Green Tea Extract Containing Piper retrofractum Fruit Ameliorates DSS-Induced Colitis via Modulating MicroRNA-21 Expression and NF-κB Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. GTP Preparation
2.2. Animals
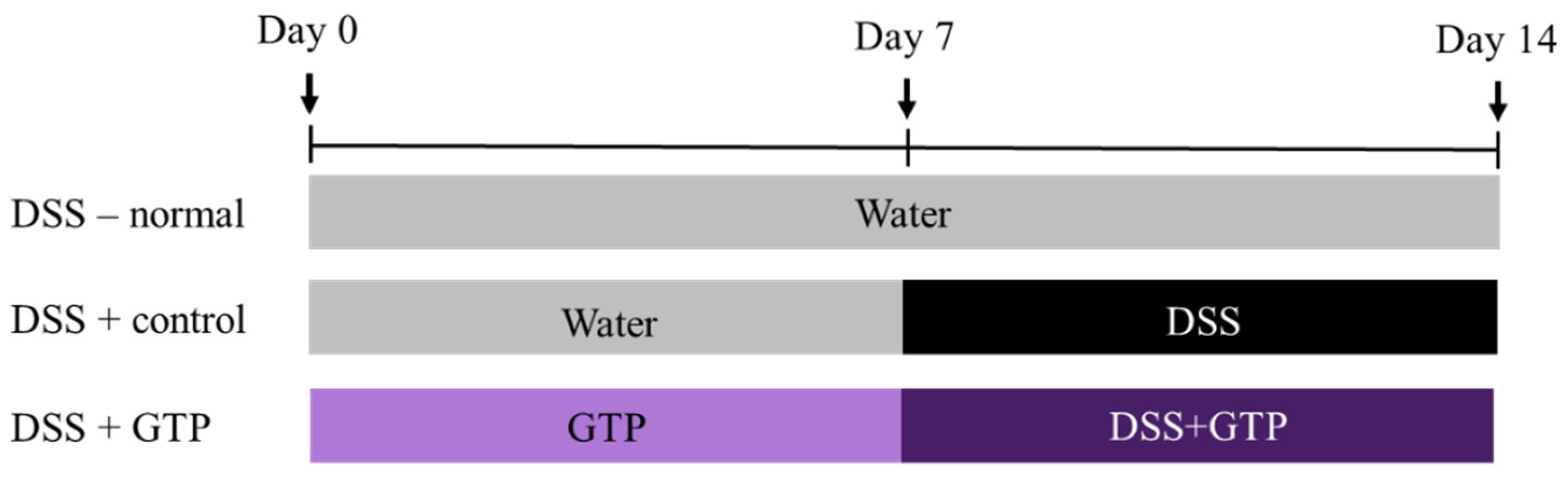
2.3. Experimental Design
2.4. Clinical Evaluation
2.5. Aspartate Aminotransferase (AST) and Alanine Aminotransaminase (ALT) Assay
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. NO Determination
2.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analysis
3. Results
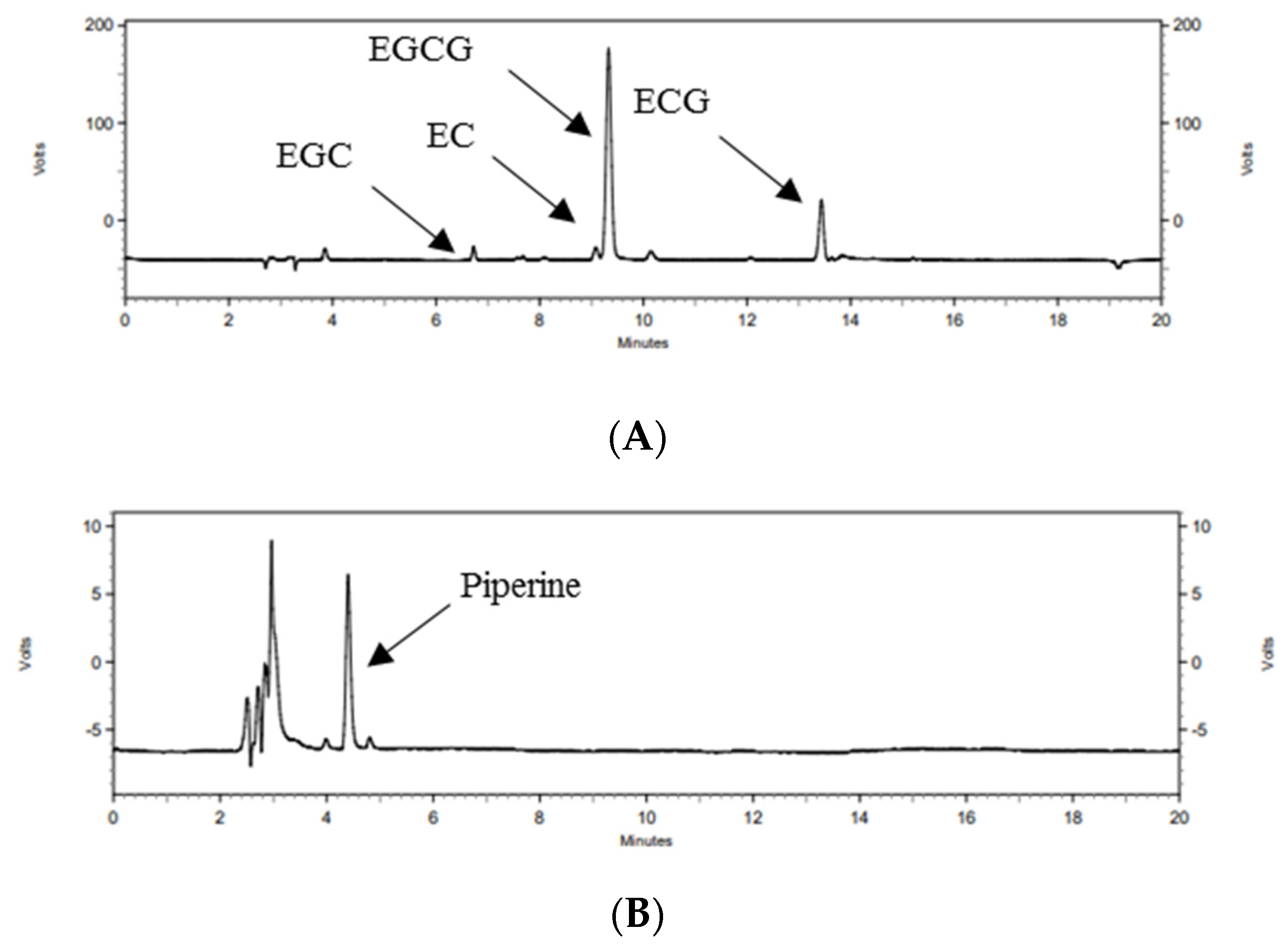
3.1. Contents of Green Tea Catechin and Piperine in GTP
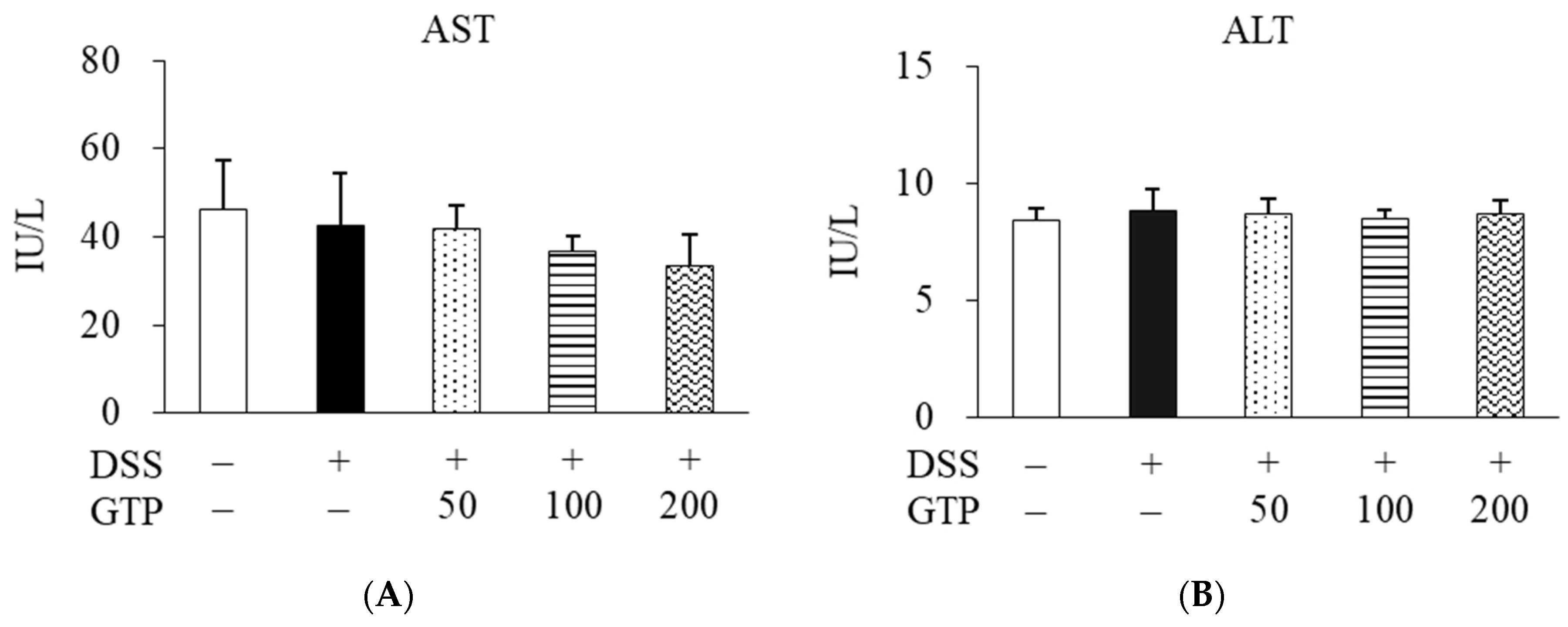
3.2. Effect of GTP on AST and ALT Activities
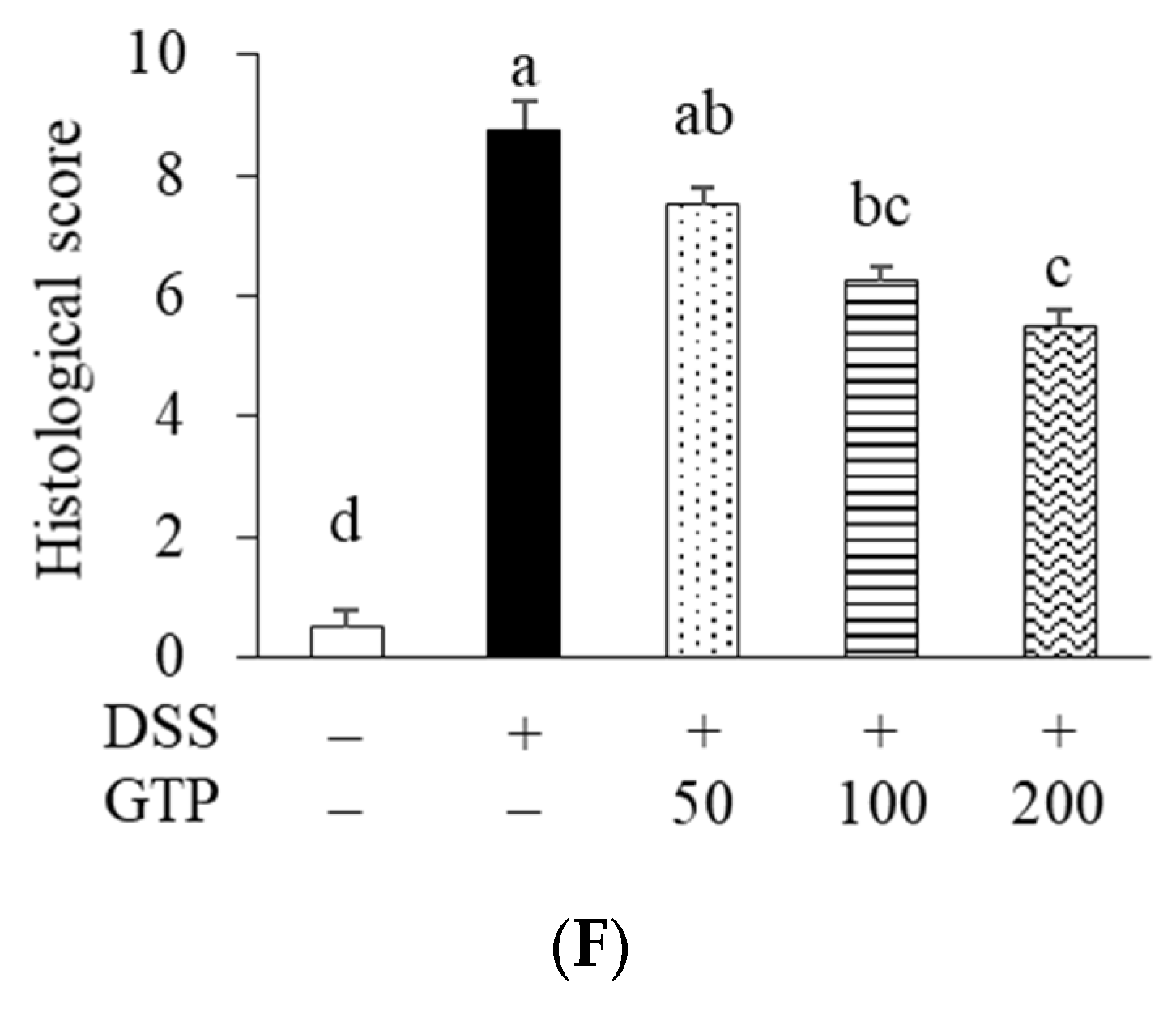
3.3. GTP Ameliorated Clinical Symptoms in DSS-Induced Colitis Mice
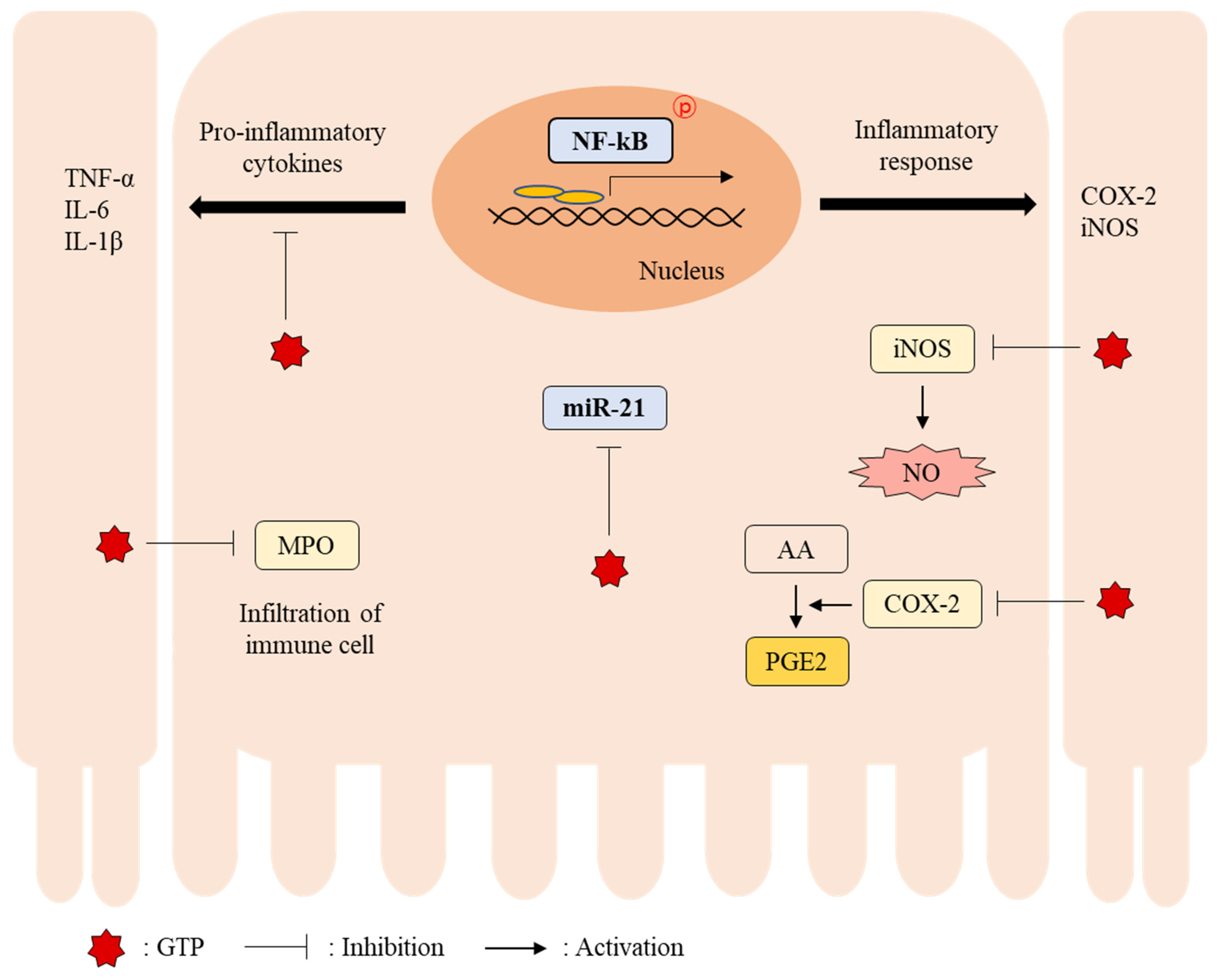
3.4. GTP Suppressed Colonic MiR-21 Expression and NF-κB Activity
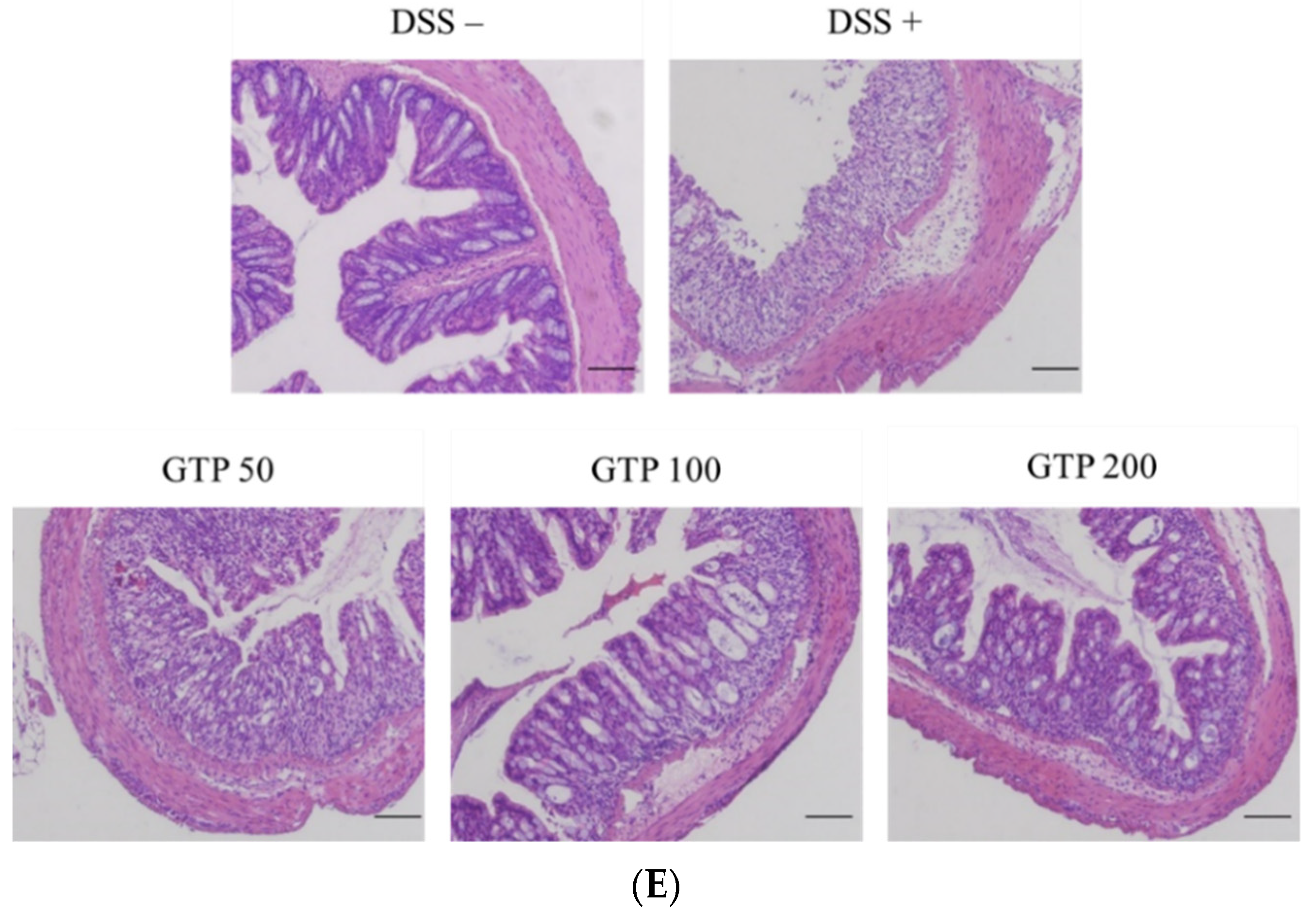
3.5. GTP Reduced Colonic Inflammation in DSS-Induced Colitis Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keshteli, A.H.; Madsen, K.L.; Dieleman, L.A. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients 2019, 11, 1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loddo, I.; Romano, C. Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, S.M.; Bornemann, P.H. Ulcerative colitis. Am. Fam Physician 2013, 87, 699–705. [Google Scholar]
- De Campos Silva, E.F.; Baima, J.P.; de Barros, J.R.; Tanni, S.E.; Schreck, T.; Saad-Hossne, R.; Sassaki, L.Y. Risk factors for ulcerative colitis-associated colorectal cancer: A retrospective cohort study. Medicine 2020, 99, e21686. [Google Scholar] [CrossRef]
- Fodor, A.; Lazar, A.L.; Buchman, C.; Tiperciuc, B.; Orasan, O.H.; Cozma, A. MicroRNAs: The Link Between the Metabolic Syndrome and Oncogenesis. Int. J. Mol. Sci. 2021, 22, 6337. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Liang, Y.; Yang, J.; Xia, Y.; Chen, H.; Han, H.; Yang, Y.; Wu, W.; Gao, R.; Qin, H. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS ONE 2013, 8, e66814. [Google Scholar] [CrossRef] [Green Version]
- Thorlacius-Ussing, G.; Schnack Nielsen, B.; Andersen, V.; Holmstrøm, K.; Pedersen, A.E. Expression and Localization of miR-21 and miR-126 in Mucosal Tissue from Patients with Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2017, 23, 739–752. [Google Scholar] [CrossRef] [Green Version]
- James, J.P.; Riis, L.B.; Malham, M.; Høgdall, E.; Langholz, E.; Nielsen, B.S. MicroRNA Biomarkers in IBD-Differential Diagnosis and Prediction of Colitis-Associated Cancer. Int. J. Mol. Sci. 2020, 21, 7893. [Google Scholar] [CrossRef]
- Bocchetti, M.; Ferraro, M.G.; Ricciardiello, F.; Ottaiano, A.; Luce, A.; Cossu, A.M.; Scrima, M.; Leung, W.Y.; Abate, M.; Stiuso, P.; et al. The Role of microRNAs in Development of Colitis-Associated Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 3967. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.Y.; Chung, K.S.; Shin, J.S.; Park, G.; Jang, Y.P.; Lee, K.T. Anti-Colitic Effects of Ethanol Extract of Persea americana Mill. through Suppression of Pro-Inflammatory Mediators via NF-κB/STAT3 Inactivation in Dextran Sulfate Sodium-Induced Colitis Mice. Int. J. Mol. Sci. 2019, 20, 177. [Google Scholar] [CrossRef] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Oz, H.S.; Chen, T.; de Villiers, W.J. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [Green Version]
- Bao, N.; Ochir, S.; Sun, Z.; Borjihan, G.; Yamagishi, T. Occurrence of piperidine alkaloids in Piper species collected in different areas. J. Nat. Med. 2014, 68, 211–214. [Google Scholar] [CrossRef]
- Amaliyah, S.; Pangesti, D.P.; Masruri, M.; Sabarudin, A.; Sumitro, S.B. Green synthesis and characterization of copper nanoparticles using Piper retrofractum Vahl extract as bioreductor and capping agent. Heliyon 2020, 6, e04636. [Google Scholar] [CrossRef]
- Hu, D.; Wang, Y.; Chen, Z.; Ma, Z.; You, Q.; Zhang, X.; Liang, Q.; Tan, H.; Xiao, C.; Tang, X.; et al. The protective effect of piperine on dextran sulfate sodium induced inflammatory bowel disease and its relation with pregnane X receptor activation. J. Ethnopharmacol. 2015, 169, 109–123. [Google Scholar] [CrossRef]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.D.; Hong, J.; Kim, D.H.; Mishin, V.M.; Yang, C.S. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J. Nutr. 2004, 134, 1948–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- López-García, G.; Cilla, A.; Barberá, R.; Alegría, A.; Recio, M.C. Effect of a Milk-Based Fruit Beverage Enriched with Plant Sterols and/or Galactooligosaccharides in a Murine Chronic Colitis Model. Foods 2019, 8, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Lee, M.S.; Chang, E.; Jung, S.; Ko, H.; Lee, E.; Lee, S.; Kim, C.T.; Kim, I.H.; Kim, Y. Tartary Buckwheat Extract Attenuated the Obesity-Induced Inflammation and Increased Muscle PGC-1a/SIRT1 Expression in High Fat Diet-Induced Obese Rats. Nutrients 2019, 11, 654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Lee, M.S.; Jung, S.; Son, H.Y.; Park, S.; Kang, B.; Kim, S.Y.; Kim, I.H.; Kim, C.T.; Kim, Y. Ginger Extract Ameliorates Obesity and Inflammation via Regulating MicroRNA-21/132 Expression and AMPK Activation in White Adipose Tissue. Nutrients 2018, 10, 1567. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ma, X.; Becker Buscaglia, L.E.; Barker, J.R.; Li, Y. MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 2011, 3, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Du, L.; Li, J.; Song, H. Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem. Toxicol. 2016, 96, 70–78. [Google Scholar] [CrossRef]
- Hatab, H.M.; Abdel Hamid, F.F.; Soliman, A.F.; Al-Shafie, T.A.; Ismail, Y.M.; El-Houseini, M.E. A combined treatment of curcumin, piperine, and taurine alters the circulating levels of IL-10 and miR-21 in hepatocellular carcinoma patients: A pilot study. J. Gastrointest. Oncol. 2019, 10, 766–776. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bosso, H.; Salzedas-Pescinini, L.M.; de Alvares Goulart, R. Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases. Complement. Ther. Med. 2019, 43, 148–153. [Google Scholar] [CrossRef]
- Varthya, S.B.; Sarma, P.; Bhatia, A.; Shekhar, N.; Prajapat, M.; Kaur, H.; Thangaraju, P.; Kumar, S.; Singh, R.; Siingh, A.; et al. Efficacy of green tea, its polyphenols and nanoformulation in experimental colitis and the role of non-canonical and canonical nuclear factor kappa beta (NF-kB) pathway: A preclinical in-vivo and in-silico exploratory study. J. Biomol. Struct. Dyn. 2021, 39, 5314–5326. [Google Scholar] [CrossRef] [PubMed]
- Gerges Geagea, A.; Rizzo, M.; Eid, A.; Hajj Hussein, I.; Zgheib, Z.; Zeenny, M.N.; Jurjus, R.; Uzzo, M.L.; Spatola, G.F.; Bonaventura, G.; et al. Tea catechins induce crosstalk between signaling pathways and stabilize mast cells in ulcerative colitis. J. Biol. Regul. Homeost. Agents 2017, 31, 865–877. [Google Scholar] [PubMed]
- Bitzer, Z.T.; Elias, R.J.; Vijay-Kumar, M.; Lambert, J.D. (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss. Mol. Nutr. Food Res. 2016, 60, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, B.S.; Aguilera Olvera, R.; Singh, V.; Xiao, X.; Kennett, M.J.; Joe, B.; Lambert, J.D.; Vijay-Kumar, M. Epigallocatechin-3-Gallate Inhibition of Myeloperoxidase and Its Counter-Regulation by Dietary Iron and Lipocalin 2 in Murine Model of Gut Inflammation. Am. J. Pathol. 2016, 186, 912–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Shi, F.; Zhu, J.; Shao, Y.; Gong, W.; Zhou, G.; Wu, H.; She, J.; Shi, W. Piperine, a functional food alkaloid, exhibits inhibitory potential against TNBS-induced colitis via the inhibition of IκB-α/NF-κB and induces tight junction protein (claudin-1, occludin, and ZO-1) signaling pathway in experimental mice. Hum. Exp. Toxicol. 2020, 39, 477–491. [Google Scholar] [CrossRef]
- Gupta, R.A.; Motiwala, M.N.; Dumore, N.G.; Danao, K.R.; Ganjare, A.B. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J. Ethnopharmacol. 2015, 164, 239–246. [Google Scholar] [CrossRef]



| Score | Weight Loss (%) | Stool Consistency | Rectal Bleeding |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1−5% | ||
| 2 | 5−10% | Loose | |
| 3 | 10−20% | ||
| 4 | >20% | Diarrhea | Gross bleeding |
| Name | GenBank No. | Primer Sequence (5′–3′) |
|---|---|---|
| β-actin | NM_007393 | F: GGACCTGACAGACTACCTCA |
| R: GTTGCCAATAGTGATGACCT | ||
| COX-2 | AF378830 | F: ACAGTAACATCAAACCGACC |
| R: GTGGAACCATTTCTAGGACA | ||
| IL-1β | M15131 | F: TCCTCCTTGCCTCTGATGGG |
| R: CATCCCCCACACGTTGACAG | ||
| IL-6 | NM_031168 | F: CCTTCCTACCCCAATTTCCA |
| R: TAACGCACTAGGTTTGCCGA | ||
| iNOS | NM_001313922 | F: CCACAGCAATATAGGCTCAT |
| R: GGATTTCAGCCTCATGGTAA | ||
| TNF-α | NM_013693 | F: AGCACAGAAAGCATGATCCG |
| R: GCCACAAGCAGGAATGAGAA |
| Compound | Content (mg/g) |
|---|---|
| EGCG | 522.04 ± 9.84 |
| ECG | 111.22 ± 2.06 |
| EGC | 105.67 ± 2.51 |
| EC | 53.06 ± 1.09 |
| Piperine | 2.05 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-S.; Lee, J.; Kim, Y. Green Tea Extract Containing Piper retrofractum Fruit Ameliorates DSS-Induced Colitis via Modulating MicroRNA-21 Expression and NF-κB Activity. Nutrients 2022, 14, 2684. https://doi.org/10.3390/nu14132684
Lee M-S, Lee J, Kim Y. Green Tea Extract Containing Piper retrofractum Fruit Ameliorates DSS-Induced Colitis via Modulating MicroRNA-21 Expression and NF-κB Activity. Nutrients. 2022; 14(13):2684. https://doi.org/10.3390/nu14132684
Chicago/Turabian StyleLee, Mak-Soon, Jumi Lee, and Yangha Kim. 2022. "Green Tea Extract Containing Piper retrofractum Fruit Ameliorates DSS-Induced Colitis via Modulating MicroRNA-21 Expression and NF-κB Activity" Nutrients 14, no. 13: 2684. https://doi.org/10.3390/nu14132684
APA StyleLee, M.-S., Lee, J., & Kim, Y. (2022). Green Tea Extract Containing Piper retrofractum Fruit Ameliorates DSS-Induced Colitis via Modulating MicroRNA-21 Expression and NF-κB Activity. Nutrients, 14(13), 2684. https://doi.org/10.3390/nu14132684





