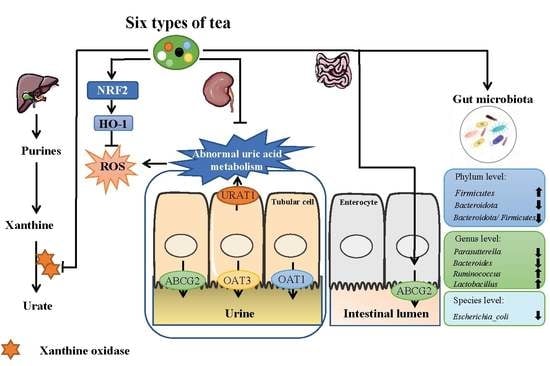
Tea (Camellia sinensis) Ameliorates Hyperuricemia via Uric Acid Metabolic Pathways and Gut Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Tea Water Extracts (TWEs)
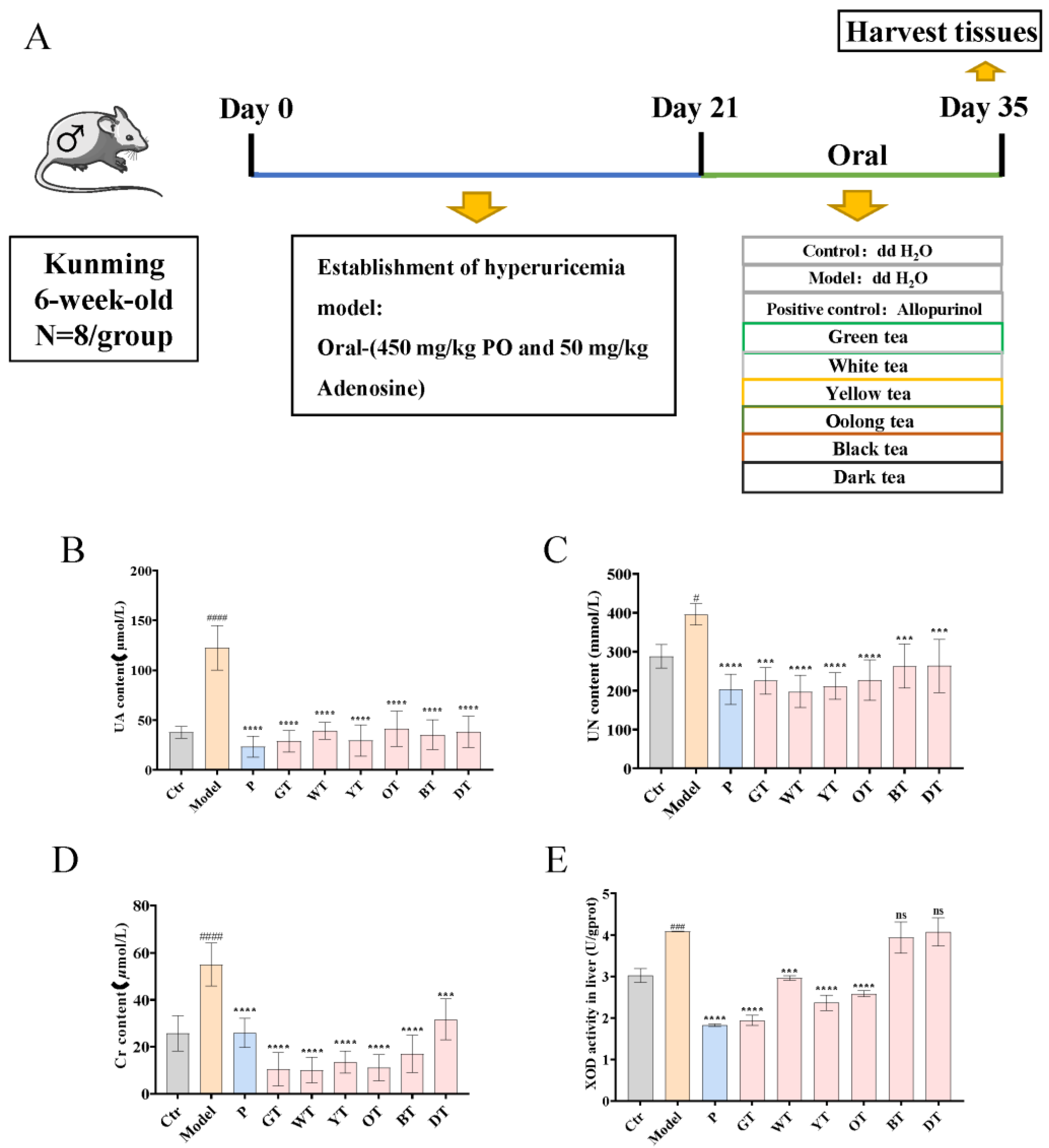
2.2. Animal Experiments
2.3. Biochemical Analysis
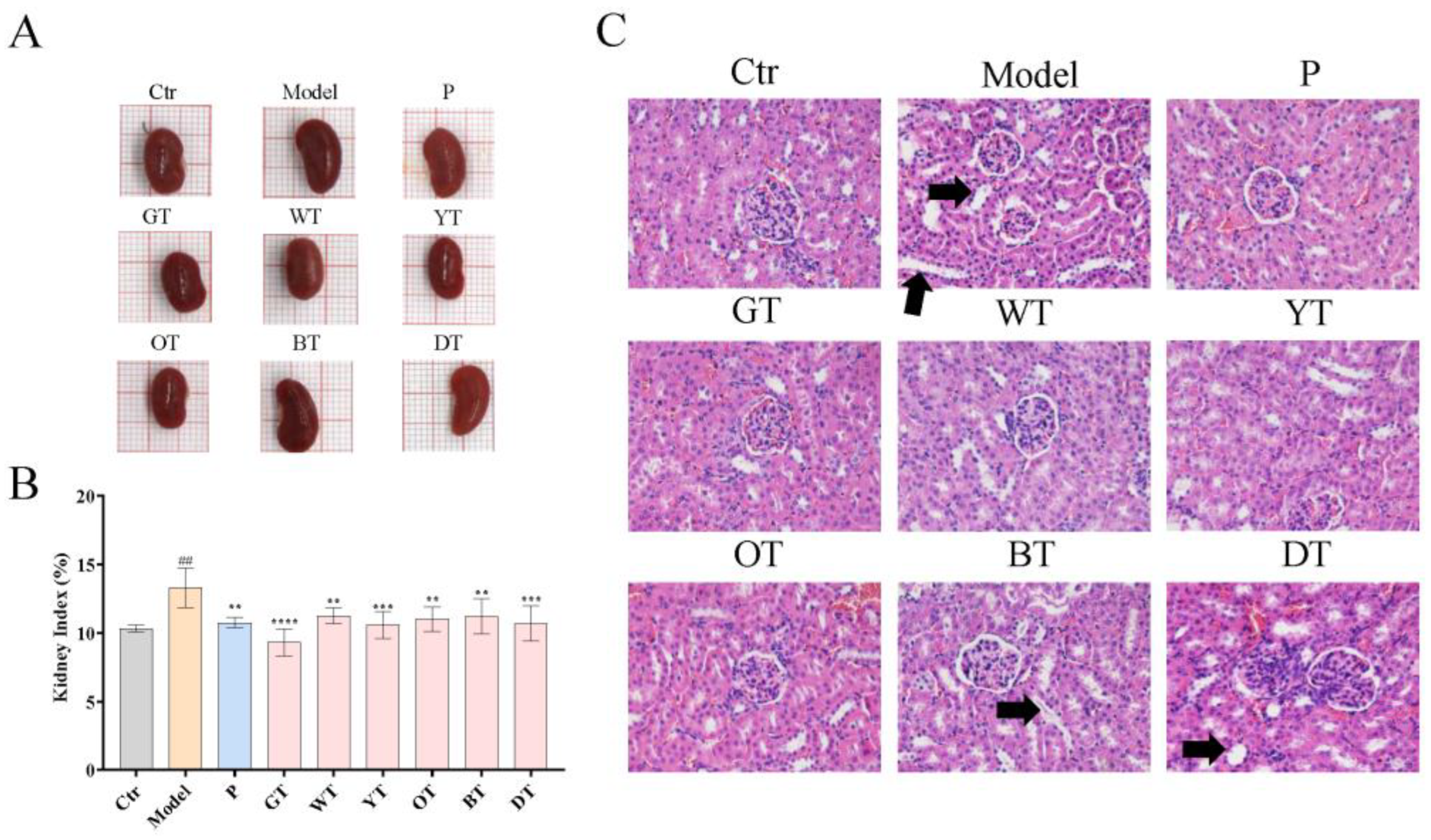
2.4. Calculation of Kidney Index
2.5. Histopathological Evaluation
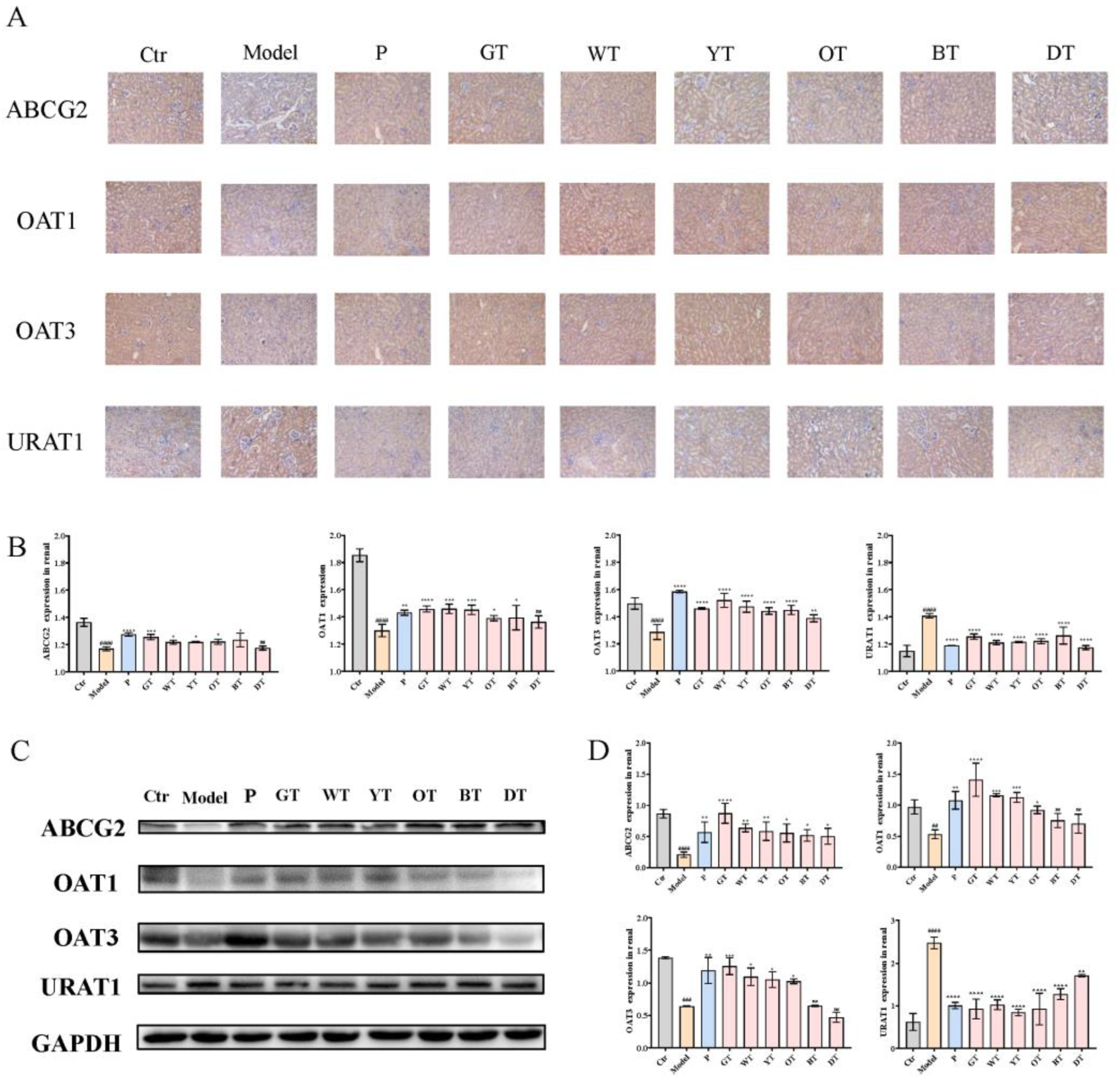
2.6. Immunohistochemistry
2.7. Western Blot Analysis
2.8. High-Throughput Sequencing and Analysis of Fecal Microbiota
2.9. Statistical Analysis
3. Results
3.1. TWEs Relieved Uric Acid Metabolism Disorder in HUA Mice
3.2. TWEs Reduced Hepatic Uric Acid Production in HUA Mice by Inhibiting XOD
3.3. TWEs Alleviated HUA by Regulating Uric Acid Transporters in Renal
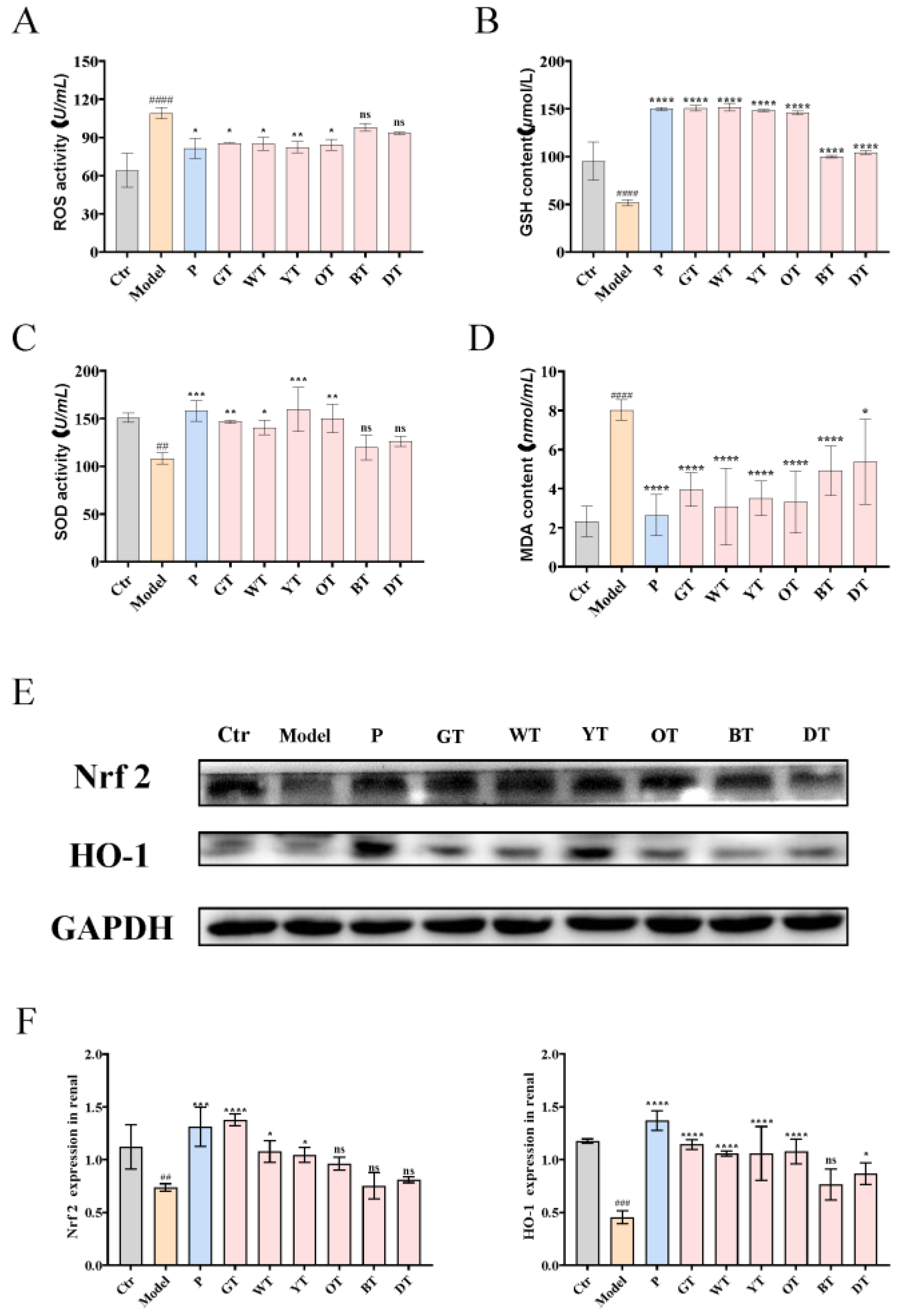
3.4. TWEs Alleviated HUA-Induced Renal Injury by Inhibiting Oxidative Stress via Nrf2/HO-1 Pathway
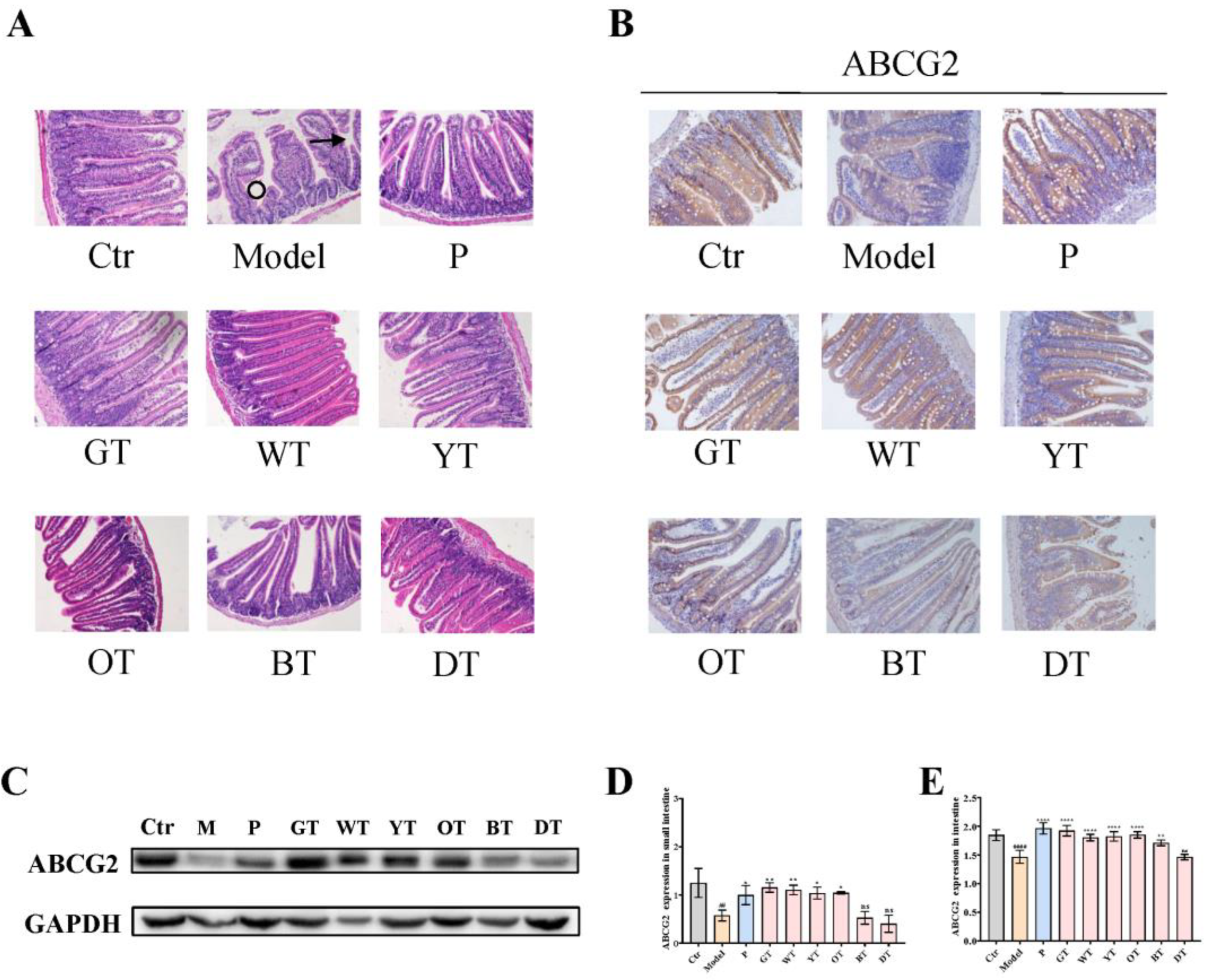
3.5. TWEs Promoted Intestinal Uric Acid Excretion by Upregulating ABCG2
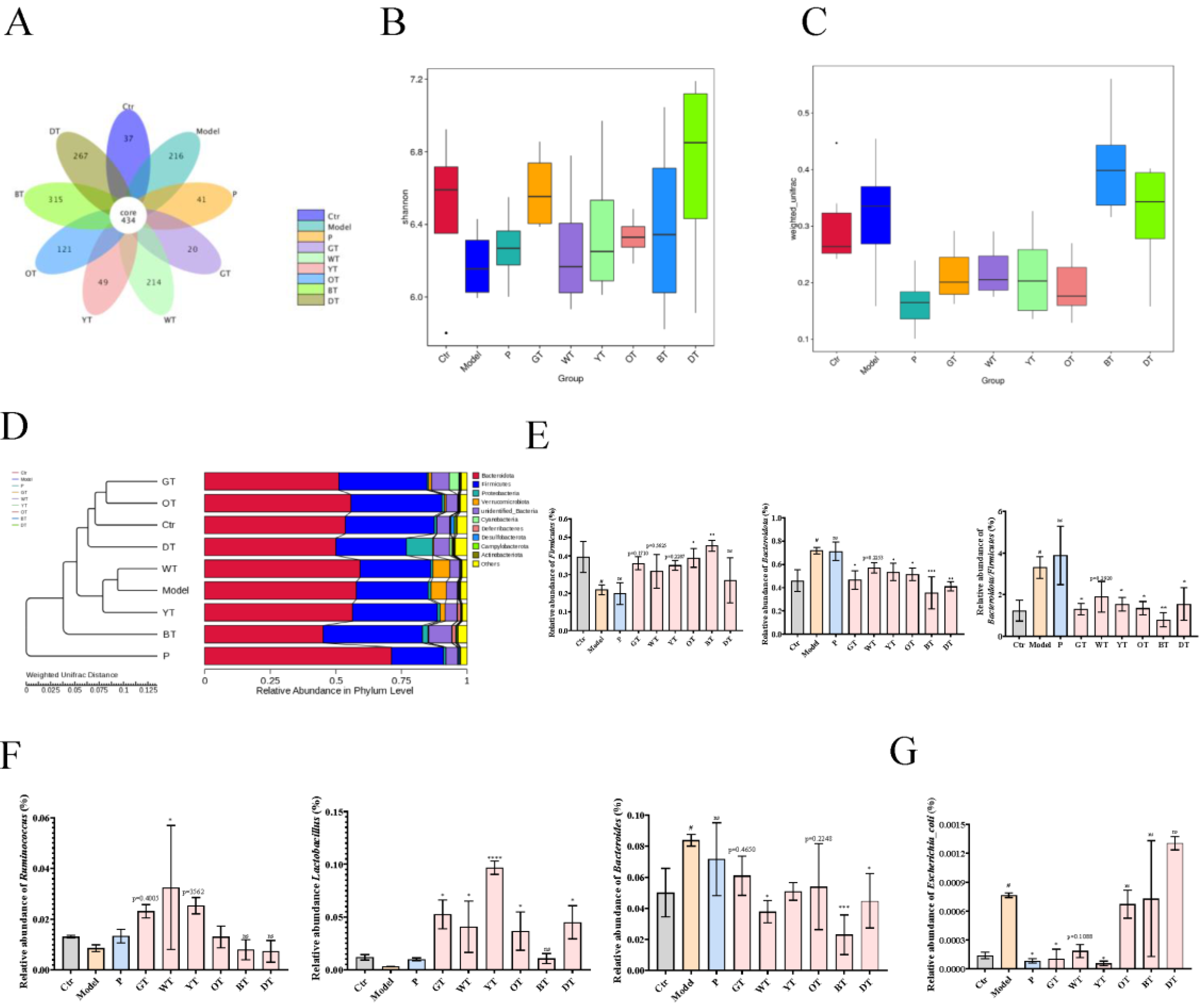
3.6. TWEs Alleviated Uric Acid Metabolism Disorders by Regulating Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HUA | hyperuricemia |
| TWEs | tea water extracts |
| WB | Western blot |
| IHC | immunohistochemical |
| URAT1 | urate anion transporter 1 |
| GLUT9 | glucose transporter 9 |
| OAT1 | organic anion transporters 1 |
| OAT3 | organic anion transporters 3 |
| ABCG2 | ATP-binding cassette sub-family G member 2 |
| XOD | xanthine oxidase |
| XDH | xanthine dehydrogenase |
| Nrf2 | nuclear factor erythroid2-related factor 2 |
| HO-1 | Heme Oxygenase 1 |
| ROS | reactive oxygen species |
| GSH | reduced glutathione |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| UA | uric acid |
| UN | urea nitrogen |
| Cr | creatine |
| CMC-Na | carboxymethylcellulose sodium |
| BW | body weight |
| UPGMA | unweighted pair-group method with arithmetic means. |
References
- Bursill, D.; Taylor, W.J.; Terkeltaub, R.; Kuwabara, M.; Merriman, T.R.; Grainger, R.; Pineda, C.; Louthrenoo, W.; Edwards, N.L.; Andres, M.; et al. Gout, Hyperuricemia, and Crystal-Associated Disease Network Consensus Statement Regarding Labels and Definitions for Disease Elements in Gout. Arthritis Care Res. 2019, 71, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, T.; Ci, X.; Zhao, F.; Sun, Y.; Li, Y.; Liu, R.; Wu, W.; Yi, X.; Liu, C. The effect of polymorphism of uric acid transporters on uric acid transport. J. Nephrol. 2019, 32, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brody, H. Tea. Nature 2019, 566, S1. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Li, Y.; Li, M.; Wang, Y.; Zhang, L.; Wan, X.; Yang, X. Tea aroma formation from six model manufacturing processes. Food Chem. 2019, 285, 347–354. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Sun, L.; Lai, X.; Li, Q.; Zhang, W.; Xiang, L.; Sun, S.; Cao, F. Six types of tea reduce high-fat-diet-induced fat accumulation in mice by increasing lipid metabolism and suppressing inflammation. Food Funct. 2019, 10, 2061–2074. [Google Scholar] [CrossRef]
- Turkozu, D.; Sanlier, N. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 1681–1687. [Google Scholar] [CrossRef]
- Chen, G.; Chen, R.; Chen, D.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Tea Polysaccharides as Potential Therapeutic Options for Metabolic Diseases. J. Agric. Food Chem. 2019, 67, 5350–5360. [Google Scholar] [CrossRef]
- Liu, W.; Wan, C.; Huang, Y.; Li, M. Effects of tea consumption on metabolic syndrome: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 34, 2857–2866. [Google Scholar] [CrossRef]
- Peluso, I.; Teichner, A.; Manafikhi, H.; Palmery, M. Camellia sinensis in asymptomatic hyperuricemia: A meta-analysis of tea or tea extract effects on uric acid levels. Crit. Rev. Food Sci. Nutr. 2017, 57, 391–398. [Google Scholar] [CrossRef]
- Xu, C. Hyperuricemia and nonalcoholic fatty liver disease: From bedside to bench and back. Hepatol. Int. 2016, 10, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Chen, D.; Wu, H. Effects of Pu-erh ripened tea on hyperuricemic mice studied by serum metabolomics. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1068–1069, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Tai, L.L.; Wan, X.C.; Li, D.X.; Zhao, Y.Q.; Xu, Y. Comparative effects of green and black tea extracts on lowering serum uric acid in hyperuricemic mice. Pharm. Biol. 2017, 55, 2123–2128. [Google Scholar] [CrossRef]
- Wu, D.; Chen, R.; Zhang, W.; Lai, X.; Sun, L.; Li, Q.; Zhang, Z.; Cao, J.; Wen, S.; Lai, Z.; et al. Tea and its components reduce the production of uric acid by inhibiting xanthine oxidase. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Lai, X.; Li, Q.; Sun, L.; Chen, R.; Sun, S.; Cao, F. Regulation of Catechins in Uric Acid Metabolism Disorder Related Human Diseases. Mini-Rev. Med. Chem. 2020, 20, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, L.; Chen, R.; Wen, S.; Li, Q.; Lai, X.; Zhang, Z.; Cao, F.; Sun, S. Chinese Tea Alleviates CCl4-Induced Liver Injury through the NF-kappaBorNrf2Signaling Pathway in C57BL-6J Mice. Nutrients 2022, 14, 972. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Feng, M.; Pan, W.; Zheng, X.; Xie, X.; Hu, B.; Teng, C.; Wang, Y.; Liu, Z.; Wu, J.; et al. Inhibitory Effects of Six Types of Tea on Aging and High-Fat Diet-Related Amyloid Formation Activities. Antioxidants 2021, 10, 1513. [Google Scholar] [CrossRef]
- Liu, R.; Han, C.; Wu, D.; Xia, X.; Gu, J.; Guan, H.; Shan, Z.; Teng, W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2015, 2015, 762820. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jin, L.; Liu, J.; Wang, W.; Yu, H.; Li, J.; Chen, Q.; Wang, T. Effect and mechanism of dioscin from Dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. J. Ethnopharmacol. 2018, 214, 29–36. [Google Scholar] [CrossRef]
- Lu, J.; Dalbeth, N.; Yin, H.; Li, C.; Merriman, T.R.; Wei, W.H. Mouse models for human hyperuricaemia: A critical review. Nat. Rev. Rheumatol. 2019, 15, 413–426. [Google Scholar] [CrossRef]
- Kawakami, Y.; Yasuda, A.; Hayashi, M.; Akiyama, M.; Asai, T.; Hosaka, T.; Arai, H. Acute effect of green tea catechins on uric acid metabolism after alcohol ingestion in Japanese men. Clin. Rheumatol. 2021, 40, 2881–2888. [Google Scholar] [CrossRef] [PubMed]
- Jatuworapruk, K.; Srichairatanakool, S.; Ounjaijean, S.; Kasitanon, N.; Wangkaew, S.; Louthrenoo, W. Effects of Green Tea Extract on Serum Uric Acid and Urate Clearance in Healthy Individuals. J. Clin. Rheumatol. 2014, 20, 310–313. [Google Scholar] [CrossRef]
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, L.; Zhang, P.; Zhi, J.; Xing, R.; He, L. Lycium barbarum polysaccharides protect mice from hyperuricaemia through promoting kidney excretion of uric acid and inhibiting liver xanthine oxidase. Pharm. Biol. 2020, 58, 944–949. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, W.J.; Hyun, J.M.; Lee, J.S.; Kwon, J.G.; Seo, C.; Song, M.J.; Choi, C.W.; Hong, S.S.; Park, K.; et al. Salvia plebeia Extract Inhibits Xanthine Oxidase Activity In Vitro and Reduces Serum Uric Acid in an Animal Model of Hyperuricemia. Planta Med. 2017, 83, 1335–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhao, M.; Su, G.; Lin, L.; Wang, Y. Effect of Soy Sauce on Serum Uric Acid Levels in Hyperuricemic Rats and Identification of Flazin as a Potent Xanthine Oxidase Inhibitor. J. Agric. Food Chem. 2016, 64, 4725–4734. [Google Scholar] [CrossRef]
- Lin, J.-K.; Chen, P.-C.; Ho, C.-T.; Lin-Shiau, S.-Y. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3’-digallate, (-)-epigallocatechin-3-gallate, and propyl gallate. J. Agric. Food Chem. 2000, 48, 2736–2743. [Google Scholar] [CrossRef]
- Chen, G.; Tan, M.L.; Li, K.K.; Leung, P.C.; Ko, C.H. Green tea polyphenols decreases uric acid level through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Ethnopharmacol. 2015, 175, 14–20. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, P.; Pan, Z.; Xu, H.; Luo, Y.; Wang, X. Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS-SPME/GC-MS. Food Chem. 2013, 141, 259–265. [Google Scholar] [CrossRef]
- Su, H.Y.; Yang, C.; Liang, D.; Liu, H.F. Research Advances in the Mechanisms of Hyperuricemia-Induced Renal Injury. BioMed Res. Int. 2020, 2020, 5817348. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Ge, J.; Wang, Y.; Yan, Q.; Wu, C.; Yu, H.; Yang, M.; Yang, H.; Zou, J. Tea Polyphenol Attenuates Oxidative Stress-Induced Degeneration of Intervertebral Discs by Regulating the Keap1/Nrf2/ARE Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6684147. [Google Scholar] [CrossRef]
- Yamagata, K. Protective Effect of Epigallocatechin Gallate on Endothelial Disorders in Atherosclerosis. J. Cardiovasc. Pharmacol. 2020, 75, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Deng, Z.; Zou, Y.; Liu, C.; Fu, H.; Gu, Y.; Chang, H. Theaflavin alleviates oxidative injury and atherosclerosis progress via activating microRNA-24-mediated Nrf2/HO-1 signal. Phytother. Res. 2021, 35, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zhao, L.; Wang, C.; Hossen, I.; Raka, R.N.; Zhang, H. Stevia residue extract increases intestinal uric acid excretion via interactions with intestinal urate transporters in hyperuricemic mice. Food Funct. 2019, 10, 7900–7912. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Q.; Li, H.; Wen, C.; He, Z. Alterations of the Gut Microbiome Associated with the Treatment of Hyperuricaemia in Male Rats. Front. Microbiol. 2018, 9, 2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Li, X.Y.; Shen, L. Modulation effect of tea consumption on gut microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 981–987. [Google Scholar] [CrossRef]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [Green Version]
- Henning, S.M.; Yang, J.; Hsu, M.; Lee, R.P.; Grojean, E.M.; Ly, A.; Tseng, C.H.; Heber, D.; Li, Z. Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice. Eur. J. Nutr. 2018, 57, 2759–2769. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhong, H.; Chen, F.; Regenstein, J.; Hu, X.; Cai, L.; Feng, F. The gut microbiota as a target to control hyperuricemia pathogenesis: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2021, 62, 3979–3989. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin modulates gut microbiota and improves renal function in rats with uric acid nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Chen, R.; Li, Q.; Lai, X.; Sun, L.; Zhang, Z.; Wen, S.; Sun, S.; Cao, F. Tea (Camellia sinensis) Ameliorates Hyperuricemia via Uric Acid Metabolic Pathways and Gut Microbiota. Nutrients 2022, 14, 2666. https://doi.org/10.3390/nu14132666
Wu D, Chen R, Li Q, Lai X, Sun L, Zhang Z, Wen S, Sun S, Cao F. Tea (Camellia sinensis) Ameliorates Hyperuricemia via Uric Acid Metabolic Pathways and Gut Microbiota. Nutrients. 2022; 14(13):2666. https://doi.org/10.3390/nu14132666
Chicago/Turabian StyleWu, Dan, Ruohong Chen, Qiuhua Li, Xingfei Lai, Lingli Sun, Zhenbiao Zhang, Shuai Wen, Shili Sun, and Fanrong Cao. 2022. "Tea (Camellia sinensis) Ameliorates Hyperuricemia via Uric Acid Metabolic Pathways and Gut Microbiota" Nutrients 14, no. 13: 2666. https://doi.org/10.3390/nu14132666
APA StyleWu, D., Chen, R., Li, Q., Lai, X., Sun, L., Zhang, Z., Wen, S., Sun, S., & Cao, F. (2022). Tea (Camellia sinensis) Ameliorates Hyperuricemia via Uric Acid Metabolic Pathways and Gut Microbiota. Nutrients, 14(13), 2666. https://doi.org/10.3390/nu14132666