Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk: A Systematic Review of Prospective Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Screening and Data Extraction
2.4. Quality Assessment
3. Results
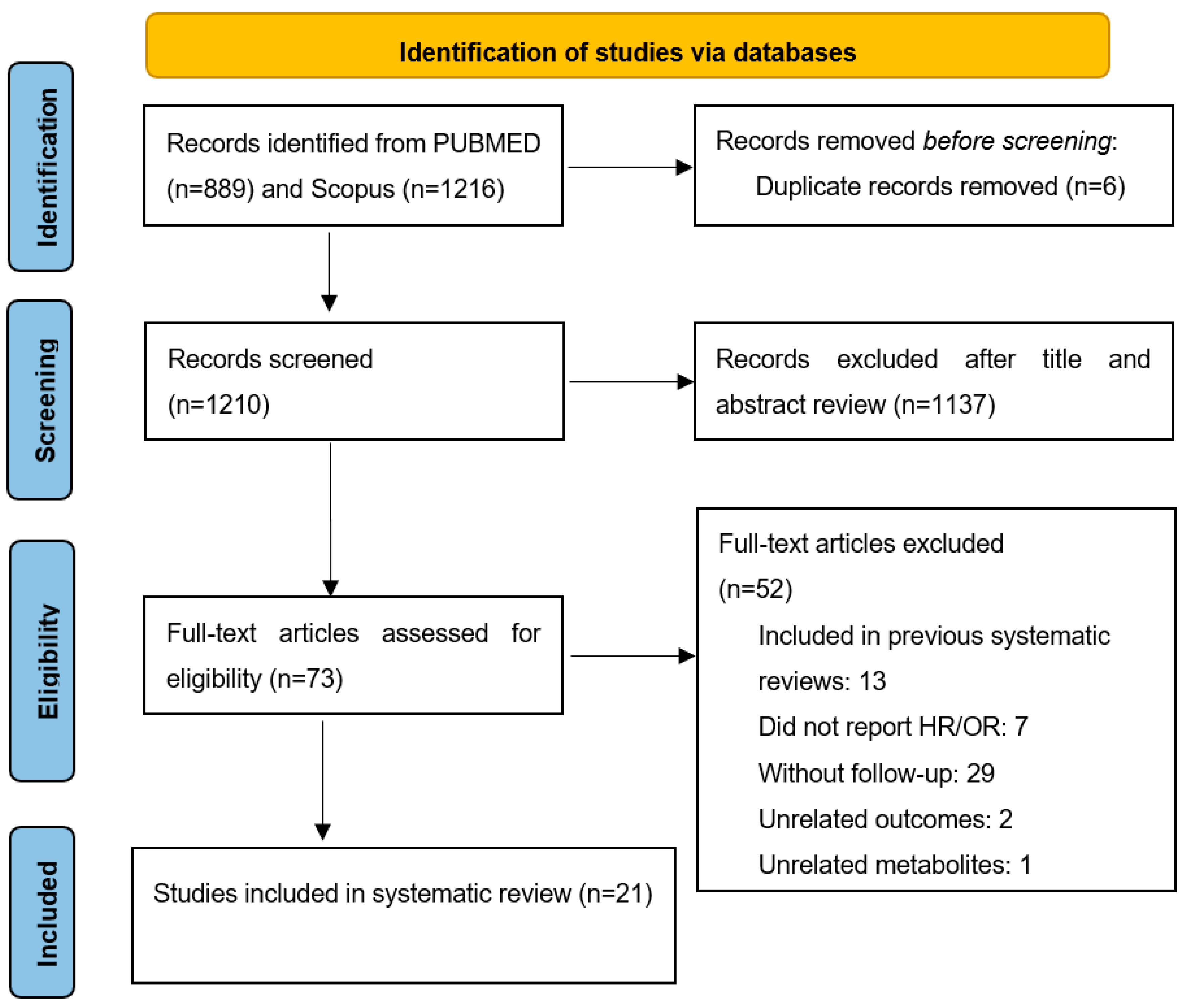
3.1. Selection of Studies
Literature Search
3.2. Study Characteristics
3.3. Metabolites Associated with CVD Risk
3.3.1. Total CVD
3.3.2. MACE
3.3.3. CAD, Major Vascular Event, AF, HF, MI, Stroke, Coronary Revascularization
3.4. Metabolites Associated with CVD Mortality and All-Cause Mortality
3.4.1. TMAO
3.4.2. Secondary Bile Acids
3.4.3. Tryptophan and Indole Derivatives
4. Discussion
4.1. TMAO
4.2. BCAAs
4.3. Secondary Bile Acids
4.4. Tryptophan and Indole Derivatives
4.5. SCFA
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.J.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Schooley, R.T.; Spilker, B.A.; et al. Biomarkers and Surrogate Endpoints: Preferred Definitions and Conceptual Framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Leon-Mimila, P.; Wang, J.; Huertas-Vazquez, A. Relevance of Multi-Omics Studies in Cardiovascular Diseases. Front. Cardiovasc. Med. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taegtmeyer, H.; Young, M.E.; Lopaschuk, G.D.; Abel, E.D.; Brunengraber, H.; Darley-Usmar, V.; des Rosiers, C.; Gerszten, R.; Glatz, J.F.; Griffin, J.L.; et al. Assessing Cardiac Metabolism. Circ. Res. 2016, 118, 1659–1701. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Bäckhed, F. Diet-Microbiota Interactions as Moderators of Human Metabolism. Nature 2016, 535, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; O’donovan, A.N.; Caplice, N.M.; Ross, R.P.; Stanton, C. Exploring the Gut Microbiota and Cardiovascular Disease. Metabolites 2021, 11, 493. [Google Scholar] [CrossRef]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Canela, M.; Hruby, A.; Clish, C.B.; Liang, L.; Martínez-González, M.A.; Hu, F.B. Comprehensive Metabolomic Profiling and Incident Cardiovascular Disease: A Systematic Review. J. Am. Heart Assoc. 2017, 6, e005705. [Google Scholar] [CrossRef] [Green Version]
- Heianza, Y.; Ma, W.; Manson, J.A.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut Microbe-Generated Metabolite Trimethylamine-N-Oxide as Cardiovascular Risk Biomarker: A Systematic Review and Dose-Response Meta-Analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating Trimethylamine N-Oxide and the Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis of 11 Prospective Cohort Studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Galliazzo, S.; Molaro, M.; Visconti, E.; Pennella, B.; Gaudio, G.V.; Lupi, A.; Grandi, A.M.; Squizzato, A. TMAO as a Biomarker of Cardiovascular Events: A Systematic Review and Meta-Analysis. Intern. Emerg. Med. 2021, 16, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Vajdi, M.; Asghari-Jafarabadi, M. Gut Microbiota-Associated Metabolite Trimethylamine N-Oxide and the Risk of Stroke: A Systematic Review and Dose-Response Meta-Analysis. Nutr. J. 2020, 19, 1–15. [Google Scholar] [CrossRef]
- McGranaghan, P.; Saxena, A.; Rubens, M.; Radenkovic, J.; Bach, D.; Schleußner, L.; Pieske, B.; Edelmann, F.; Trippel, T.D. Predictive Value of Metabolomic Biomarkers for Cardiovascular Disease Risk: A Systematic Review and Meta-Analysis. Biomarkers 2020, 25, 101–111. [Google Scholar] [CrossRef]
- Ke, C.; Pan, C.W.; Zhang, Y.; Zhu, X.; Zhang, Y. Metabolomics Facilitates the Discovery of Metabolic Biomarkers and Pathways for Ischemic Stroke: A Systematic Review. Metabolomics 2019, 15, 152. [Google Scholar] [CrossRef]
- Yao, M.E.; Liao, P.D.; Zhao, X.J.; Wang, L. Trimethylamine-N-Oxide Has Prognostic Value in Coronary Heart Disease: A Meta-Analysis and Dose-Response Analysis. BMC Cardiovasc. Disord. 2020, 20, 7–9. [Google Scholar] [CrossRef]
- Winther, S.A.; Øllgaard, J.C.; Hansen, T.W.; von Scholten, B.J.; Reinhard, H.; Ahluwalia, T.S.; Wang, Z.; Gæde, P.; Parving, H.H.; Hazen, S.; et al. Plasma Trimethylamine N-Oxide and Its Metabolic Precursors and Risk of Mortality, Cardiovascular and Renal Disease in Individuals with Type 2-Diabetes and Albuminuria. PLoS ONE 2021, 16, e0244402. [Google Scholar] [CrossRef]
- Ringel, C.; Dittrich, J.; Gaudl, A.; Schellong, P.; Beuchel, C.F.; Baber, R.; Beutner, F.; Teren, A.; Engel, C.; Wirkner, K.; et al. Association of Plasma Trimethylamine N-Oxide Levels with Atherosclerotic Cardiovascular Disease and Factors of the Metabolic Syndrome. Atherosclerosis 2021, 335, 62–67. [Google Scholar] [CrossRef]
- Israr, M.Z.; Bernieh, D.; Salzano, A.; Cassambai, S.; Yazaki, Y.; Heaney, L.M.; Jones, D.J.L.; Ng, L.L.; Suzuki, T. Association of Gut-Related Metabolites with Outcome in Acute Heart Failure. Am. Heart J. 2021, 234, 71–80. [Google Scholar] [CrossRef]
- Papandreou, C.; Bulló, M.; Hernández-Alonso, P.; Ruiz-Canela, M.; Li, J.; Guasch-Ferré, M.; Toledo, E.; Clish, C.; Corella, D.; Estruch, R.; et al. Choline Metabolism and Risk of Atrial Fibrillation and Heart Failure in the PREDIMED Study. Clin. Chem. 2021, 67, 288–297. [Google Scholar] [CrossRef]
- Croyal, M.; Saulnier, P.-J.; Aguesse, A.; Gand, E.; Ragot, S.; Roussel, R.; Halimi, J.-M.; Ducrocq, G.; Cariou, B.; Montaigne, D.; et al. Plasma Trimethylamine N-Oxide and Risk of Cardiovascular Events in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, J.; Li, Y.; Hu, Y.; Franke, A.A.; Liang, L.; Hu, F.B.; Chan, A.T.; Mukamal, K.J.; Rimm, E.B.; et al. Gut Microbiota-Derived Metabolites and Risk of Coronary Artery Disease: A Prospective Study among US Men and Women. Am. J. Clin. Nutr. 2021, 114, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nemet, I.; Wang, Z.; Lai, H.T.M.; de Oliveira Otto, M.C.; Lemaitre, R.N.; Fretts, A.M.; Sotoodehnia, N.; Budoff, M.; Didonato, J.A.; et al. Longitudinal Plasma Measures of Trimethylamine N-Oxide and Risk of Atherosclerotic Cardiovascular Disease Events in Community-Based Older Adults. J. Am. Heart Assoc. 2021, 10, e020646. [Google Scholar] [CrossRef] [PubMed]
- Amrein, M.; Li, X.S.; Walter, J.; Wang, Z.; Zimmermann, T.; Strebel, I.; Honegger, U.; Leu, K.; Schäfer, I.; Twerenbold, R.; et al. Gut Microbiota-Dependent Metabolite Trimethylamine N-Oxide (TMAO) and Cardiovascular Risk in Patients with Suspected Functionally Relevant Coronary Artery Disease (FCAD). Clin. Res. Cardiol. 2022, 111, 692–704. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Ye, Z.S.; Xia, N.G.; Xu, Y. TMAO as a Novel Predictor of Major Adverse Vascular Events and Recurrence in Patients with Large Artery Atherosclerotic Ischemic Stroke. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221090503. [Google Scholar] [CrossRef]
- Fretts, A.M.; Hazen, S.L.; Jensen, P.; Budoff, M.; Sitlani, C.M.; Wang, M.; de Oliveira Otto, M.C.; DiDonato, J.A.; Lee, Y.; Psaty, B.M.; et al. Association of Trimethylamine N -Oxide and Metabolites With Mortality in Older Adults. JAMA Netw. Open 2022, 5, e2213242. [Google Scholar] [CrossRef]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Murabito, J.M.; Rhee, E.P.; Ho, J.E.; Jacques, P.F.; Ghorbani, A.; Magnusson, M.; Souza, A.L.; et al. Distinct Metabolomic Signatures Are Associated with Longevity in Humans. Nat. Commun. 2015, 6, 6791. [Google Scholar] [CrossRef]
- Alonso, A.; Yu, B.; Sun, Y.V.; Chen, L.Y.; Loehr, L.R.; O’Neal, W.T.; Soliman, E.Z.; Boerwinkle, E. Serum Metabolomics and Incidence of Atrial Fibrillation (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2019, 123, 1955–1961. [Google Scholar] [CrossRef]
- Huang, J.; Weinstein, S.J.; Moore, S.C.; Derkach, A.; Hua, X.; Liao, L.M.; Gu, F.; Mondul, A.M.; Sampson, J.N.; Albanes, D.; et al. Serum Metabolomic Profiling of All-Cause Mortality: A Prospective Analysis in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study Cohort. Am. J. Epidemiol. 2018, 187, 1721–1732. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.C.; Chang, J.C.H.; Lin, C.N.; Lee, C.C.; Chen, Y.T.; Chu, P.H.; Kou, G.; Lu, Y.A.; Yang, C.W.; Chen, Y.C. Serum Indoxyl Sulfate Predicts Adverse Cardiovascular Events in Patients with Chronic Kidney Disease. J. Formos. Med. Assoc. 2019, 118, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Frazier, R.; Cai, X.; Lee, J.; Bundy, J.D.; Jovanovich, A.; Chen, J.; Deo, R.; Lash, J.P.; Anderson, A.H.; Go, A.S.; et al. Deoxycholic Acid and Risks of Cardiovascular Events, ESKD, and Mortality in CKD: The CRIC Study. Kidney Med. 2021, 4, 100387. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Sokol, H. Gut Microbiota-Derived Metabolites as Key Actors in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Heiss, G.; Alexander, D.; Grams, M.E.; Boerwinkle, E. Associations between the Serum Metabolome and All-Cause Mortality among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2016, 183, 650–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 May 2022).
- Roncal, C.; Martínez-Aguilar, E.; Orbe, J.; Ravassa, S.; Fernandez-Montero, A.; Saenz-Pipaon, G.; Ugarte, A.; Estella-Hermoso de Mendoza, A.; Rodriguez, J.A.; Fernández-Alonso, S.; et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobias, D.K.; Lawler, P.R.; Harada, P.H.; Demler, O.V.; Ridker, P.M.; Manson, J.A.E.; Cheng, S.; Mora, S. Circulating Branched-Chain Amino Acids and Incident Cardiovascular Disease in a Prospective Cohort of US Women. Circ. Genom. Precis. Med. 2018, 11, e002157. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, R.; Paynter, N.P.; Giulianini, F.; Manson, J.E.; Zhao, Y.; Chen, J.C.; Vitolins, M.Z.; Albert, C.A.; Clish, C.; Rexrode, K.M. Metabolomic Profiles Associated with All-Cause Mortality in the Women’s Health Initiative. Int. J. Epidemiol. 2019, 49, 289–300. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Wang, Y.; You, H.; Hui, P.; Zheng, Y.; Du, J. Increased Branched-Chain Amino Acid Levels Are Associated with Long-Term Adverse Cardiovascular Events in Patients with STEMI and Acute Heart Failure. Life Sci. 2018, 209, 167–172. [Google Scholar] [CrossRef]
- Wang, C.H.; Cheng, M.L.; Liu, M.H.; Shiao, M.S.; Hsu, K.H.; Huang, Y.Y.; Lin, C.C.; Lin, J.F. Increased P-Cresyl Sulfate Level Is Independently Associated with Poor Outcomes in Patients with Heart Failure. Heart Vessel. 2016, 31, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, Atherosclerosis and Cardiovascular Disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, J.; Dou, P.; Gao, M.; Kong, X.; Li, C.; Liu, Z.; Huang, T. Assessment of Causal Direction between Gut Microbiota-Dependent Metabolites and Cardiometabolic Health: A Bi-Directional Mendelian Randomisation Analysis. Diabetes 2019, 68, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The Gut Microbiome in Coronary Artery Disease and Heart Failure: Current Knowledge and Future Directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef] [Green Version]
- Hazen, S.L.; Brown, J.M. Eggs as a Dietary Source for Gut Microbial Production of Trimethylamine-N-Oxide. Am. J. Clin. Nutr. 2014, 100, 741–743. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Malsam, J.; Satoh, A.; Pelletier, L.; Warren, G. Golgin Tethers Define Subpopulations of COPI Vesicles. Science (1979) 2005, 307, 1095–1098. [Google Scholar] [CrossRef]
- Stamler, J.; Daviglus, M.L.; Garside, D.B.; Ma, A.R.; Dyer, P.; Greenland, J.D.; Neaton, F. Relationship of Baseline Serum Cholesterol Levels in 3 Large Cohorts of Younger Men to Long-Term Coronary, Cardiovascular, and All-Cause Mortality and to Longevity. JAMA 2000, 284, 311–318. [Google Scholar] [CrossRef]
- Konopelski, P.; Mogilnicka, I. Biological Effects of Indole-3-Propionic Acid, a Gut Microbiota-Derived Metabolite, and Its Precursor Tryptophan in Mammals’ Health and Disease. Int. J. Mol. Sci. 2022, 23, 1222. [Google Scholar] [CrossRef]
- Pertovaara, M.; Raitala, A.; Lehtimäki, T.; Karhunen, P.J.; Oja, S.S.; Jylhä, M.; Hervonen, A.; Hurme, M. Indoleamine 2,3-Dioxygenase Activity in Nonagenarians Is Markedly Increased and Predicts Mortality. Mech. Ageing Dev. 2006, 127, 497–499. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, T.; Takeda, N.; Maeda, K.; Shibata, M.; Tatematsu, A. Accumulation of Furancarboxylic Acids in Uremic Serum as Inhibitors of Drug Binding. Clin. Chim. Acta 1988, 173, 127–138. [Google Scholar] [CrossRef]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The Uremic Solute Indoxyl Sulfate Induces Oxidative Stress in Endothelial Cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The Uremic Solutes P-Cresol and Indoxyl Sulfate Inhibit Endothelial Proliferation and Wound Repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Salazar, N.; Margolles, A.; González, S.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Suárez, A. Free Fatty Acids Profiles Are Related to Gut Microbiota Signatures and Short-Chain Fatty Acids. Front. Immunol. 2017, 8, 823. [Google Scholar] [CrossRef]
| First Author | Year/Country | Title | Journal | Study Design | N, Follow-Up Time | Baseline Characteristics of Participants | Assay Method and Metabolite Targets | Biological Sample | Main Outcome | Statistical Analysis | Covariates in Fully Adjusted Model | Adjusted HR or OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amrein, M. [25] | 2022/Switzerland | Gut microbiota-dependent metabolite trimethylamine N-oxide (TMAO) and cardiovascular risk in patients with suspected functionally relevant coronary artery disease (fCAD) | Clin Res Cardiol | Cohort | 1726 (223 deaths, 115 deaths due to CVD, 88 MI), 5 years | Patients with suspected functionally relevant CAD | LC-MS/MS; TMAO | Serum (non-fasting) | All-cause mortality, CVD mortality, MI | Cox regression | Age, sex, history of CAD, age, gender, BMI, smoking history, positive cardiovascular family history, hypertension, hypercholesterolemia, history of diabetes, history of stroke/TIA, history of CAD, previous MI, history of heart failure, cystatin-C | Per log2-transformed all-cause mortality HR 1.11 (95% CI 0.99–1.26), CVD mortality HR 1.19 (95% CI 1.01–1.40), MI HR 1.17 (95% CI 0.95–1.45) |
| Chen, Y.Y. [26] | 2022/China | TMAO as a novel predictor of major adverse vascular events and recurrence in patients with large artery atherosclerotic ischemic stroke | Clin Appl Thromb Hemost | Cohort | 291 (number of major vascular event cases not indicated), 3 months | Patients with ischemic stroke | LC-MS/MS; TMAO | Plasma (fasting); anticoagulant not indicated | Major vascular event (transient ischemic attack, recurrent ischemic stroke, MI) | Cox regression | Age, sex, hypertension, T2D, smoking, creatinine | >median vs. <median HR 3.12 (95% CI 1.02–9.61) |
| Fretts, A.M. [27] | 2022/USA | Association of trimethylamine N-Oxide and metabolites with mortality in older adults | JAMA Network Open | Cohort | 5333 (4791 total deaths), 13.2 years | 20% of participants had CHD and 15% had T2D at baseline | LC-MS/MS; TMAO | Plasma (fasting); anticoagulant: EDTA | All-cause mortality | Cox regression | Age, sex, race, ethnicity, educational level, household income, smoking status, BMI, physical activity, treated hypertension, instrumental activities of daily living, self-reported health status, SBP, HDL-C, prevalent AF, prevalent CHD, history of MI, prevalent T2D, prevalent COPD, foods consumption | 5th vs. 1st quintile all-cause mortality HR 1.30 (95% CI 1.17–1.44) |
| Liu, J. [23] | 2021/USA | Gut microbiota–derived metabolites and risk of coronary artery disease: a prospective study among US men and women | Am J Clin Nutr | Nested case—control | 1216 (608 CAD), not indicated | Participants were free of diabetes, cardiovascular disease, and cancer | LC-MS/MS; TMAO | Plasma (fasting and non-fasting); anticoagulant: EDTA | CAD | Conditional logistic regression | Age, sex, month of sample collection, fasting status at time of collection, smoking status, alcohol intake, physical activity, BMI, menopause status, family history of MI, aspirin use, T2D, hypertension, dyslipidemia, foods consumption | 3rd vs. 1st tertile OR 1.23 (95% CI 0.89–1.70) |
| Lee, Y. [24] | 2021/USA | Longitudinal plasma measures of trimethylamine N-Oxide and risk of atherosclerotic cardiovascular disease events in community-based older adults | J Am Heart Assoc | Cohort | 4131 (1766 ASCVD), 15.0 years | Participants free of CVD at baseline | LC-MS/MS; TMAO | Plasma (fasting); anticoagulant: EDTA | ASCVD | Cox regression | Age, sex, race, study site, education, income, health status, smoking status, alcohol intake, physical activity, BMI, WC, lipid-lowering medication, antihypertensive medication, antibiotics, T2D, HDL-C, LDL-C, TG, CRP, SBP, DBP, diet, eGFR | 5th vs. 1st quintile HR 0.98 (95% CI 0.80–1.20) |
| Frazier, R. [32] | 2021/USA | Deoxycholic acid and risks of cardiovascular events, ESKD, and mortality in CKD: The CRIC study | Kidney Med | Cohort | 3147 (512 CVD), 6.7 years; (575 HF), 7.0 years; (411 all-cause mortality), 7.9 years | Patients with chronic kidney disease but free of CVD at baseline | LC-MS/MS; deoxycholic acid | Serum (fasting) | CVD, HF, all-cause mortality | Cox regression | Age, sex, race, study site, eGFR, urinary protein, T2D, SBP, antihypertensive medications, smoking status, history of CVD, TChol, statin use, Il-6, CRP, fibroblast growth factor 23, parathyroid hormone, phosphate, calcium, albumin | >median vs. <median CVD HR 1.52 (0.74–3.12), HF 1.22 (95% CI 0.63–2.38), mortality HR 2.13 (95% CI 1.25–3.64) |
| Winther, S.A. [18] | 2021/Denmark | Plasma trimethylamine N-oxide and its metabolic precursors and risk of mortality, cardiovascular and renal disease in individuals with type 2-diabetes and albuminuria | PLoS One | Cohort | 311 (106 deaths 116 CVD, 44 deaths due to CVD), 21.9 years (6.8 years for death, 6.5 years for CVD) | Patients with T2D but free of CVD at baseline | LC-MS/MS; TMAO | Plasma (non-fasting); anticoagulant not indicated | CVD (cardiovascular mortality, non-fatal MI, ischaemic CVD, non-fatal stroke, amputation due to ischemia and cardiac or peripheral revascularization), CVD mortality and all-cause mortality | Cox regression | Age, sex, HbA1c, SBP, BMI, TChol, smoking, urinary albumin excretion rate and eGFR at baseline. | Per SD all-cause mortality HR 1.02 (95% CI 0.83–1.26), CV mortality 0.98 (95% CI 0.70–1.37), CVD 1.11 (95% CI 0.93–1.33) |
| Ringel, C. [19] | 2021/Germany | Association of plasma trimethylamine N-oxide levels with atherosclerotic cardiovascular disease and factors of the metabolic syndrome | Atherosclerosis | 3 Cohorts | 1666 (99 deaths in LIFE-CAD), 9 years; (26 deaths in LIFE-AMI), 9 years; (194 deaths in CULPRIT-SHOCK), 30 days | Patients with ≥50% stenosis in at least one major coronary artery, and patients with angiographically normal coronary arteries (“LIFE-CAD”); patients with AMI (“LIFE-AMI”); patients with AMI with CS (“CULPRIT-SHOCK”) | LC-MS/MS; TMAO | Plasma (fasting status not indicated); anticoagulant: EDTA | All-cause mortality during 30 days and long term | Cox regression (LIFE-CAD, LIFE-AMI); logistic regression (CULPRIT-SHOCK) | Age, sex, BMI, T2D, eGFR, smoking status, SBP, DBP, high-sensitivity CRP, HDL-C, LDL-C, white blood cell count) and in LIFE-CAD additionally for presence of CAD. | Per SD (LIFE-CAD) HR 1.24 (95% CI 1.01–1.51), (LIFE-AMI) HR 1.07 (95% CI 0.70–1.63), (CULPRIT-SHOCK) OR 1.14 (95% CI 0.86–1.51) |
| Israr, M.Z. [20] | 2021/UK | Association of gut-related metabolites with outcome in acute heart failure | Am Heart J | Cohort | 806 (62 deaths), 30 days; (213 deaths), 1 year; (98 deaths/HF), 30 days; (313 deaths/HF), 1 year | Patients with acute HF at baseline | UHPLC-MS/MS; TMAO | Plasma (fasting status not indicated); anticoagulant: EDTA | All-cause mortality (death) and a composite of death and/or rehospitalization caused by HF (death/HF) at 30 days and 1 year | Cox regression | Sex, age, previous medical history (HF, ischemic heart disease, hypertension, and diabetes mellitus), NYHA class, smoking status, edema, AF, SBP, DBP, heart rate, hemoglobin, respiratory rate, blood sodium, and (log) N-terminal proBNP. | TMAO per SD death HR 1.39 (95% CI 1.05–1.84), 1 year HR 1.26 (95% CI 1.08–1.47); death/HF HR 1.38 (95% CI 1.10–1.73), 1 year HR 1.25 (95% CI 1.09–1.42) |
| Papandreou, C. [21] | 2021/Spain | Choline metabolism and risk of atrial fibrillation and heart failure in the PREDIMED study | Clin Chem | 2 Nested case-control | 1127 (509 AF); 752 (326 HF), 10 years | Almost half of participants had T2D and were free of CVD at baseline | LC-MS/MS; TMAO | Plasma (fasting); anticoagulant: EDTA | AF and HF | Conditional logistic regression | Smoking, family history of premature CHD, physical activity, alcohol intake, BMI, intervention group, dyslipidemia, hypertension, T2D, medication use | TMAO per SD AF OR 1.04 (95% CI 0.92–1.17); HF OR 0.91 (95% CI 0.77–1.08) TMAO 4th vs. 1st quartile AF OR 1.02 (95% CI 0.72–1.44); HF OR 0.72 (95% CI 0.45–1.15) |
| Balasubramanian, R. [40] | 2020/USA | Metabolomic profiles associated with all-cause mortality in the Women’s Health Initiative | Int J Epidemiol | 2 Cohorts (discovery and replication sets) | 943 (417 all-cause mortality (discovery)); 1355 (685 all-cause mortality (replication)), 10 years | 10.9% and 14.8% of participants had T2D in the discovery and replication sets, respectively, and were free of CVD at baseline | LC-MS/MS; 470 metabolites (tryptophan) | Plasma (fasting); anticoagulant: EDTA | All-cause mortality | Cox regression | Age, WHI arm and CHD, BMI, SBP, hypertension treatment, T2D, smoking status, TChol, HDL-C | Tryptophan per SD WHI-HT HR 0.87 (95% CI 0.81–0.94); WHI-OS HR 0.82 (95% CI 0.75–0.89) |
| Croyal, M. [22] | 2020/France | Plasma trimethylamine N-Oxide and risk of cardiovascular events in patients with type 2 diabetes | J Clin Endocrinol Metab | Cohort | 1463 (403 MACEs and 538 deaths), 7.1 years | Patients with T2D at baseline | LC-MS/MS; TMAO | Plasma (non-fasting); anticoagulant not indicated | MACE (CV death, non-fatal MI and non-fatal stroke and all-cause mortality) and all-cause mortality | Cox regression | For MACE: sex, age, personal history of MI, eGFR, uACR, NT-proBNP; for all-cause mortality: sex, age, sinus rhythm, SBP, TNF receptor 1, angiopoietin-like 2 | MACE 4th vs. 1st quartile HR 1.29 (95% CI 1.02–1.64), all-cause mortality 4th vs. 1st quartile HR 1.16 (95% CI 0.95–1.42) |
| Alonso, A. [29] | 2019/USA | Serum metabolomics and incidence of atrial fibrillation (from the Atherosclerosis Risk in Communities Study) | Am J Cardiol | Cohort | 3922 (608 AF), 20.4 years | 14% of participants had T2D and were free of CVD at baseline | GC-MS/MS, LC-MS/MS; glycocholenate sulfate, glycolithocholate sulfate | Serum (fasting) | AF | Cox regression | Age, sex and race, smoking, BMI, SBP, use of antihypertensive medication, T2D, prevalent heart failure, and prevalent CHD, eGFR | Glycocholenate sulfate per SD HR 1.12 (95% CI 1.04–1.21) Glycolithocholate sulfate per SD HR 1.07 (95% CI 0.99–1.15) |
| Roncal, C. [38] | 2019/Spain | Trimethylamine-N-oxide (TMAO) predicts cardiovascular mortality in peripheral artery disease | Sci Rep | Cohort | 262 (135 MACE, 101 all-cause mortality and 39 cardiovascular mortality, 4 years | Patients with PAD at baseline | UHPLC-MS/MS; TMAO | Plasma (fasting); anticoagulant: citrate | All-cause mortality, CVD mortality, MACE (amputation, stroke, myocardial infarction and all-cause mortality) | Cox regression | Sex, age, hs-CRP, smoking, T2D, hypertension, dyslipidemia, HDL-C, eGFR (<60 mL/min/1.73 m2) | All-cause mortality per log unit HR 1.09 (95% CI 0.94–1.26), CV mortality TMAO per log unit HR 1.52 (95% CI 1.27–1.82), TMAO >2.26 μmol/L HR 3.36 (95% CI 1.68–6.70), MACE per log unit HR 1.08 (95% CI 0.95–1.23) |
| Fan, P.C. [31] | 2019/Taiwan | Serum indoxyl sulfate predicts adverse cardiovascular events in patients with chronic kidney disease | J Formos Med Assoc | Cohort | 147 (47 MACE), 3 years | Patients with chronic kidney disease but free of CVD at baseline | LC-MS/MS; indoxyl sulfate | Serum (fasting) | MACE (all-cause mortality, admission for HF and attack of acute coronary syndrome) | Cox regression | Age, sex, T2D, hypertension, SBP, left ventricular ejection fraction, hemoglobin, creatinine, albumin, hsCRP, LDL, TChol, medications | Per log unit HR 1.45 (95% CI 1.02–2.06) |
| Tobias, D.K. [39] | 2018/USA | Circulating branched-chain amino acids and incident cardiovascular disease in a prospective cohort of US women | Circ Genom Precis Med | Cohort | 27041 (2207 CVD), 18.6 years | Participants free of CVD at baseline | NMR; BCAAs | Plasma (fasting in 72.9%); anticoagulant: EDTA | CVD (MI, stroke, and coronary revascularization) | Cox regression | Age, randomized treatment assignments, fasting status at blood draw, menopausal status, current hormone therapy use, family history of MI, Caucasian race/ethnicity, smoking status, current 15+ c/d), AHEI diet quality score, alcohol intake, total physical activity MET-hrs/wk, cholesterol, hypertension, BMI | BCAAs per SD total CVD HR 1.13 (95% CI 1.08–1.18), MI HR 1.16 (95% CI 1.06–1.26); revascularization HR 1.17 (95% CI 1.11–1.25), stroke HR 1.07 (95% CI 0.99–1.15) Isoleucine per SD total CVD HR 1.14 (95% CI 1.09–1.19), MI HR 1.19 (95% CI 1.09–1.31); revascularization HR 1.24 (95% CI 1.16–1.32), stroke HR 1.05 (95% CI 0.97–1.13) Leucine per SD total CVD HR 1.06 (95% CI 1.02 -1.11), MI HR 1.05 (95% CI 0.97–1.15); revascularization HR 1.08 (95% CI 1.02–1.14), stroke HR 1.04 (95% CI 0.97–1.12) Valine per SD total CVD HR 1.13 (95% CI 1.08–1.18), MI HR 1.16 (95% CI 1.06–1.27 ); revascularization HR 1.16 (95% CI 1.09–1.24), stroke HR 1.06 (95% CI 0.99–1.15) |
| Huang, J. [30] | 2018/USA | Serum metabolomic profiling of all-cause mortality: A prospective analysis in the alpha-tocopherol, beta-carotene cancer prevention (ATBC) study cohort | Am J Epidemiol | Cohort | 620 (435 deaths (197 CVD and 107 cancer), 28 years | Participants free of CVD at baseline | UHPLC-MS/MS, GC-MS/MS; 406 metabolites (glycocholate, glycocholenate_sulfate, glycodeoxycholate, glycohyocholate, glycolithocholate_sulfate, glycoursodeoxycholate, taurocholenate_sulfate, taurodeoxycholate, taurolithocholate_3_sulfate, tauroursodeoxycholate ) | Serum (fasting) | All-cause mortality | Cox regression | Age, BMI, number of cigarettes per day, TChol, HDL-C, hypertension, T2D, serum creatinine | No significant associations. HR with 95% CI were not indicated |
| Du, X. [41] | 2018/China | Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure | Life Sci | Cohort | 138 (32 deaths and 21 hospitalizations for heart failure), 3 years | Patients with STEMI and AHF at baseline | LC-MS/MS; 26 amino acids (BCAAs) | Plasma (non-fasting); anticoagulant not indicated | Adverse cardiac events (composite of death and HF hospitalization) | Cox regression | Age, sex, history of T2D, history of hypertension, current smoking, and Killip class | Per SD HR 2.67 (95% CI: 2.41–4.41) |
| Yu, B. [36] | 2016/USA | Associations between the serum metabolome and all-cause mortality among African Americans in the atherosclerosis risk in communities (ARIC) study | Am J Epidemiol | Cohort | 1887 (671 deaths), 22.5 years | 10.9% of participants had T2D and 6.3% had CVD at baseline | GC-MS/MS, LC-MS/MS; 600 metabolites (glycocholate) | Serum (fasting) | All-cause mortality, CVD mortality | Cox regression | Age, sex, BMI, SBP, antihypertensive medication use, diabetes status, current smoking status, prevalent cardiovascular disease status, HDL-C, TChol, and eGFR | Glycocholate per SD all-cause mortality HR 1.12 (95% CI 1.07–1.16), CV mortality HR 1.14 (95% CI 1.07–1.22) |
| Wang, C. [42] | 2016/Taiwan | Increased p-cresyl sulfate level is independently associated with poor outcomes in patients with heart failure | Heart Vessels | Cohort | 187 (35 composite event of death or HF-related re-hospitalization), 2.3 years | 136 patients with HF and 51 participants without HF at baseline | LC-MS/MS; indoxyl sulfate | Plasma (non-fasting); anticoagulant: EDTA | Composite event of death or HF-related re-hospitalization | Cox regression | Age, LVEF, T2D, eGFR, and BNP | Indoxyl sulfate per μM HR 1.01 (95% CI 0.98–1.04) |
| Cheng, S. [28] | 2015/USA | Distinct metabolomic signatures are associated with longevity in humans | Nat Commun | 2 Cohorts (discovery and replication sets) | 2327 (358 CVD, 439 all-cause mortality (discovery)), 13.6 years; 325 (36 all-cause mortality (replication)), 15.8 years | 10% of participants had T2D and were free of CVD at baseline | LC-MS/MS; 217 metabolites (glycocholate, glycodeoxycholates, deoxycholates) | Plasma (fasting); anticoagulant: EDTA | CVD (coronary heart disease, HF, or stroke) and all-cause mortality | Cox regression | For CVD and all-cause mortality: age, sex, BMI, SBP, anti-hypertensive treatment, diabetes, smoking status, and total/HDL cholesterol | No significant associations. HR with 95% CI were not indicated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Gimenez, R.; Ahmed-Khodja, W.; Molina, Y.; Peiró, O.M.; Bonet, G.; Carrasquer, A.; Fragkiadakis, G.A.; Bulló, M.; Bardaji, A.; Papandreou, C. Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk: A Systematic Review of Prospective Cohort Studies. Nutrients 2022, 14, 2654. https://doi.org/10.3390/nu14132654
Sanchez-Gimenez R, Ahmed-Khodja W, Molina Y, Peiró OM, Bonet G, Carrasquer A, Fragkiadakis GA, Bulló M, Bardaji A, Papandreou C. Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk: A Systematic Review of Prospective Cohort Studies. Nutrients. 2022; 14(13):2654. https://doi.org/10.3390/nu14132654
Chicago/Turabian StyleSanchez-Gimenez, Raul, Wahiba Ahmed-Khodja, Yesica Molina, Oscar M. Peiró, Gil Bonet, Anna Carrasquer, George A. Fragkiadakis, Mònica Bulló, Alfredo Bardaji, and Christopher Papandreou. 2022. "Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk: A Systematic Review of Prospective Cohort Studies" Nutrients 14, no. 13: 2654. https://doi.org/10.3390/nu14132654
APA StyleSanchez-Gimenez, R., Ahmed-Khodja, W., Molina, Y., Peiró, O. M., Bonet, G., Carrasquer, A., Fragkiadakis, G. A., Bulló, M., Bardaji, A., & Papandreou, C. (2022). Gut Microbiota-Derived Metabolites and Cardiovascular Disease Risk: A Systematic Review of Prospective Cohort Studies. Nutrients, 14(13), 2654. https://doi.org/10.3390/nu14132654








