Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases
Abstract
:1. Introduction
2. Materials and Methods
- a.
- Discussing bacteria or bacterial metabolites and at least one of the diseases of choice;
- b.
- Including findings from human studies with the support of preclinical data;
- c.
- Published in a peer-reviewed journal;
- d.
- Available in full-text;
- e.
- Written in English.
- f.
- Published within the time frame of January 2017–January 2022. Papers published before January 2017 were included if they were referred to in another paper.
3. Evidence of Linking the Microbiome–Gut–Brain Axis to Brain Disorders
3.1. Microbiome
3.2. Microbiota–Gut–Brain Axis (MGBA)
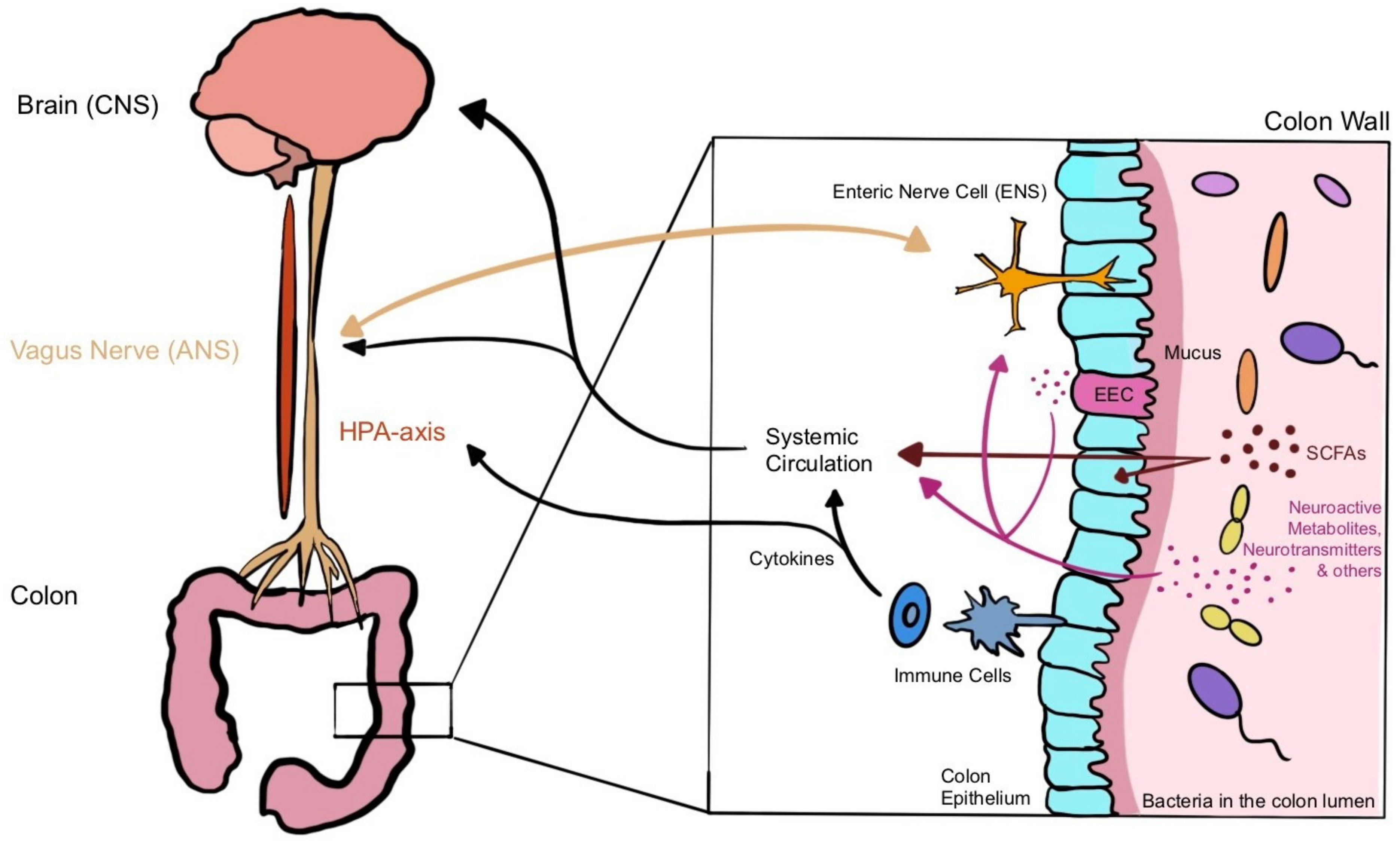
3.2.1. Chemical Signalling
Short-Chain Fatty Acids (SCFAs)
Amino Acids and Neurotransmitters
3.2.2. Immune System Signalling
3.2.3. Neural Signalling
4. Changes in Gut Microbiota and Metabolites in Brain-Related Pathologies
4.1. Attention Deficit Disorder with Hyperactivity
4.2. Autistic Spectrum Disorder
4.3. Schizophrenia
4.4. Alzheimer’s Disease
4.5. Parkinson Disease
4.6. Depression
4.7. Bipolar Disorder
5. Discussion
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | serotonin |
| AD | Alzheimer’s disease |
| ADHD | attention deficit hyperactivity disorder |
| ADT | arogenate dehydratase |
| ANS | autonomic nervous system |
| APOE | apolipoprotein E |
| APP | amyloid precursor protein |
| ASD | autism spectrum disorder |
| Aβ | amyloid-beta |
| BBB | blood–brain barrier |
| BD | bipolar disorder |
| BDNF | brain-derived neurotrophic factor |
| CNS | central nervous system |
| CREB | cAMP response element-binding protein |
| CRP | C-reactive protein |
| DA | dopamine |
| EEC | enteroendocrine cell |
| ENS | enteric nervous system |
| FMT | faecal microbiota transplant |
| GABA | gamma-Aminobutyric acid |
| GF | germ-free |
| GFAP | glial fibrillary acidic protein |
| GI | gastrointestinal |
| GLP1 | glucagon-like peptide-1 |
| GOGAT | oxoglutarate aminotransferase |
| GPCR | G-protein-coupled receptor |
| HDAC | histone deacetylase |
| HPA-axis | hypothalamic-pituitary-adrenal axis |
| HPHPA | 3-(3-hydroxyphenyl)-3-hydroxypropionic acid |
| IFN-γ | Interferon gamma |
| IL | interleukin |
| LBP | lipopolysaccharide binding protein |
| LDA | linear discriminant analysis |
| LPS | lipopolysaccharide |
| MDD | major depressive disorder |
| MGBA | Microbiota–Gut–Brain Axis |
| NE | noradrenaline |
| NFTs | neurofibrillary tangles |
| PAMP | pathogen-associated molecular pattern |
| p-cresol | para-cresol |
| PD | Parkinson’s Disease |
| PET | positron emission tomography |
| PYY | peptide YY |
| REM-sleep | rapid eye movement sleep |
| sAD | sporadic Alzheimer’s disease |
| sCD14 | soluble cluster of differentiation 14 |
| SCFA | short-chain fatty acid |
| SCZ | schizophrenia |
| TGF-β | transforming growth factor beta |
| TLR | Toll-like receptor |
| TNF-α | tumour necrosis factor alpha |
References
- Banaschewski, T.; Becker, K.; Döpfner, M.; Holtmann, M.; Rösler, M.; Romanos, M. Attention-Deficit/Hyperactivity Disorder. Dtsch. Arztebl. Int. 2017, 114, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polanczyk, G. The Worldwide Prevalence of ADHD: A Systematic Review and Metaregression Analysis. Am. J. Psychiatry 2007, 164, 942. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.K.; Rose, D.; Ashwood, P. The Gut Microbiota and Dysbiosis in Autism Spectrum Disorders. Curr. Neurol. Neurosci. Rep. 2018, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Firth, J.; Vieta, E. Bipolar Disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef]
- Grøntvedt, G.R.; Schroder, T.N.; Sando, S.B.; White, L.; Brathen, G.; Doeller, C.F. Alzheimer’s disease. Curr. Biol. 2018, 28, R645–R649. [Google Scholar] [CrossRef] [Green Version]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Raza, C.; Anjum, R.; Shakeel, N.U.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019, 226, 77–90. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2019, 36, 1–12. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; López-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Microbiota-Brain-Gut Axis and Neurodegenerative Diseases. Curr. Neurol. Neurosci. Rep. 2017, 17, 94. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Sidhu, M.; van der Poorten, D. The gut microbiome. Aust. Fam. Physician 2017, 46, 206–211. [Google Scholar]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef]
- Capuco, A.; Urits, I.; Hasoon, J.; Chun, R.; Gerald, B.; Wang, J.K.; Kassem, H.; Ngo, A.L.; Abd-Elsayed, A.; Simopoulos, T.; et al. Current Perspectives on Gut Microbiome Dysbiosis and Depression. Adv. Ther. 2020, 37, 1328–1346. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; De Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2017, 3, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hills, R.D., Jr.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Shin, C. The Microbiota-Gut-Brain Axis in Neuropsychiatric Disorders: Pathophysiological Mechanisms and Novel Treatments. Curr. Neuropharmacol. 2018, 16, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-gut brain axis involvement in neuropsychiatric disorders. Expert Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Bull-Larsen, S.; Mohajeri, M.H. The Potential Influence of the Bacterial Microbiome on the Development and Progression of ADHD. Nutrients 2019, 11, 2805. [Google Scholar] [CrossRef] [Green Version]
- Boonchooduang, N.; Louthrenoo, O.; Chattipakorn, N.; Chattipakorn, S.C. Possible links between gut–microbiota and attention-deficit/hyperactivity disorders in children and adolescents. Eur. J. Nutr. 2020, 59, 3391–3403. [Google Scholar] [CrossRef]
- Tran, S.M.-S.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Aarts, E.; Ederveen, T.; Naaijen, J.; Zwiers, M.P.; Boekhorst, J.; Timmerman, H.M.; Smeekens, S.P.; Netea, M.G.; Buitelaar, J.K.; Franke, B.; et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS ONE 2017, 12, e0183509. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 2046. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693 Pt B, 201–206. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, C.; Knudsen, J.; Leutscher, P.D.C.; Lauritsen, M.B.; Nyegaard, M.; Hagstrøm, S.; Sørensen, S. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic literature review. Gut Microbes 2020, 11, 1172–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posner, J.; Polanczyk, G.V.; Sonuga-Barke, E. Attention-deficit hyperactivity disorder. Lancet 2020, 395, 450–462. [Google Scholar] [CrossRef]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef]
- Dam, S.A.; Mostert, J.C.; Szopinska-Tokov, J.W.; Bloemendaal, M.; Amato, M.; Arias-Vasquez, A. The Role of the Gut-Brain Axis in Attention-Deficit/Hyperactivity Disorder. Gastroenterol. Clin. N. Am. 2019, 48, 407–431. [Google Scholar] [CrossRef]
- Drechsler, R.; Brem, S.; Brandeis, D.; Grünblatt, E.; Berger, G.; Walitza, S. ADHD: Current Concepts and Treatments in Children and Adolescents. Neuropediatrics 2020, 51, 315–335. [Google Scholar] [CrossRef]
- Prehn-Kristensen, A.; Zimmermann, A.; Tittmann, L.; Lieb, W.; Schreiber, S.; Baving, L.; Fischer, A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS ONE 2018, 13, e0200728. [Google Scholar] [CrossRef]
- Wang, L.-J.; Yang, C.-Y.; Chou, W.-J.; Lee, M.-J.; Chou, M.-C.; Kuo, H.-C.; Yeh, Y.-M.; Lee, S.-Y.; Huang, L.-H.; Li, S.-C. Gut microbiota and dietary patterns in children with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2020, 29, 287–297. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Zhou, Y.-Y.; Zhou, G.-L.; Li, Y.-C.; Yuan, J.; Li, X.-H.; Ruan, B. Gut microbiota profiles in treatment-naïve children with attention deficit hyperactivity disorder. Behav. Brain Res. 2018, 347, 408–413. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, diagnosis and therapy. J. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Settanni, C.R.; Bibbò, S.; Ianiro, G.; Rinninella, E.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A. Gastrointestinal involvement of autism spectrum disorder: Focus on gut microbiota. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 599–622. [Google Scholar] [CrossRef]
- Peralta-Marzal, L.N.; Prince, N.; Bajic, D.; Roussin, L.; Naudon, L.; Rabot, S.; Garssen, J.; Kraneveld, A.D.; Perez-Pardo, P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci. 2021, 22, 10052. [Google Scholar] [CrossRef]
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the Gut Microbiome in Autism Spectrum Disorders. Adv. Exp. Med. Biol. 2019, 1118, 253–269. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; Riba, G.V.G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [Green Version]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.-F.; Shen, Y.-Q. Dysbiosis of gut microbiota and microbial metabolites in Parkinson’s Disease. Ageing Res. Rev. 2018, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Kushak, R.I.; Winter, H.S.; Buie, T.M.; Cox, S.B.; Phillips, C.; Ward, N.L. Analysis of the Duodenal Microbiome in Autistic Individuals: Association with Carbohydrate Digestion. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e110–e116. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Finegold, S.M. Real-Time PCR Quantitation of Clostridia in Feces of Autistic Children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finegold, S.M.; Summanen, P.H.; Downes, J.; Corbett, K.; Komoriya, T. Detection of Clostridium perfringens toxin genes in the gut microbiota of autistic children. Anaerobe 2017, 45, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Pulikkan, J.; Maji, A.; Dhakan, D.B.; Saxena, R.; Mohan, B.; Anto, M.M.; Agarwal, N.; Grace, T.; Sharma, V.K. Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders. Microb. Ecol. 2018, 76, 1102–1114. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Kang, D.-W.; Park, J.G.; Ilhan, Z.E.; Wallstrom, G.; LaBaer, J.; Adams, J.B.; Krajmalnik-Brown, R. Reduced Incidence of Prevotella and Other Fermenters in Intestinal Microflora of Autistic Children. PLoS ONE 2013, 8, e68322. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Marder, S.R.; Cannon, T.D. Schizophrenia. N. Engl. J. Med. 2019, 381, 1753–1761. [Google Scholar] [CrossRef]
- Rodrigues-Amorim, D.; Rivera-Baltanás, T.; Regueiro, B.; Spuch, C.; Heras, M.E.D.L.; Méndez, R.V.-N.; Nieto-Araujo, M.; Barreiro-Villar, C.; Olivares, J.M.; Agís-Balboa, R.C. The role of the gut microbiota in schizophrenia: Current and future perspectives. World J. Biol. Psychiatry 2018, 19, 571–585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Kang, Y.; Zhuo, C.; Huang, X.-F.; Song, X. The gut microbiota promotes the pathogenesis of schizophrenia via multiple pathways. Biochem. Biophys. Res. Commun. 2019, 512, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. The role of the gut microbiome in the development of schizophrenia. Schizophr. Res. 2021, 234, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Kosciolek, T.; Maldonado, Y.; Daly, R.E.; Martin, A.S.; McDonald, D.; Knight, R.; Jeste, D.V. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr. Res. 2019, 204, 23–29. [Google Scholar] [CrossRef]
- Xu, R.; Wu, B.; Liang, J.; He, F.; Gu, W.; Li, K.; Luo, Y.; Chen, J.; Gao, Y.; Wu, Z.; et al. Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain Behav. Immun. 2020, 85, 120–127. [Google Scholar] [CrossRef]
- Zhu, F.; Ju, Y.; Wang, W.; Wang, Q.; Guo, R.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 2020, 11, 1612. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Mirzaei, R.; Bouzari, B.; Hosseini-Fard, S.R.; Mazaheri, M.; Ahmadyousefi, Y.; Abdi, M.; Jalalifar, S.; Karimitabar, Z.; Teimoori, A.; Keyvani, H.; et al. Role of microbiota-derived short-chain fatty acids in nervous system disorders. Biomed. Pharmacother. 2021, 139, 111661. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Bin Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef]
- Sochocka, M.; Donskow-Łysoniewska, K.; Diniz, B.S.; Kurpas, D.; Brzozowska, E.; Leszek, J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019, 56, 1841–1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostanciklioğlu, M. The role of gut microbiota in pathogenesis of Alzheimer’s disease. J. Appl. Microbiol. 2019, 127, 954–967. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M. The Role of the Microbiota-Gut-Brain Axis in the Health and Illness Condition: A Focus on Alzheimer’s Disease. J. Alzheimers Dis. 2021, 81, 1345–1360. [Google Scholar] [CrossRef]
- Kesika, P.; Suganthy, N.; Sivamaruthi, B.S.; Chaiyasut, C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021, 264, 118627. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Megur, A.; Baltriukienė, D.; Bukelskienė, V.; Burokas, A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef]
- Benakis, C.; Martin-Gallausiaux, C.; Trezzi, J.-P.; Melton, P.; Liesz, A.; Wilmes, P. The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 2020, 61, 1–9. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Gerhardt, S.; Mohajeri, M.H. Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Chaudhuri, K.R.; Reynolds, R.; Tan, E.-K.; Pettersson, S. The role of gut dysbiosis in Parkinson’s disease: Mechanistic insights and therapeutic options. Brain 2021, 144, 2571–2593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-Y.; Li, H.-X.; Xu, R.-C.; Miao, W.-T.; Dai, M.-Y.; Ding, S.-T.; Liu, H.-D. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 2021, 69, 101347. [Google Scholar] [CrossRef]
- Rani, L.; Mondal, A.C. Unravelling the role of gut microbiota in Parkinson’s disease progression: Pathogenic and therapeutic implications. Neurosci. Res. 2021, 168, 100–112. [Google Scholar] [CrossRef]
- Menozzi, E.; Macnaughtan, J.; Schapira, A.H.V. The gut-brain axis and Parkinson disease: Clinical and pathogenetic relevance. Ann. Med. 2021, 53, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Behl, T.; Bungau, S.; Kumar, A.; Uddin, M.S.; Mehta, V.; Zengin, G.; Mathew, B.; Shah, M.A.; Arora, S. Dysregulation of the Gut-Brain Axis, Dysbiosis and Influence of Numerous Factors on Gut Microbiota Associated Parkinson’s Disease. Curr. Neuropharmacol. 2021, 19, 233–247. [Google Scholar] [CrossRef]
- Gallop, A.; Weagley, J.; Paracha, S.-U.; Grossberg, G. The Role of The Gut Microbiome in Parkinson’s Disease. J. Geriatr. Psychiatry Neurol. 2021, 34, 253–262. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F.; Qin, B. Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P.; et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.-D.; Xiao, Q. Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Wang, P.; Chen, Z.; Sui, X.; Xie, X.; Zhang, J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci. Lett. 2019, 707, 134297. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Athira, K.V.; Bandopadhyay, S.; Samudrala, P.K.; Naidu, V.; Lahkar, M.; Chakravarty, S. An Overview of the Heterogeneity of Major Depressive Disorder: Current Knowledge and Future Prospective. Curr. Neuropharmacol. 2020, 18, 168–187. [Google Scholar] [CrossRef] [PubMed]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2017, 72, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.K.; Bundgaard-Nielsen, C.; Hjerrild, S.; Nielsen, R.E.; Leutscher, P.; Sørensen, S. Gut microbiota variations in patients diagnosed with major depressive disorder—A systematic review. Brain Behav. 2021, 11, e02177. [Google Scholar] [CrossRef]
- Łoniewski, I.; Misera, A.; Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Kaźmierczak-Siedlecka, K.; Misiak, B.; Marlicz, W.; Samochowiec, J. Major Depressive Disorder and gut microbiota—Association not causation. A scoping review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 106, 110111. [Google Scholar] [CrossRef]
- Bastiaanssen, T.F.S.; Cussotto, S.; Claesson, M.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harv. Rev. Psychiatry 2020, 28, 26–39. [Google Scholar] [CrossRef]
- Knuesel, T.; Mohajeri, M.H. The Role of the Gut Microbiota in the Development and Progression of Major Depressive and Bipolar Disorder. Nutrients 2021, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Han, B.; Ding, M.; Wen, Y.; Ma, M.; Zhang, L.; Qi, X.; Cheng, B.; Li, P.; Kafle, O.P.; et al. Identifying psychiatric disorder-associated gut microbiota using microbiota-related gene set enrichment analysis. Brief. Bioinform. 2020, 21, 1016–1022. [Google Scholar] [CrossRef]
- Zheng, P.; Yang, J.; Li, Y.; Wu, J.; Liang, W.; Yin, B.; Tan, X.; Huang, Y.; Chai, T.; Zhang, H.; et al. Gut Microbial Signatures Can Discriminate Unipolar from Bipolar Depression. Adv. Sci. 2020, 7, 1902862. [Google Scholar] [CrossRef]
- Rong, H.; Xie, X.-H.; Zhao, J.; Lai, W.-T.; Wang, M.-B.; Xu, D.; Liu, Y.-H.; Guo, Y.-Y.; Xu, S.-X.; Deng, W.-F.; et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef]
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257. [Google Scholar] [CrossRef]
- Liu, R.T.; Rowan-Nash, A.D.; Sheehan, A.E.; Walsh, R.F.; Sanzari, C.M.; Korry, B.J.; Belenky, P. Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain Behav. Immun. 2020, 88, 308–324. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.-J.; Fan, S.-H.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.E.; Chen, H.-C.; Chou, H.-C.L.; Chen, I.-M.; Lee, M.-S.; Chuang, L.-C.; Liu, Y.-W.; Lu, M.-L.; Chen, C.-H.; Wu, C.-S.; et al. Exploration of microbiota targets for major depressive disorder and mood related traits. J. Psychiatr. Res. 2019, 111, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Freund, N.; Juckel, G. Bipolar Disorder: Its Etiology and How to Model in Rodents. Methods Mol. Biol. 2019, 2011, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, L.; Pettorruso, M.; Vellante, F.; Di Carlo, F.; Ceci, F.; Santovito, M.; Di Muzio, I.; Fornaro, M.; Ventriglio, A.; Tomasetti, C.; et al. Gut Microbiota and Bipolar Disorder: An Overview on a Novel Biomarker for Diagnosis and Treatment. Int. J. Mol. Sci. 2021, 22, 3723. [Google Scholar] [CrossRef]
- Sublette, M.E.; Cheung, S.; Lieberman, E.; Hu, S.; Mann, J.J.; Uhlemann, A.; Miller, J.M. Bipolar disorder and the gut microbiome: A systematic review. Bipolar Disord. 2021, 23, 544–564. [Google Scholar] [CrossRef]
- Fries, G.R.; Walss-Bass, C.; Bauer, M.E.; Teixeira, A.L. Revisiting inflammation in bipolar disorder. Pharmacol. Biochem. Behav. 2019, 177, 12–19. [Google Scholar] [CrossRef]
- Flowers, S.A.; Ward, K.M.; Clark, C.T. The Gut Microbiome in Bipolar Disorder and Pharmacotherapy Management. Neuropsychobiology 2019, 79, 43–49. [Google Scholar] [CrossRef]
- Lu, Q.; Lai, J.; Lu, H.-F.; Ng, C.; Huang, T.; Zhang, H.; Ding, K.; Wang, Z.; Jiang, J.; Hu, J.; et al. Gut Microbiota in Bipolar Depression and Its Relationship to Brain Function: An Advanced Exploration. Front. Psychiatry 2019, 10, 784. [Google Scholar] [CrossRef]
- Hu, S.; Li, A.; Huang, T.; Lai, J.; Li, J.; Sublette, M.E.; Lu, H.; Lu, Q.; Du, Y.; Hu, Z.; et al. Gut Microbiota Changes in Patients with Bipolar Depression. Adv. Sci. 2019, 6, 1900752. [Google Scholar] [CrossRef]
- Coello, K.; Hansen, T.; Sørensen, N.; Munkholm, K.; Kessing, L.V.; Pedersen, O.; Vinberg, M. Gut microbiota composition in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Brain Behav. Immun. 2019, 75, 112–118. [Google Scholar] [CrossRef]
- Xiao, S.; Jiang, S.; Qian, D.; Duan, J. Modulation of microbially derived short-chain fatty acids on intestinal homeostasis, metabolism, and neuropsychiatric disorder. Appl. Microbiol. Biotechnol. 2020, 104, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Meng, L.; Shen, L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition 2022, 93, 111499. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.; Houser, M.C.; Pereira, P.A.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host–Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, C.-H.; Lane, H.-Y. d-glutamate and Gut Microbiota in Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 2676. [Google Scholar] [CrossRef] [Green Version]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [Green Version]
- Subhramanyam, C.S.; Wang, C.; Hu, Q.; Dheen, S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019, 94, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Boche, D.; Perry, V.H.; Nicoll, J.A.R. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Manosso, L.M.; Lin, J.; Carlessi, A.S.; Recco, K.C.; Quevedo, J.; Gonçalves, C.L.; Réus, G.Z. Sex-related patterns of the gut-microbiota-brain axis in the neuropsychiatric conditions. Brain Res. Bull. 2021, 171, 196–208. [Google Scholar] [CrossRef]
- Valeri, F.; Endres, K. How biological sex of the host shapes its gut microbiota. Front. Neuroendocr. 2021, 61, 100912. [Google Scholar] [CrossRef] [PubMed]
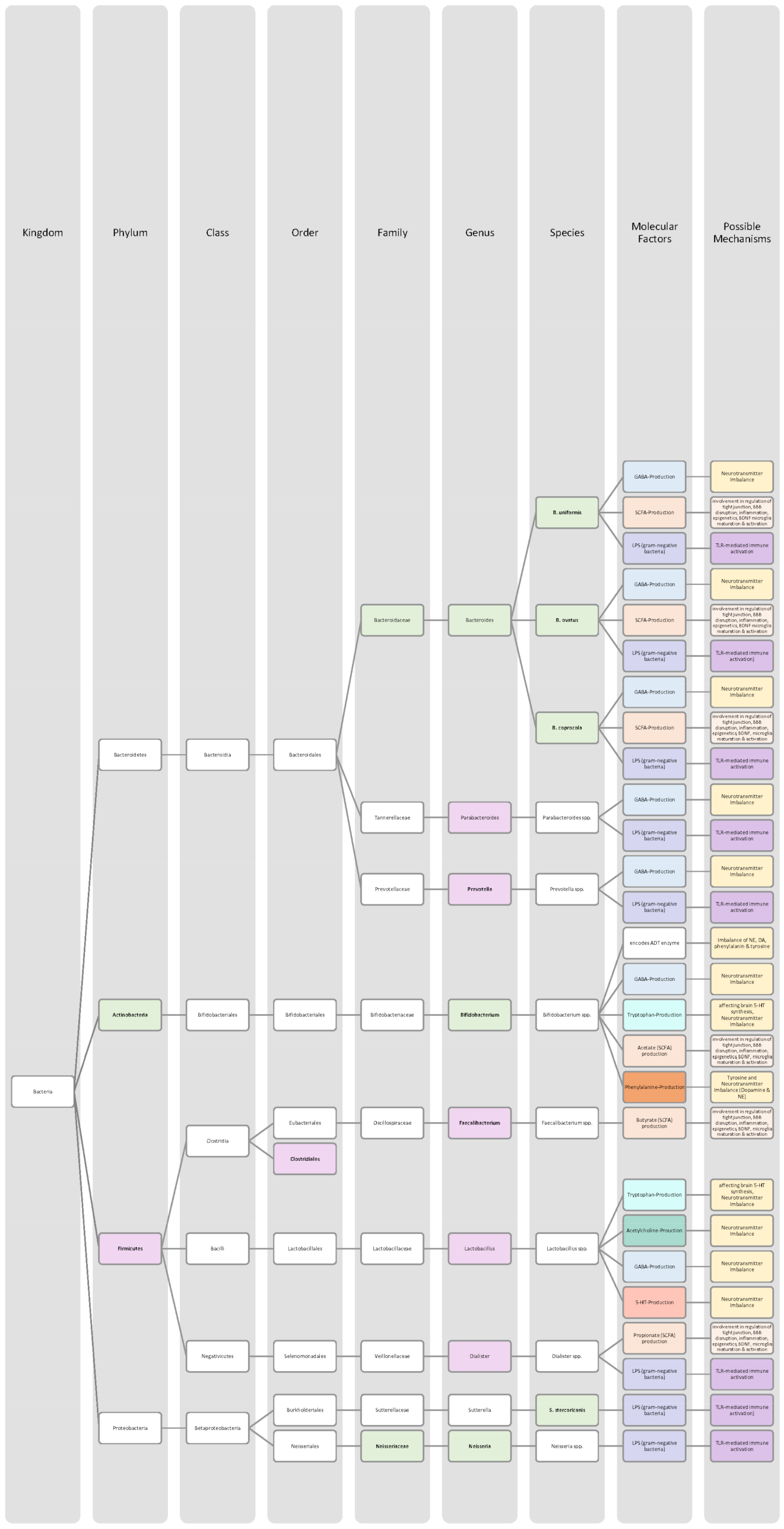



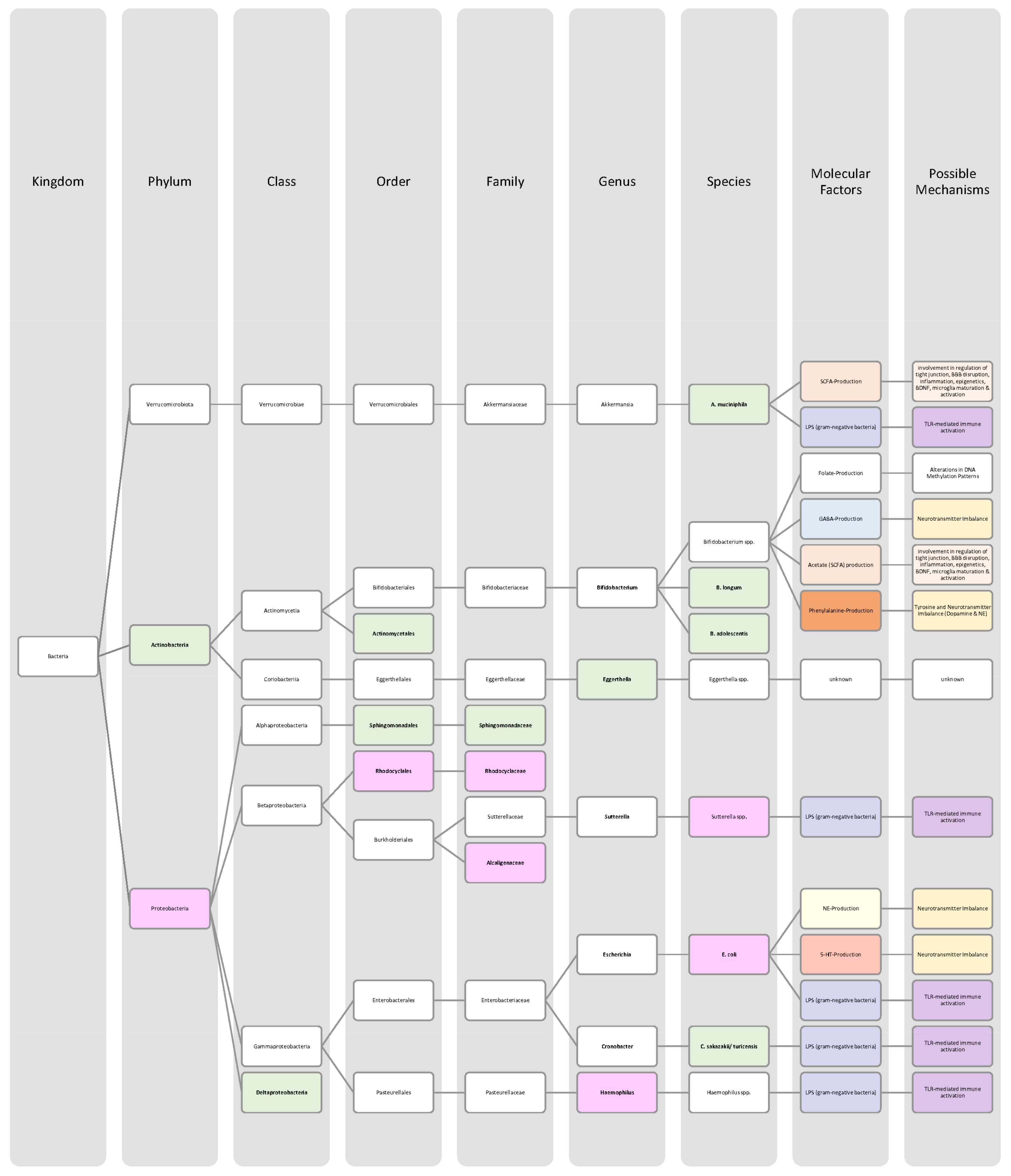
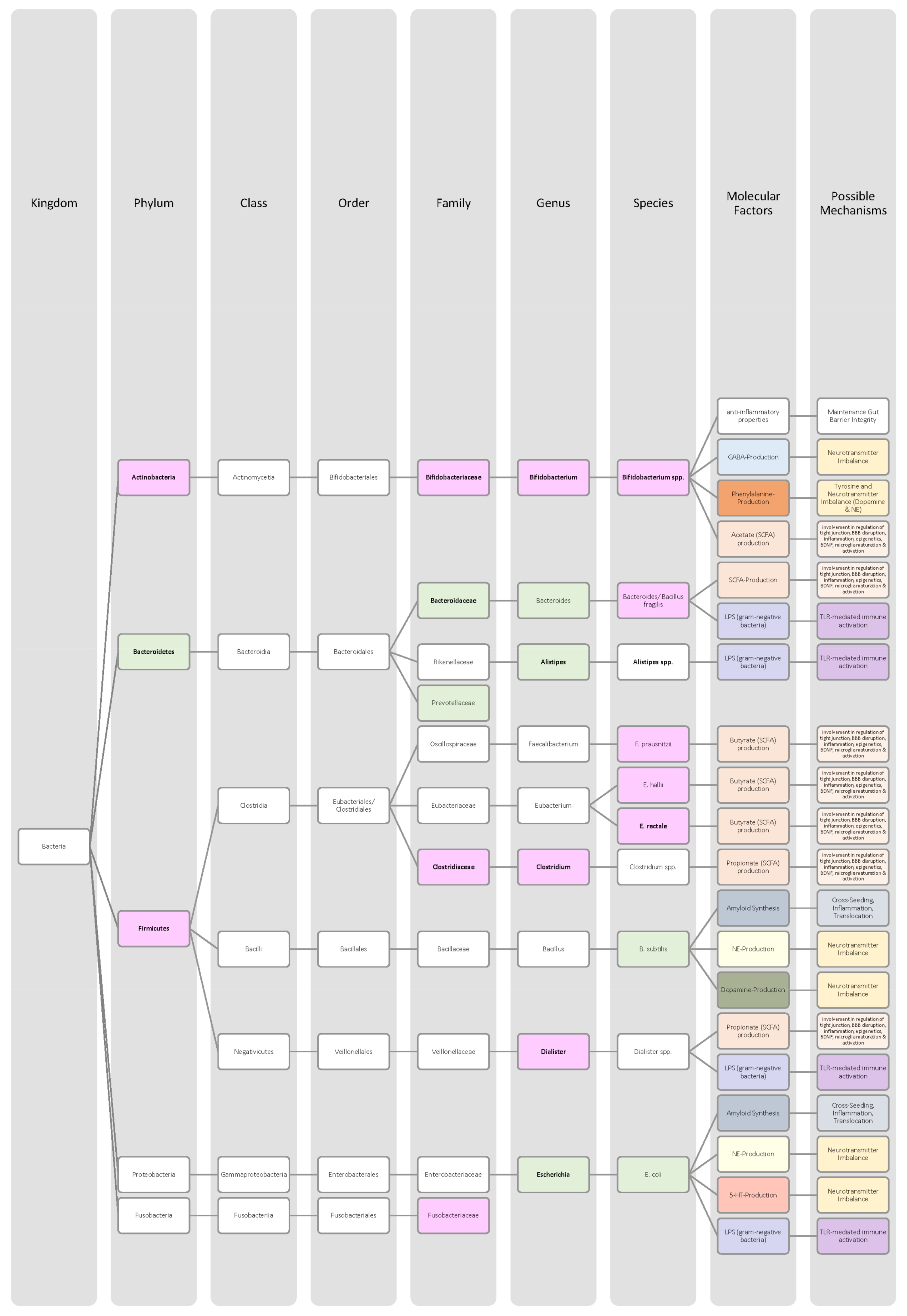
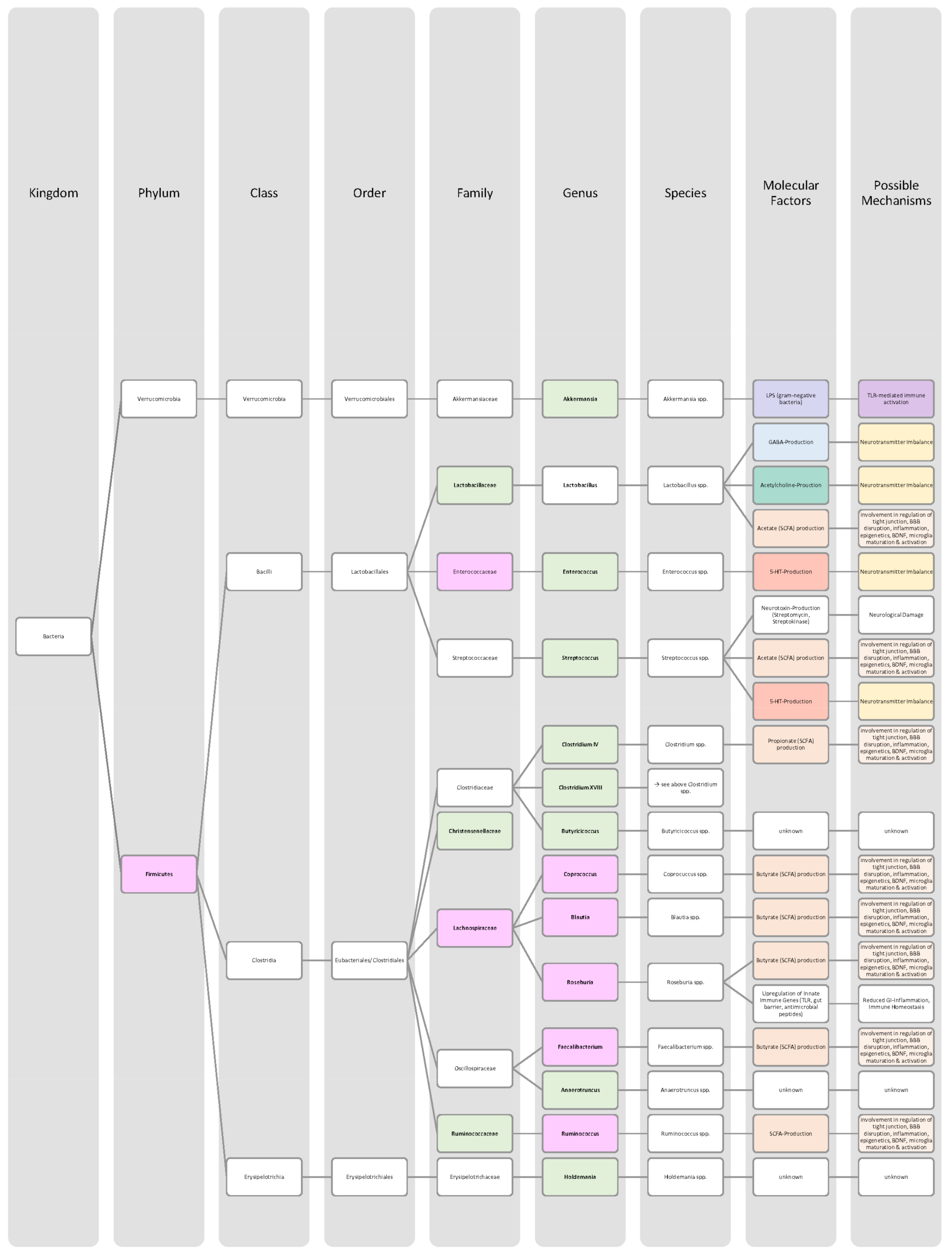
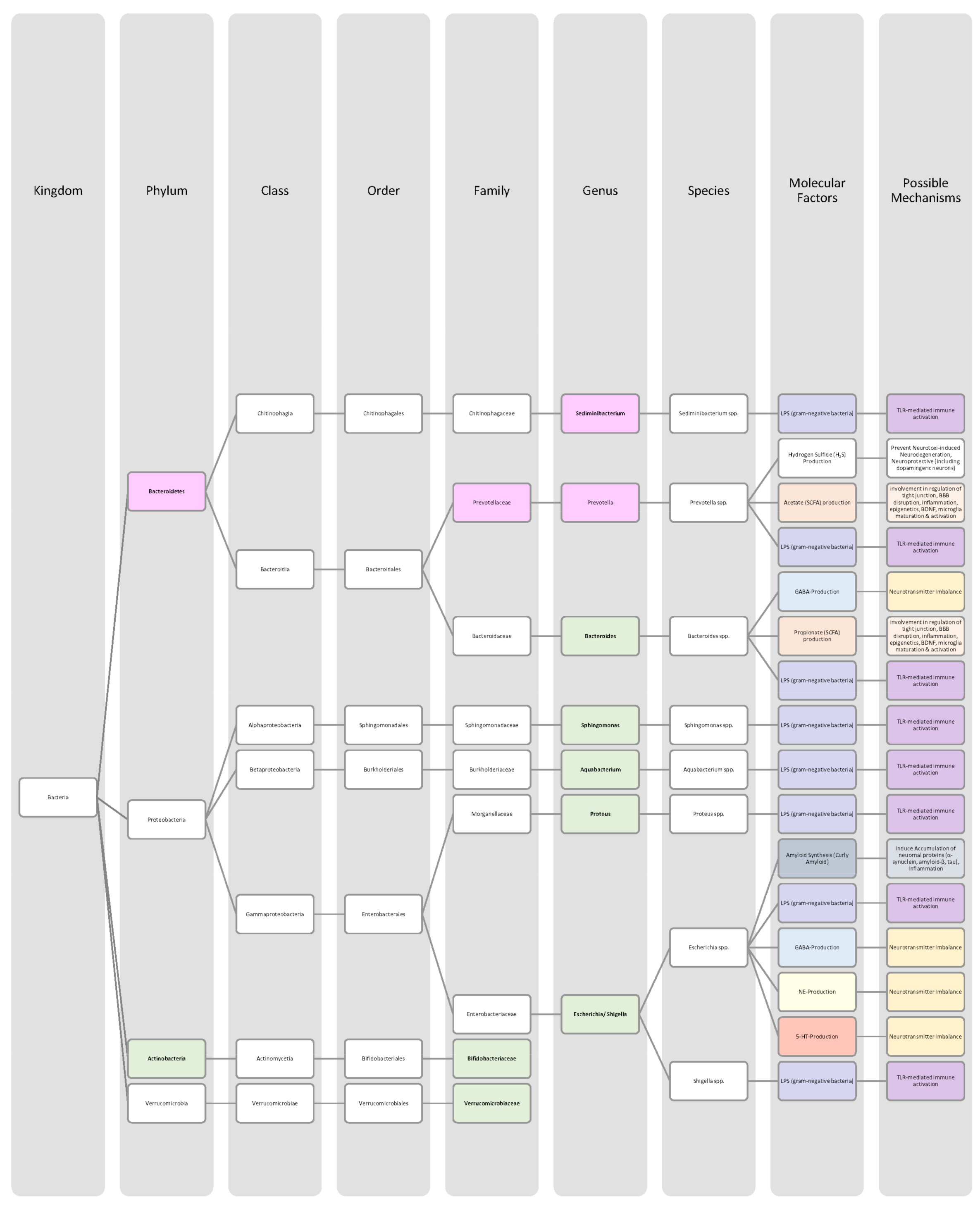

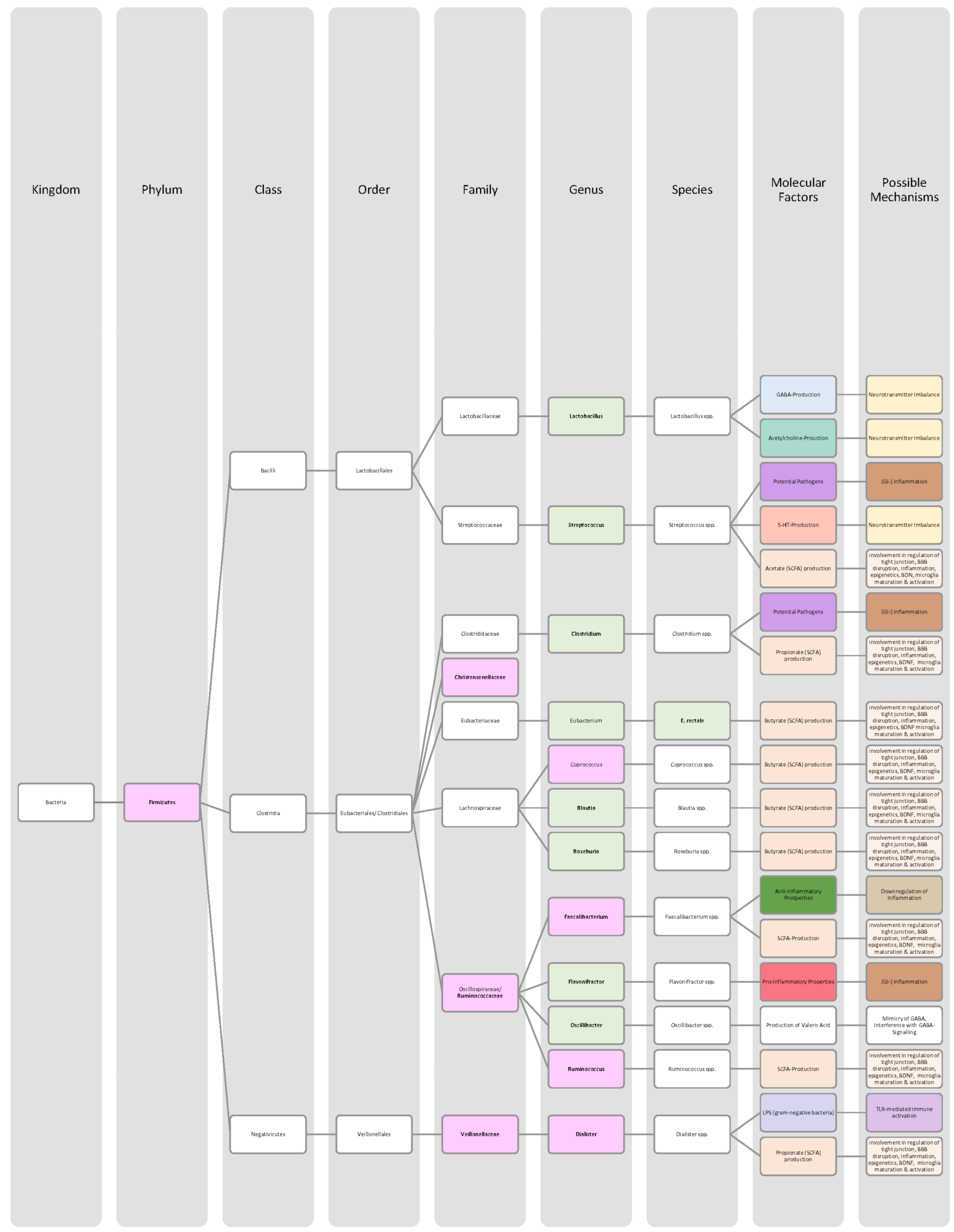
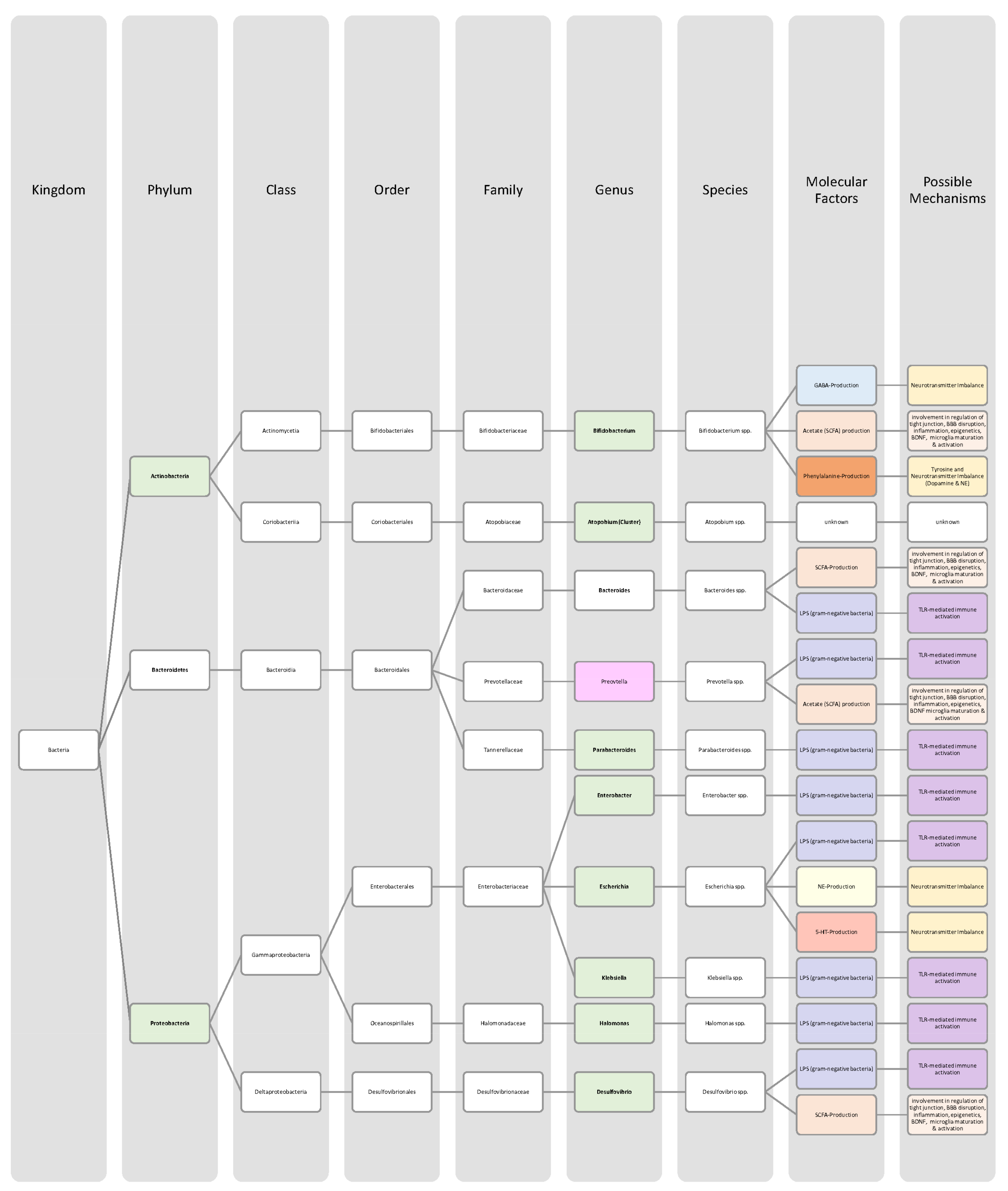

| Neurotransmitter | Bacteria |
|---|---|
| GABA | Lactobacillus spp. [18,20] Bifidobacterium spp. [18,20] |
| Acetylcholine | Lactobacillus spp. [18,20] |
| Noradrenaline | Bacillus spp. [18,20] Escherichia spp. [18,20] Saccharomyces spp. [18,20] |
| Serotonin | Streptococcus spp. [18,20] Candida spp. [18,20] Enterococcus spp. [18,20] Escherichia spp. [18,20] |
| Dopamine | Bacillus spp. [18,20] |
| Bacteria in ADHD Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | p = 0.002 | 96 (♀ 42; ♂ 54) ADHD: 19 Control: 77 | ADHD: 19.5 Control: 27,1 | [27],[30] | |
| Bacteroidaceae (family) | ↑ | - | 31 (♀ 0; ♂ 31) ADHD: 14 Control: 17 | ADHD: 11.9 Control: 13.1 | [28],[38] | |
| Bacteroides (genus) | ↑ | - | 31 (♀ 0; ♂ 31) ADHD: 14 Control: 17 | ADHD: 11.9 Control: 13.1 | [28],[38] | |
| Bacteroides coprocola (species) | ↓ | p = 0.028 | 60 (♀ 19; ♂ 41) ADHD: 30 Control: 30 | ADHD: 8.4 Control: 9.3 | [28],[39] | |
| Bacteroides ovatus (species) | ↑ | p = 0.023 | 60 (♀ 19; ♂ 41) ADHD: 30 Control: 30 | ADHD: 8,4 Control: 9.3 | [28],[39] | |
| Bacteroides uniformis (species) | ↑ | p = 0.021 | 60 (♀ 19; ♂ 41) ADHD: 30 Control: 30 | ADHD: 8.4 Control: 9.3 | [28],[39] | |
| Bacteroidetes (phylum) | p = 0.166 | 96 (♀ 42; ♂ 54) ADHD: 19 Control: 77 | ADHD: 19.5 Control: 27,1 | [30] | ||
| Bifidobacterium (genus) | ↑ | p = 0.002 | 96 (♀ 42; ♂ 54) ADHD: 19 Control: 77 | ADHD: 19.5 Control: 27.1 | [27,28],[30] | |
| Clostridiales (order) | ↓ | p = 0.003 | 96 (♀ 42; ♂ 54) ADHD: 19 Control: 77 | ADHD: 19.5 Control: 27.1 | [30] | |
| Dialister (genus) | ↓ | - | - | - | [33] | |
| Faecalibacterium (genus) | ↓ | LDA value > 2 | 83 (♀ 23; ♂ 60) ADHD: 51 Control: 32 | ADHD: 8.47 Control: 8.5 | [28,33],[40] | |
| Firmicutes (phylum) | ↓ | p = 0.001 | 96 (♀ 42; ♂ 54) ADHD: 19 Control: 77 | ADHD: 19.5 Control: 27.1 | [30] | |
| Lactobacillus (genus) | ↓ | - | - | - | [33] | |
| Neisseria (genus) | ↑ | p < 0.05 | 31 (♀ 0; ♂ 31) ADHD: 14 Control: 17 | ADHD: 11.9 Control: 13.1 | [28],[38] | |
| Neisseriaceae (family) | ↑ | p < 0.05 | 31 (♀ 0; ♂ 31) ADHD: 14 Control: 17 | ADHD: 11.9 Control: 13.1 | [28],[38] | |
| Parabacteroides (genus) | ↓ | - | - | - | [33] | |
| Prevotella (genus) | ↓ | p < 0.05 | 31 (♀ 0; ♂ 31) ADHD: 14 Control: 17 | ADHD: 11.9 Control: 13.1 | [33],[38] | |
| Proteobacteria (phylum) | - | 31 (♀ 0; ♂ 31) ADHD: 14 Control: 17 | ADHD: 11.9 Control: 13.1 | [28],[38] | ||
| Sutterella stercoricanis (species) | ↑ | p = 0.001 | 60 (♀ 19; ♂ 41) ADHD: 30 Control: 30 | ADHD: 8,4 Control: 9.3 | [28],[39] |
| Bacteria in ASD Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | p = 0.360 * | - | - | [45,47],[48] | |
| Alistipes (genus) | ↓ | p< 0.01 | 80 (♀ 21; ♂ 59) ASD: 40 Control: 40 | ASD: 10 Control: 7 | [3,44],[49] | |
| Alistipes (genus) | ↑ | p = 0.07 | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [47],[50] | |
| Bacteroides (genus) | ↑ | p < 0.001 * | - | - | [45],[48] | |
| Bacteroides vulgatus (species) | ↑ | p = 0.007 | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [3,47],[50],[51] | |
| Bacteroidetes (phylum) | ↑ | p = 0.001 | 41 (♀ 12; ♂ 29) ASD: 33 Control: 8 | ASD: 2–13 Control: 2–13 | [46,51],[52] | |
| Bacteroidetes (phylum) | ↓ | p = 0.002 * | - | - | [3,43,44,45,46,47],[48] | |
| Betaproteobacteria (class) | ↑ | - | - | - | [44,45,47] | |
| Bifidobacterium (genus) | ↓ | p < 0.001 * | - | - | [3,44,45,46,47],[48] | |
| Bilophila (genus) | ↓ | p < 0.01 | 80 (♀ 21; ♂ 59) ASD: 40 Control: 40 | ASD: 10 Control: 7 | [3,44],[49] | |
| Burkholderia (genus) | ↑ | p = 0.03 | 40 (♀ 11; ♂ 29) ASD: 21 Control: 19 | ASD: 14.43 Control: 16.05 | [3,44],[53] | |
| Clostridium (genus) | ↑ | p < 0.001 * | - | - | [3,43,44,45,46,47],[48],[51] | |
| Clostridium bolteae (species) | ↑ | p = 0.01 | 23 (♀ -; ♂ -) ASD: 15 Control: 8 | ASD: - Control: - | [3,47],[54] | |
| Clostridium perfringens (species) | ↑ | p = 0.031 | 46 (♀ -; ♂ -) ASD: 33 Control: 13 | ASD: 2–9 Control: 2–9 | [44,45],[55] | |
| Coprococcus (genus) | ↓ | p < 0.001 * | - | - | [3,43,44,47],[48] | |
| Corynebacterium (genus) | ↑ | p < 0.01 | 80 (♀ 21; ♂ 59) ASD: 40 Control: 40 | ASD: 10 Control: 7 | [3,44],[49] | |
| Desulfovibrio (genus) | ↑ | p = 0.011 | 41 (♀ 12; ♂ 29) ASD: 33 Control: 8 | ASD: 2–13 Control: 2–13 | [3,43,44,45,47,51],[52] | |
| Dialister (genus) | ↓ | p = 0.760 * | - | - | [3,44],[48] | |
| Dialister (genus) | ↑ | - | - | - | [45] | |
| Dorea (genus) | ↑ | p < 0.01 | 80 (♀ 21; ♂ 59) ASD: 40 Control: 40 | ASD: 10 Control: 7 | [3,44],[49] | |
| Enterobacteriaceae (family) | ↑ | p = 0.21 | 54 (♀ 11; ♂ 43) ASD: 30 Control: 24 | ASD: 9.5 Control: 9.5 | [44,47],[56] | |
| Enterococcus (genus) | ↓ | - | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [44,45,47],[50] | |
| Escherichia coli (species) | ↓ | p = 0.03 | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [44],[50] | |
| Eubacterium (genus) | ↓ | LDA > 2.0 | 50 (♀ 9; ♂ 41) ASD: 30 Control: 20 | ASD: 4.43 Control: 4.28 | [45],[57] | |
| Faecalibacterium (genus) | ↑ | p < 0.001 * | - | - | [44,45],[48] | |
| Firmicutes (phylum) | ↓ | p = 0.001 | 41 (♀ 12; ♂ 29) ASD: 33 Control: 8 | ASD: 2–13 Control: 2–13 | [44,46],[52] | |
| Firmicutes (phylum) | ↑ | p < 0.001 * | - | - | [3,43,44,45,47],[48] | |
| Fusobacteria (phylum) | ↓ | p = 0.430 * | - | - | [44],[48] | |
| Lachnospiraceae (family) | ↓ | p = 0.1023 | 50 (♀ 9; ♂ 41) ASD: 30 Control: 20 | ASD: 4.43 Control: 4.28 | [45],[57] | |
| Lachnospiraceae (family) | ↑ | - | - | - | [44] | |
| Lactobacillaceae (family) | ↑ | p = 0.018 | 54 (♀ 11; ♂ 43) ASD: 30 Control: 24 | ASD: 9.5 Control: 9.5 | [51],[56] | |
| Lactobacillus (genus) | ↓ | - | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [44,47],[50] | |
| Lactobacillus (genus) | ↑ | p < 0.01 | 80 (♀ 21; ♂ 59) ASD: 40 Control: 40 | ASD: 10 Control: 7 | [3,43,44,45],[49],[51] | |
| Neisseria (genus) | ↓ | p = 0.01 | 40 (♀ 11; ♂ 29) ASD: 21 Control: 19 | ASD: 14.43 Control: 16.05 | [3,44],[53] | |
| Parabacteroides (genus) | ↑ | p < 0.001 * | - | - | [44],[48] | |
| Parabacteroides (genus) | ↓ | p <0.01 | 80 (♀ 21; ♂ 59) ASD: 40 Control: 40 | ASD: 10 Control: 7 | [3,44],[49] | |
| Prevotella (genus) | ↓ | p < 0.05 | 40 (♀ 5; ♂ 35) ASD: 20 Control: 20 | ASD: 6.7 Control: 8.3 | [3,43,44,46,47],[58] | |
| Prevotella copri (species) | ↑ | p = 0.04 | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [44],[50] | |
| Roseburia (genus) | ↑ | p = 0.003 | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [44],[50] | |
| Ruminococcaceae (family) | ↓ | p < 0.001 | 50 (♀ 9; ♂ 41) ASD: 30 Control: 20 | ASD: 4.43 Control: 4.28 | [45],[57] | |
| Ruminococcus (genus) | ↑ | p = 0.170 * | - | - | [45],[48] | |
| Ruminococcus torques (species) | ↑ | p = 0.08 | 54 (♀ -; ♂ -) ASD: 23 Control: 9 | ASD: - Control: - | [3],[59] | |
| Streptococcus (genus) | ↓ | p = 0.04 | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [44],[50] | |
| Sutterella (genus) | ↓ | p = 0.480 * | - | - | [44],[48] | |
| Sutterella (genus) | ↑ | p = 0.05 | 54 (♀ -; ♂ -) ASD: 23 Control: 9 | ASD: - Control: - | [3,43,44],[59] | |
| Sutterellaceae (family) | ↑ | - | 30 (♀ 16; ♂ 14) ASD: 10 Control: 10 | ASD: 4–10 Control: 4–10 | [47],[50] | |
| Veillonella (genus) | ↓ | p = 0.460 * | - | - | [3,44],[48] | |
| Veillonellaceae (family) | ↑ | p = 0.008 | 54 (♀ 11; ♂ 43) ASD: 30 Control: 24 | ASD: 9.5 Control: 9.5 | [51],[56] | |
| Veillonellaceae (unclassified genus of this family) | ↓ | p = 0.04 | 40 (♀ 5; ♂ 35) ASD: 20 Control: 20 | ASD: 6.7 Control: 8.3 | [3,43,44],[58] |
| Bacteria in Schizophrenia Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | p = 0.0478 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Actinomycetales (order) | ↑ | p = 0.0025 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Akkermansia muciniphila (species) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65],[66] | |
| Alcaligenaceae (family) | ↓ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Alkaliphilus oremlandii (species) | ↑ | p = 0.008 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Anaerococcus (genus) | ↑ | p = 0.007 | 50 (♀ 21; ♂ 29) SCZ: 25 Control: 25 | SCZ: 52.9 Control: 54.7 | [64] | |
| Bacteroides plebeius (species) | ↑ | p = 0.0038 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Bifidobacterium adolescentis (species) | ↑ | p = 0.003 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65],[66] | |
| Bifidobacterium longum (species) | ↑ | p = 0.0075 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Bifidobacterium (genus) | ↑ | p = 0.0062 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Bifidobacterium (genus) | ↓ | p = 0.006 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [62],[66] | |
| Clostridium (genus) | ↓ | p = 0.0002 | 50 (♀ 21; ♂ 29) SCZ: 25 Control: 25 | SCZ: 52.9 Control: 54.7 | [64] | |
| Clostridium coccoides (species) | ↑ | p < 0.001 | - | - | [62] | |
| Clostridium perfringens (species) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Clostridium symbiosum (species) | ↑ | p = 0.0166 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Cronobacter sakazakii/turicensis (species) | ↑ | p = 0.0387 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Deltaproteobacteria (class) | ↑ | p = 0.002 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Eggerthella (genus) | ↑ | p = 0.00307 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Enterococcaceae (family) | ↓ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Enterococcus (genus) | ↓ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Enterococcus faecium (species) | ↑ | p = 0.0035 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Escherichia coli (species) | ↓ | p < 0.001 | - | - | [62],[66] | |
| Eubacterium siraeum (species) | ↑ | p = 0.0008 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Haemophilus (genus) | ↓ | p = 0.004 | 50 (♀ 21; ♂ 29) SCZ: 25 Control: 25 | SCZ: 52.9 Control: 54.7 | [64] | |
| Lactobacillus fermentum (species) | ↑ | p = 0.0026 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Lactobacillus gasseri (species) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Lactobacillus (genus) | ↑ | p = 0.027 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [62],[66] | |
| Leuconostocaceae (family) | ↓ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Megasphaera (genus) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Megasphaera elsdeniis (species) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Proteobacteria (phylum) | ↓ | - | 50 (♀ 21; ♂ 29) SCZ: 25 Control: 25 | SCZ: 52.9 Control: 54.7 | [64] | |
| Rhodocyclaceae (family) | ↓ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Rhodocyclales (order) | ↓ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Rikenellaceae (family) | ↓ | p = 0.011 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Streptococcus vestibularis (species) | ↑ | p = 0.0036 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] | |
| Sphingomonadaceae (family) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Sphingomonadales (oder) | ↑ | p < 0.001 | 168 (♀ 72; ♂ 89) SCZ: 84 Control: 84 | SCZ: 35 Control: 35 | [65] | |
| Sutterella (genus) | ↓ | p = 0.004 | 50 (♀ 21; ♂ 29) SCZ: 25 Control: 25 | SCZ: 52.9 Control: 54.7 | [64] | |
| Veillonella parvula (species) | ↑ | p = 0.004 | 171 (♀ 84; ♂ 87) SCZ: 90 Control: 81 | SCZ: 28.6 Control: 32.8 | [66] |
| Bacteria in Alzheimer’s Disease Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [75],[79] | |
| Alistipes (genus) | ↑ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [79],[80] | |
| Bacillus subtilis (species) | ↑ | - | - | - | [76] | |
| Bacteroidaceae (family) | ↑ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [79] | |
| Bacteroides (genus) | ↑ | - | - | - | [80] | |
| Bacteroides/Bacillus fragilis (species) | ↓ | - | - | - | [74],[76] | |
| Bacteroidetes (phylum) | ↑ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [74,76,78],[79],[80] | |
| Bifidobacteriaceae (family) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [79],[80] | |
| Bifidobacterium (genus) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [74,76],[79],[80] | |
| Clostridiaceae (family) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [79] | |
| Clostridium (genus) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [79] | |
| Dialister (genus) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [79] | |
| Escherichia (genus) | ↑ | p < 0.001 | 83 (♀ 44; ♂ 39) AD: 73 Control: 10 | AD: 70.5 Control: 68 | [76],[81] | |
| Escherichia coli (species) | ↑ | - | - | - | [76] | |
| Eubacterium hallii (species) | ↓ | Not significant | 83 (♀ 44; ♂ 39) AD: 73 Control: 10 | AD: 70.5 Control: 68 | [74],[81] | |
| Eubacterium rectale (species) | ↓ | p < 0.001 | 83 (♀ 44; ♂ 39) AD: 73 Control: 10 | AD: 70.5 Control: 68 | [74,76],[81] | |
| Faecalibacterium prausnitzii (species) | ↓ | Not significant | 83 (♀ 44; ♂ 39) AD: 73 Control: 10 | AD: 70.5 Control: 68 | [74],[81] | |
| Firmicutes (phylum) | ↓ | p < 0.05 | 50 (♀ 35; ♂ 15) AD: 25 Control: 25 | AD: 71.3 Control: 69.3 | [74,76],[79] | |
| Fusobacteriaceae (family) | ↓ | - | - | - | [76] | |
| Prevotellaceae (family) | ↑ | - | - | - | [76] |
| Bacteria in Parkinson’s Disease Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | p < 0.001 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [80],[89] | |
| Akkermansia (genus) | ↑ | p = 0.0001 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [51,83,84,86,88],[90] | |
| Anaerotruncus (genus) | ↑ | p = 0.047 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Aquabacterium (genus) | ↑ | p < 0.0001 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Bacteroides (genus) | ↑ | p = 0.05 | 72 (♀ 30; ♂ 42) PD: 38 Control: 34 | PD: 61.6 Control: 45.1 | [83],[92] | |
| Bacteroidetes (phylum) | ↓ | p = 0.045 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [84],[89],[93] | |
| Bifidobacteriaceae (family) | ↑ | p < 0.0001 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [84],[90] | |
| Blautia (genus) | ↓ | p = 0.018 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [51],[89] | |
| Butyricicoccus (genus) | ↑ | p = 0.034 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Christensenellaceae (family) | ↑ | p < 0.0001 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [84],[90] | |
| Clostridium IV (genus) | ↑ | p < 0.0001 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Clostridium XVIII (genus) | ↑ | p = 0.03 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Coprococcus (genus) | ↓ | p = 0.03 | 72 (♀ 30; ♂ 42) PD: 38 Control: 34 | PD: 61.6 Control: 45.1 | [51],[92] | |
| Enterococcaceae (family) | ↓ | - | 68 (♀ 26; ♂ 42) PD: 34 Control: 34 | PD: 67.7 Control: 64.6 | [51],[93] | |
| Enterococcus (genus) | ↑ | p = 0.006 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [51],[89] | |
| Escherichia-Shigella (genus) | ↑ | p = 0.038 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [51],[89] | |
| Faecalibacterium (genus) | ↓ | p < 0.05 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [51,84,86,88],[90] | |
| Firmicutes (phylum) | ↓ | p = 0.03 | 72 (♀ 30; ♂ 42) PD: 38 Control: 34 | PD: 61.6 Control: 45.1 | [84],[92] | |
| Holdemania (genus) | ↑ | p = 0.004 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Lachnospiraceae (family) | ↓ | p = 0.02 | 72 (♀ 30; ♂ 42) PD: 38 Control: 34 | PD: 61.6 Control: 45.1 | [84,86,88],[92] | |
| Lactobacillaceae (family) | ↑ | p < 0.0001 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [83,84,85,87],[90] | |
| Lactobacillus (genus) | ↑ | p < 0.0001 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [84],[90] | |
| Lactobacillus (genus) | ↓ | LDA > 2 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51,85],[91] | |
| Prevotella (genus) | ↓ | p = 0.28 | 88 (♀ 46; ♂ 42) PD: 52 Control: 36 | PD: 68.9 Control: 68.4 | [51,84,85,86,88],[94] | |
| Prevotellaceae (family) | ↓ | Not significant | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [84],[89],[93] | |
| Proteus (genus) | ↑ | p = 0.022 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [51,85],[89] | |
| Roseburia (genus) | ↓ | p < 0.05 | 327 (♀ 144; ♂ 183) PD: 197 Control: 130 | PD: 68.4 Control: 70.3 | [51,83],[90] | |
| Ruminococcaceae (family) | ↑ | p < 0.05 | 20 (♀ 8; ♂ 12) PD: 10 Control: 10 | PD: 79.5 Control: 76.5 | [84],[95] | |
| Ruminococcus (species) | ↓ | p = 0.019 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [89] | |
| Sediminibacterium (genus) | ↓ | LDA > 2 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Sphingomonas (genus) | ↑ | p < 0.05 | 90 (♀ 45; ♂ 45) PD: 45 Control: 45 | PD: 68.1 Control: 67.9 | [51],[91] | |
| Streptococcus (genus) | ↑ | p = 0.01 | 38 (♀ 16; ♂ 22) PD: 24 Control: 14 | PD: 73.75 Control: 74.64 | [51],[89] | |
| Verrucomicrobiaceae (family) | ↑ | p = 0.05 | 72 (♀ 30; ♂ 42) PD: 38 Control: 34 | PD: 61.6 Control: 45.1 | [51],[92] |
| Bacteria in Major Depressive Disorder Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [102],[105] | |
| Actinomycetaceae (family) | ↑ | - | - | - | [100],[104] | |
| Alistipes (genus) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [51,100,102],[105] | |
| Atopobium (genus) | ↑ | - | - | - | [99],[100] | |
| Bacteroides (genus) | ↑ | p = 0.007 | - | - | [102],[106] | |
| Bacteroidetes (phylum) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [101,103],[105] | |
| Bifidobacteriaceae (family) | ↑ | p = 0.004 | 382 (♀ 228; ♂ 154) MDD: 165 Control: 217 | MDD: 45.1 Control: 36.1 | [102],[107] | |
| Bifidobacterium (genus) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [99,102],[108] | |
| Bifidobacterium (genus) | ↓ | p = 0.012 | 100 (♀ 53; ♂ 47) MDD: 43 Control: 57 | MDD: 39.4 Control: 42.8 | [51,100,103,104],[109] | |
| Blautia (genus) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [100],[105] | |
| Christensenellaceae (family) | ↓ | p = 0.0395 | 90 (♀ 72; ♂ 18) MDD: 43 Control: 47 | MDD: 21.9 Control: 22.1 | [102],[110] | |
| Clostridium (genus) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [100],[108] | |
| Coprococcus (genus) | ↓ | p = 0.101 | 121 (♀ 76; ♂ 45) MDD: 58 Control: 63 | MDD: 40.6 Control: 41.8 | [100,102,104],[111] | |
| Dialister (genus) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [99],[105] | |
| Eggerthella (genus) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [99],[108] | |
| Enterobacteriaceae (family) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [51],[105] | |
| Escherichia (genus) | ↓ | - | - | - | [100],[104] | |
| Eubacterium (genus) | ↑ | p = 0.065 | 121 (♀ 76; ♂ 45) MDD: 58 Control: 63 | MDD: 40.6 Control: 41.8 | [111] | |
| Eubacterium rectale (species) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [108] | |
| Faecalibacterium (genus) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [51,99,100,102,103,104],[105] | |
| Firmicutes (phylum) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [101,103],[105] | |
| Flavonifractor (genus) | ↑ | LDA > 2 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [102],[105] | |
| Lactobacillus (genus) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [108] | |
| Oscillibacter (genus) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [100],[105] | |
| Parabacteroides (genus) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [102,105] | |
| Paraprevotella (genus) | ↑ | p = 0.041 | 67 (♀ 27; ♂ 40) MDD: 34 Control: 33 | MDD: 45.8 Control: 45.8 | [100,104],[112] | |
| Prevotella (genus) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [103],[108] | |
| Prevotellaceae (family) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [100,103,104],[105] | |
| Proteobacteria (phylum) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [103],[105] | |
| Roseburia (genus) | ↑ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [105],[111] | |
| Ruminococcaceae (family) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [102],[105] | |
| Ruminococcus (genus) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [100,103,104],[105] | |
| Streptococcus (genus) | ↑ | p < 0.01 | 61 (♀ 38; ♂ 23) MDD: 31 Control: 30 | MDD: 41.58 Control: 39.47 | [102],[108],[111,113] | |
| Sutterella (genus) | ↓ | - | 73 (♀ 51; ♂ 22) MDD: 36 Control: 37 | MDD: 45.83 Control: 41.19 | [102],[113] | |
| Sutterellaceae (family) | ↓ | - | - | - | [100,102],[104] | |
| Veillonellaceae (family) | ↓ | p < 0.05 | 76 (♀ 34; ♂ 42) MDD: 46 Control: 30 | MDD: 26.2 Control: 26.8 | [100,104],[105] |
| Bacteria in Bipolar Disorder Patients | Increase | Decrease | Significance | Sample Size (n) | Mean Age (Years) | Source |
|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [102],[108],[116,118] | |
| Atopobium Cluster (genus) | ↑ | p < 0.001 | 63 (♀ 27; ♂ 36) BD: 36 Control: 27 | BD: 32.64 Control: 28.89 | [115,116],[119] | |
| Bacteroides (genus) | ↓ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[116] | |
| Bacteroides (genus) | ↑ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [116],[120] | |
| Bacteroidetes (phylum) | ↑ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [116],[120] | |
| Bacteroidetes (phylum) | ↓ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108] | |
| Bifidobacterium (genus) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[116] | |
| Clostridium Cluster IV (genus) | ↑ | p < 0.001 | 63 (♀ 27; ♂ 36) BD: 36 Control: 27 | BD: 32.64 Control: 28.89 | [115,116],[119] | |
| Clostridium (genus) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[116] | |
| Coprococcus (genus) | ↓ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [115,116],[120] | |
| Desulfovibrio (genus) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[116] | |
| Enterobacter (genus) | ↑ | p < 0.001 | 63 (♀ 27; ♂ 36) BD: 36 Control: 27 | BD: 32.64 Control: 28.89 | [115,116],[119] | |
| Escherichia (genus) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[115,116] | |
| Faecalibacterium (genus) | ↓ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [115,116,117,118],[120] | |
| Faecalibacterium prausnitzii (species) | ↑ | p = 0.030 | 63 (♀ 27; ♂ 36) BD: 36 Control: 27 | BD: 32.64 Control: 28.89 | [115,116],[119] | |
| Firmicutes (phylum) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108] | |
| Flavonifractor (genus) | ↑ | p < 0.05 | 190 (♀ 117; ♂ 73) BD: 113 Control: 77 | BD: 31 Control: 28 | [115,116],[121] | |
| Halomonas (genus) | ↑ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [116],[120] | |
| Klebsiella (genus) | ↑ | p < 0.05 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[115,116] | |
| Oscillibacter (genus) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[116] | |
| Parabacteroides (genus) | ↑ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [116],[120] | |
| Prevotella (genus) | ↓ | Not significant | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108] | |
| Proteobacteria (phylum) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [102],[108] | |
| Roseburia (genus) | ↓ | p < 0.05 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [115,116],[120] | |
| Ruminococcaceae (family) | ↓ | LDA > 2 | 97 (♀ 47; ♂ 50) BD: 52 Control: 45 | BD: 24.15 Control: 36.29 | [115,116,117,118],[120] | |
| Streptococcus (genus) | ↑ | p < 0.01 | 60 (♀ 31; ♂ 29) BD: 30 Control: 30 | BD: 38.40 Control: 39.47 | [108],[116] |
| ADHD | ASD | Schizophrenia | AD | PD | MDD | BD | |
|---|---|---|---|---|---|---|---|
| Actinobacteria (phylum) | ↑ | ↑ | ↑ | ↓ | ↑ | ↑ | ↑ |
| Alistipes (genus) | ↑↓ | ↑ | ↑ | ||||
| Atopobium (genus) | ↑ | ↑ | |||||
| Bacteroides (genus) | ↑ | ↑ | ↑ | ↑ | ↑ | ↑↓ | |
| Bacteroidetes (phylum) | ↑↓ | ↑ | ↓ | ↑ | ↑↓ | ||
| Bifidobacteriaceae (family) | ↓ | ↑ | ↑ | ||||
| Bifidobacterium (genus) | ↑ | ↓ | ↑↓ | ↓ | ↑↓ | ↑ | |
| Blautia (genus) | ↓ | ↑ | |||||
| Christensenellaceae (family) | ↑ | ↓ | |||||
| Clostridium (genus) | ↑ | ↓ | ↓ | ↑ | ↑ | ||
| Coprococcus (genus) | ↓ | ↓ | ↓ | ↓ | |||
| Desulfovibrio (genus) | ↑ | ↑ | |||||
| Dialister (genus) | ↓ | ↓ | ↓ | ↓ | |||
| Eggerthella (genus) | ↑ | ↑ | |||||
| Enterobacteriaceae (family) | ↑ | ↑ | |||||
| Enterococcaceae (family) | ↓ | ↓ | |||||
| Enterococcus (genus) | ↓ | ↓ | ↑ | ||||
| Escherichia (genus) | ↑ | ↓ | ↑ | ||||
| Escherichia coli (species) | ↓ | ↓ | ↑ | ||||
| Eubacterium (genus) | ↓ | ↑ | |||||
| Eubacterium rectale (species) | ↓ | ↑ | |||||
| Faecalibacterium (genus) | ↓ | ↑ | ↓ | ↓ | ↓ | ||
| Faecalibacterium prausnitzii (species) | ↓ | ↑ | |||||
| Firmicutes (phylum) | ↓ | ↑↓ | ↓ | ↓ | ↓ | ↑ | |
| Flavonifractor (genus) | ↑ | ↑ | |||||
| Lachnospiraceae (family) | ↑↓ | ↓ | |||||
| Lactobacillaceae (family) | ↑ | ↑ | |||||
| Lactobacillus (genus) | ↓ | ↑↓ | ↑ | ↑↓ | ↑ | ||
| Neisseria (genus) | ↑ | ↓ | |||||
| Oscillibacter (genus) | ↑ | ↑ | |||||
| Parabacteroides (genus) | ↓ | ↑↓ | ↑ | ↑ | |||
| Prevotella (genus) | ↓ | ↓ | ↓ | ↑ | ↓ | ||
| Prevotellaceae (family) | ↑ | ↓ | ↓ | ||||
| Proteobacteria (phylum) | ↓ | ↑ | ↑ | ||||
| Roseburia (genus) | ↑ | ↓ | ↑ | ↓ | |||
| Ruminococcaceae (family) | ↓ | ↑ | ↓ | ↓ | |||
| Ruminococcus (genus) | ↑ | ↓ | ↓ | ||||
| Streptococcus (genus) | ↓ | ↑ | ↑ | ↑ | |||
| Sutterella (genus) | ↑↓ | ↓ | ↓ | ||||
| Sutterellaceae (family) | ↑ | ↓ | |||||
| Veillonellaceae (family) | ↑ | ↓ |
| ADHD | ASD | Schizophrenia | AD | PD | MDD | BD | |
|---|---|---|---|---|---|---|---|
| General SCFA levels | ↓ [27] | ↑ [3,29,45] | ↑ [68] | ↓ [71,123] | ↓ [29,51,68,124] | ↓ [29] | |
| ↓ [29,44,46] | |||||||
| Butyrate | ↑↓ [29] | ↓ [29,68,124] | ↓ [115,116] | ||||
| Acetate | ↑↓ [29] | ↑ [68] | ↓ [125] | ↓ [29,68] | ↓ [29] | ||
| Valerate | ↑↓ [29] | ↓ [125] | |||||
| Isovaleric Acid | ↑ [29] | ↓ [125] | ↓ [29] | ||||
| Propionate | ↑↓ [29] | ↑ [68] | ↓ [125] | ↓ [29,68,124] | ↓ [29] | ||
| ↑ [68] | |||||||
| Isocaproic acid | ↑ [29] | ||||||
| Isobutyric acid | ↑ [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eicher, T.P.; Mohajeri, M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients 2022, 14, 2661. https://doi.org/10.3390/nu14132661
Eicher TP, Mohajeri MH. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients. 2022; 14(13):2661. https://doi.org/10.3390/nu14132661
Chicago/Turabian StyleEicher, Tanja Patricia, and M. Hasan Mohajeri. 2022. "Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases" Nutrients 14, no. 13: 2661. https://doi.org/10.3390/nu14132661
APA StyleEicher, T. P., & Mohajeri, M. H. (2022). Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients, 14(13), 2661. https://doi.org/10.3390/nu14132661






