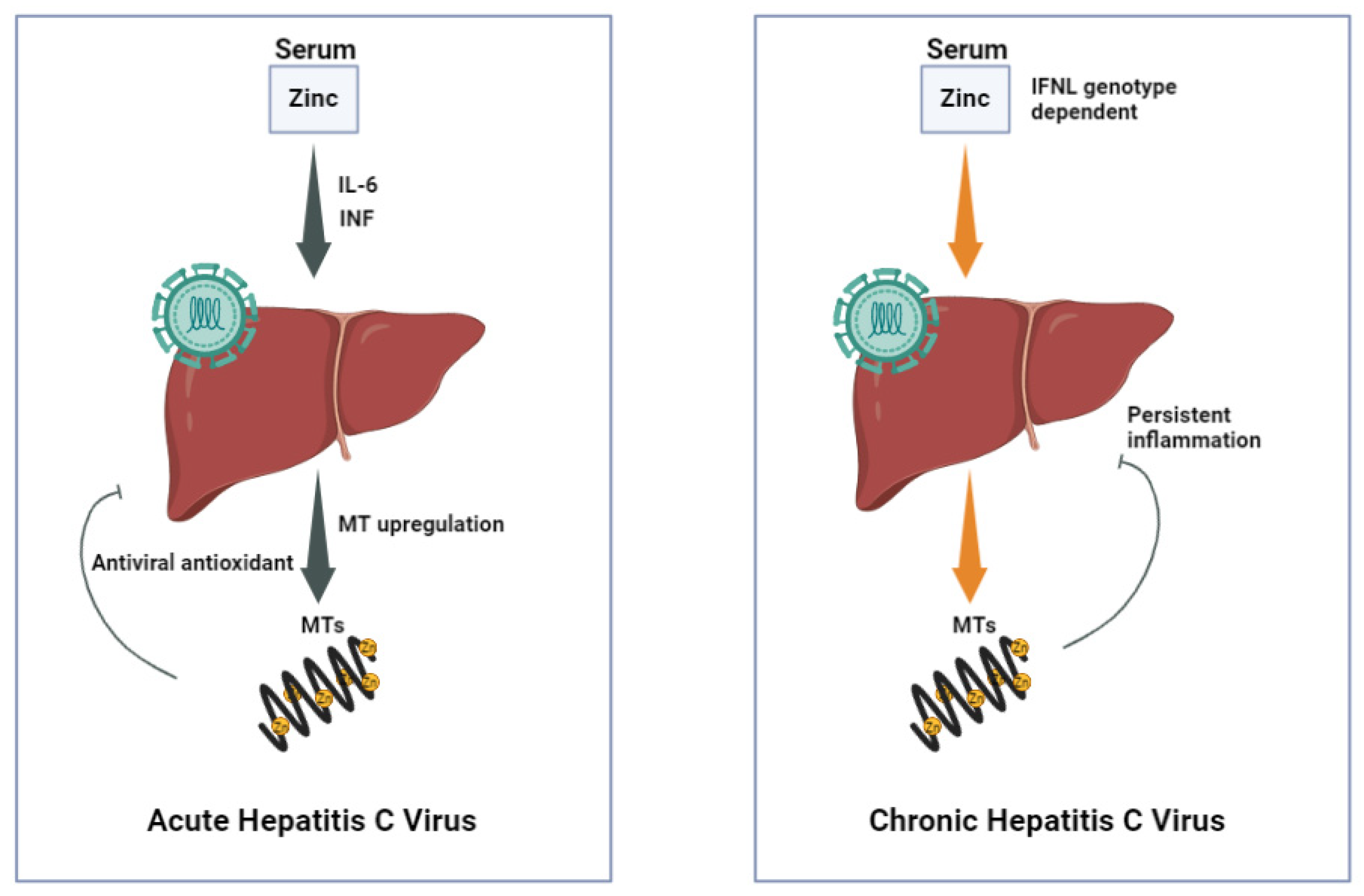

| Chronic Hepatitis | |
|---|---|
| Zinc (Zn) |
|
| Selenium (Se) | |
| Iron (Fe) |
|
| Copper (Cu) |
|
| COVID-19 | |
| Zinc (Zn) |
|
| Selenium (Se) |
|
| Copper (Cu) |
|
| Type-2 Diabetes Mellitus (T2DM) | |
| Zinc (Zn) |
|
| Selenium (Se) | |
| Magnesium (Mg) |
|
| ClinicalTials.gov Identifier | Study Title | Condition | Intervention & Findings | Status | Reference |
|---|---|---|---|---|---|
| NCT05000762 | Zinc Supplementation Improves Cardiovascular Morbidity in Patients with Diabetes Mellitus | Diabetes Mellitus, Type 2 Cardiovascular Diseases | Dietary Supplement: Zinc (Zn) Zinc gluconate 30 mg/day orally | Recruiting | [152] |
| NCT05320510 | Effect of Selenium (Se) Supplementation on Glycemic Control in Patients with Type 2 Diabetes or Prediabetes | Type 2 Diabetes Pre Diabetes | Dietary Supplement: Se-yeast The participants will be asked to take Se-yeast tablet. Dietary Supplement: Placebo The participants will be asked to take placebo-yeast tablet. | Not yet recruiting | [153] |
| NCT04636411 | Effect of Oral Magnesium Supplementation on Patients with Type 2 Diabetes | Type2 Diabetes | Dietary Supplement: Oral Magnesium (Mg) Supplementation Other: Standard Care for diabetic patients | Recruiting | [154] |
| NCT03002545 | Magnesium Supplementation in Type II Diabetes | Effect of magnesium (Mg) in diabetes | Dietary Supplement: Magnesium (Mg) citrate Dietary Supplement: Placebo Findings: oral Mg citrate supplementation reduced HbA1c levels and reduced BP in normomagnesemic persons with MetS | Completed | [155] |
| NCT05033054 | Effect of Dietary Magnesium Supplementation vs. Dapagliflozin in Patients with Diabetic Kidney Disease (DKD) | Kidney Disease, Chronic Diabetes | Dietary Supplement: EffCaMg Citrate 480 mg Drug: Dapagliflozin 10 mg Dietary Supplement: Placebo EffCaMg Citrate Drug: Placebo Dapagliflozin | Not yet recruiting | [156] |
| NCT04869579 | Selenium (Se) as a Potential Treatment for Moderately-ill, Severely-ill and Critically-ill COVID-19 Patients. | Covid19 | Drug: Selenium (as Selenious Acid)Other: Placebo | Not yet recruiting | [157] |
| NCT04877509 | Micronutrient Status Involved in Immunity in Elderly Patients with COVID-19 | Covid19 | Biological: Selenium (Se), Zinc (Zn) and Copper (Cu), Vitamin A, D, E plasma concentrations during patient hospitalization | Completed | [158] |
| NCT04941703 | CHANGE COVID-19 Severity | COVID-19 Infection | Drug: Magnesium Citrate plus probiotic | Recruiting | [159] |
| NCT04716985 | Evaluation of the Daily Intake of 0.5 L of Water Saturated with Molecular Hydrogen for 21 Days in COVID-19 Patients Treated in Ambulatory Care | SARS-CoV-2 Covid19 AMBULATORY CARE | Dietary Supplement: molecular hydrogen Dietary Supplement: placebo magnesium (Mg) | Active not recruiting | [160] |
| NCT04641195 | Vitamin D and Zinc Supplementation for Improving Treatment Outcomes Among COVID-19 Patients in India | Covid19 | Dietary Supplement: Vitamin D3 (cholecalciferol) Dietary Supplement: Zinc (zinc gluconate) Dietary Supplement: Zinc (zinc gluconate) & Vitamin D (cholecalciferol) Other: Placebo | recruiting | [161] |
| NCT04370782 | Hydroxychloroquine and Zinc with Either Azithromycin or Doxycycline for Treatment of COVID-19 in Outpatient Setting | Covid19 | Drug: Hydroxychloroquine Drug: Azithromycin Drug: Zinc Sulfate Drug: Doxycycline | Completed | [162] |
| NCT04558424 | RCT, Double Blind, Placebo to Evaluate the Effect of Zinc and Ascorbic Acid Supplementation in COVID-19 Positive Hospitalized Patients in BSMMU | Covid19 | Dietary Supplement: zinc gluconate and ascorbic acid | Not yet recruiting | [163] |
| NCT04542993 | Can SARS-CoV-2 Viral Load and COVID-19 Disease Severity Be Reduced by Resveratrol-assisted Zinc Therapy (Reszinate) | Covid19 SARS-CoV Infection | Dietary Supplement: Zinc Picolinate Dietary Supplement: Resveratrol Dietary Supplement: Zinc Picolinate Placebo Dietary Supplement: Resveratrol Placebo | Active, not recruiting | [164] |
| NCT04072822 | Trial of Anakinra (Plus Zinc) or Prednisone in Patients with Severe Alcoholic Hepatitis | Alcoholic Hepatitis | Drug: Anakinra and Zinc (Zn) Drug: Prednisone Drug: Placebos | Active, not recruiting | [165] |
| NCT01809132 | Efficacy Study of Anakinra, Pentoxifylline and Zinc Compared to Methylprednisolone in Severe Acute Alcoholic Hepatitis | Acute Alcoholic Hepatitis | Drug: Anakinra Drug: Pentoxifylline Drug: Zinc Sulfate Drug: Methylprednisolone | Completed, has results | [166] |
| NCT01355107 | Comparison of Selenium (Se) Levels in HCV- Infected Patients at Different Stages of Disease | Hepatitis C Liver Cirrhosis Carcinoma, Hepatocellular | N/A | Completed | [167] |
| NCT03349008 | Magnesium Isoglycyrrhizinate Followed by Diammonium Glycyrrhizinate and Combined with Entecavir in Chronic Hepatitis B | Chronic Hepatitis B Liver Inflammation | Drug: Entecavir Drug: Magnesium Isoglycyrrhizinate Drug: Diammonium Glycyrrhizinate Drug: Magnesium Isoglycyrrhizinate Placebo Drug: Diammonium Glycyrrhizinate Placebo | N/A | [168] |
| NCT03166280 | Hepatitis c and Vitamin D and Iron (Fe) Status | Hepatitis C | Drug: Sofosbuvir 400 mg Drug: Daclatasvir 60 mg/day | N/A | [169] |
| NCT02744560 | Effect of Spirulina on Liver Iron (Fe) Concentration in Beta Thalassemic Children with Hepatitis C | Beta Thalassemia Major | Dietary Supplement: spirulina | Completed | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumaily, K.M. The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus. Nutrients 2022, 14, 2632. https://doi.org/10.3390/nu14132632
Sumaily KM. The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus. Nutrients. 2022; 14(13):2632. https://doi.org/10.3390/nu14132632
Chicago/Turabian StyleSumaily, Khalid M. 2022. "The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus" Nutrients 14, no. 13: 2632. https://doi.org/10.3390/nu14132632
APA StyleSumaily, K. M. (2022). The Roles and Pathogenesis Mechanisms of a Number of Micronutrients in the Prevention and/or Treatment of Chronic Hepatitis, COVID-19 and Type-2 Diabetes Mellitus. Nutrients, 14(13), 2632. https://doi.org/10.3390/nu14132632






