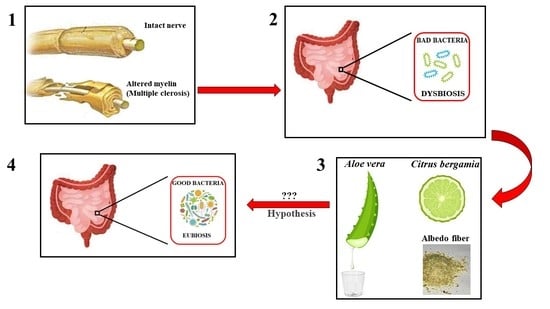
Involvement of the Intestinal Microbiota in the Appearance of Multiple Sclerosis: Aloe vera and Citrus bergamia as Potential Candidates for Intestinal Health
Abstract
:1. Introduction
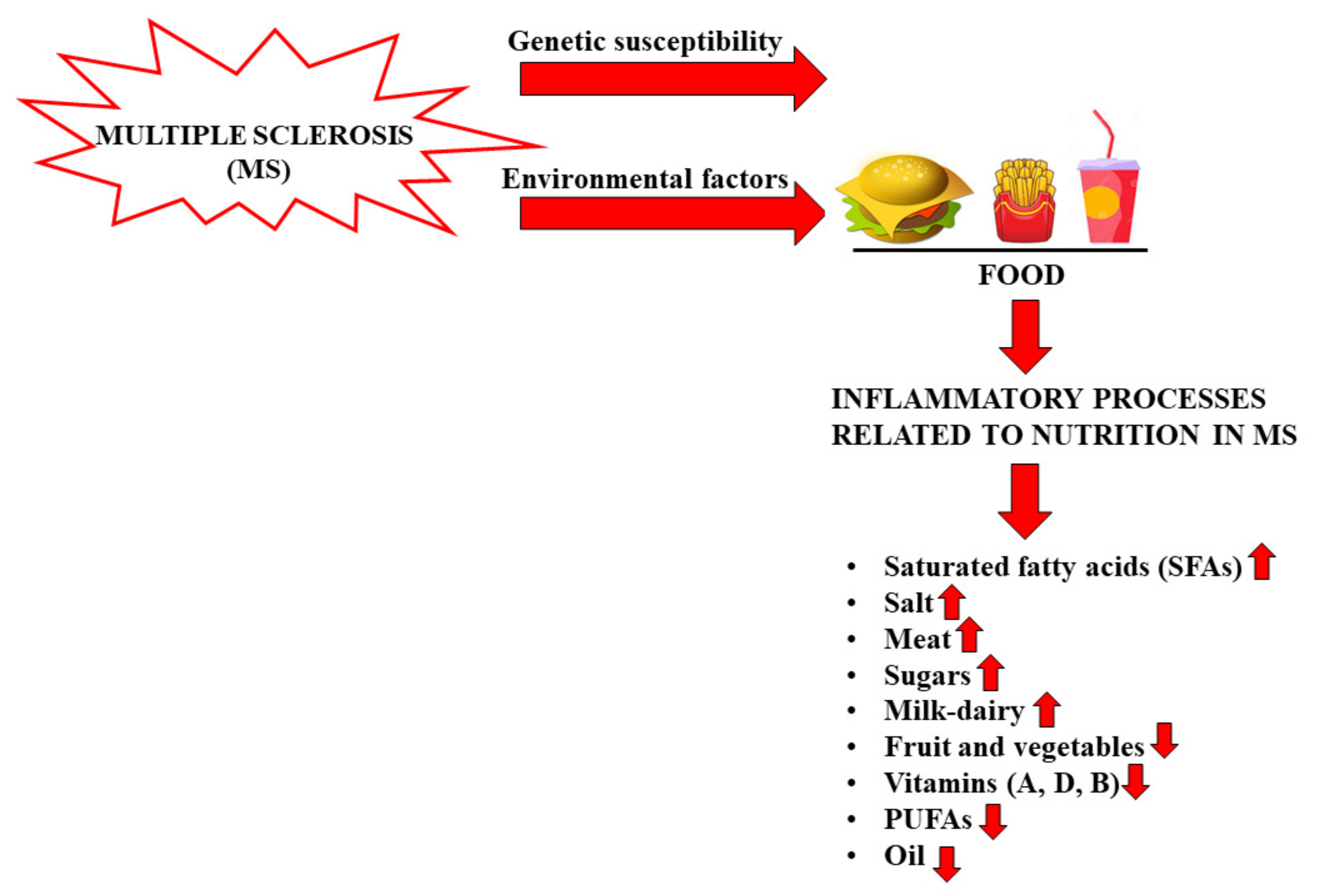
2. Nutrition and MS
Milk, Dairy Products, and MS
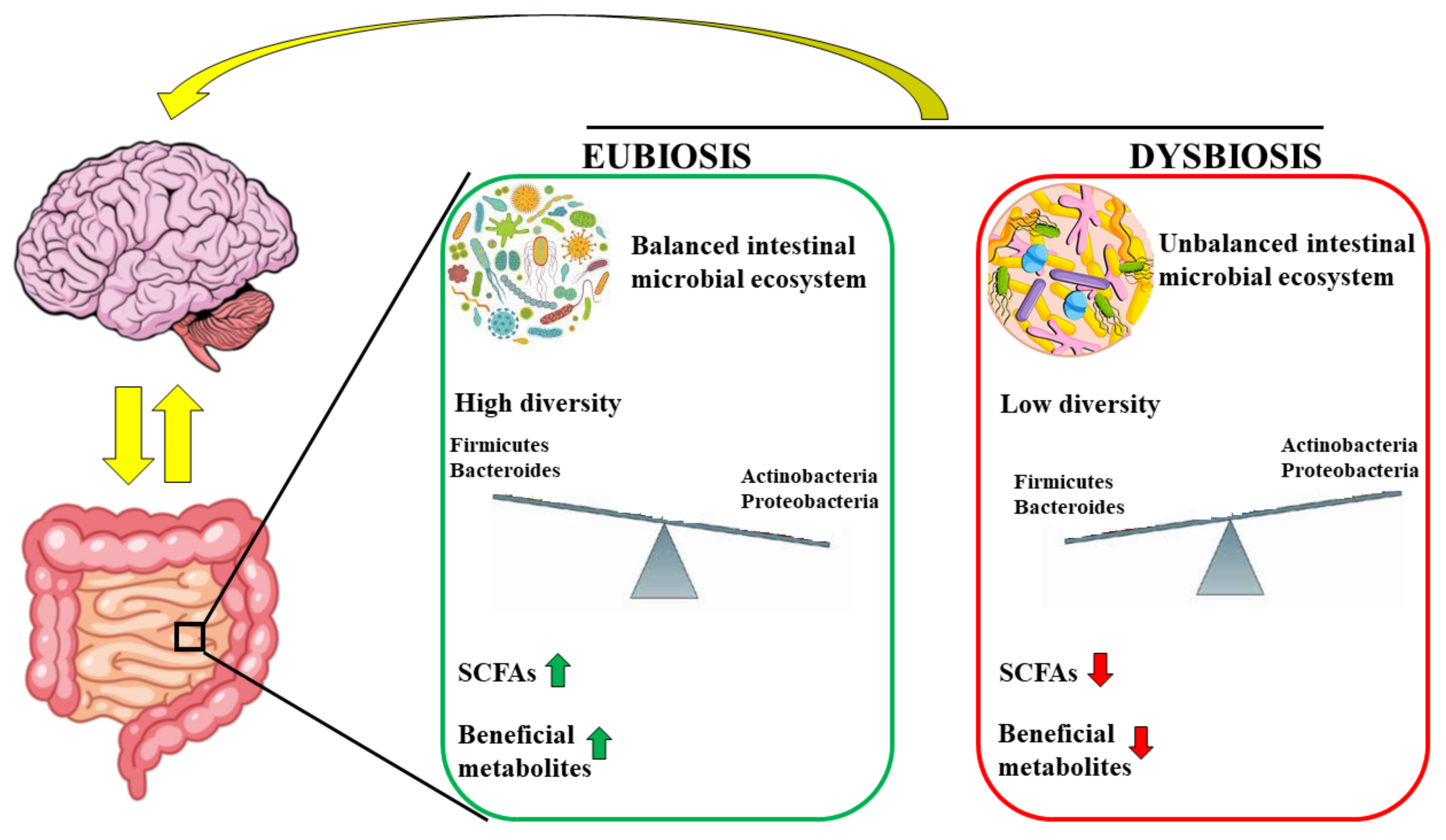
3. Gut Microbiota and Brain
3.1. Microbiota–Brain Communication
3.2. Gut Microbiota and MS
4. The Interaction of Plant Fibers with the Intestinal Microbiota
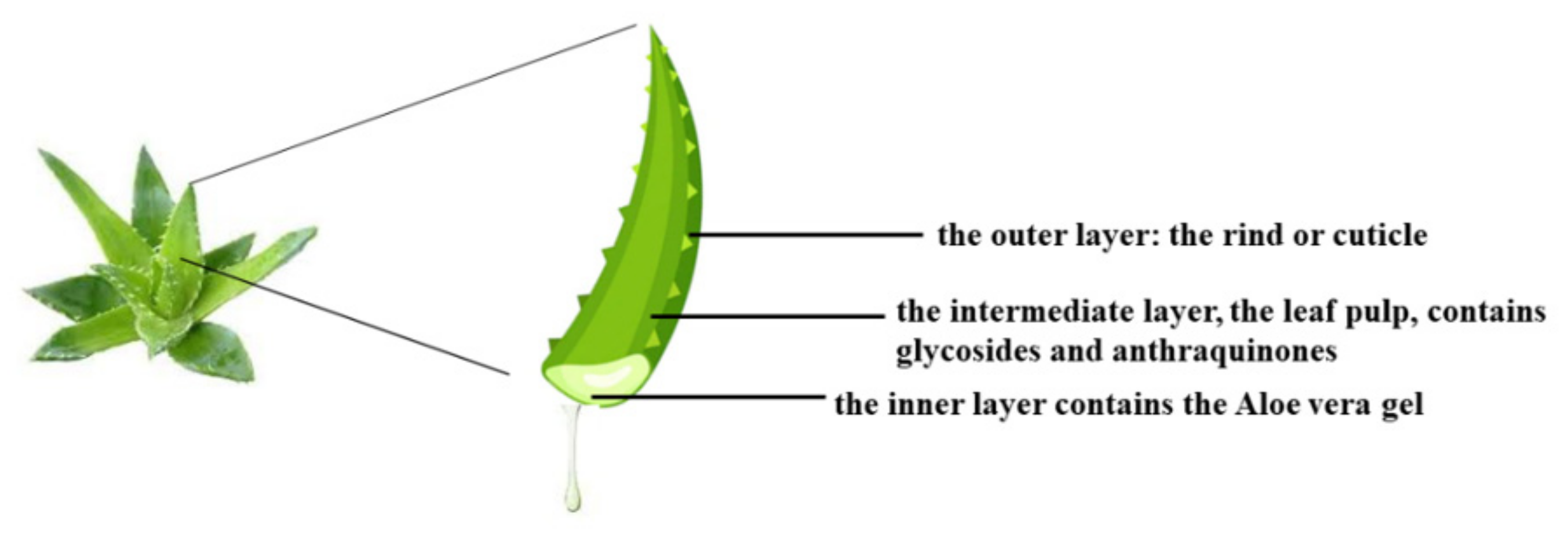
4.1. Involvement of Aloe vera with the Intestinal Microbiota
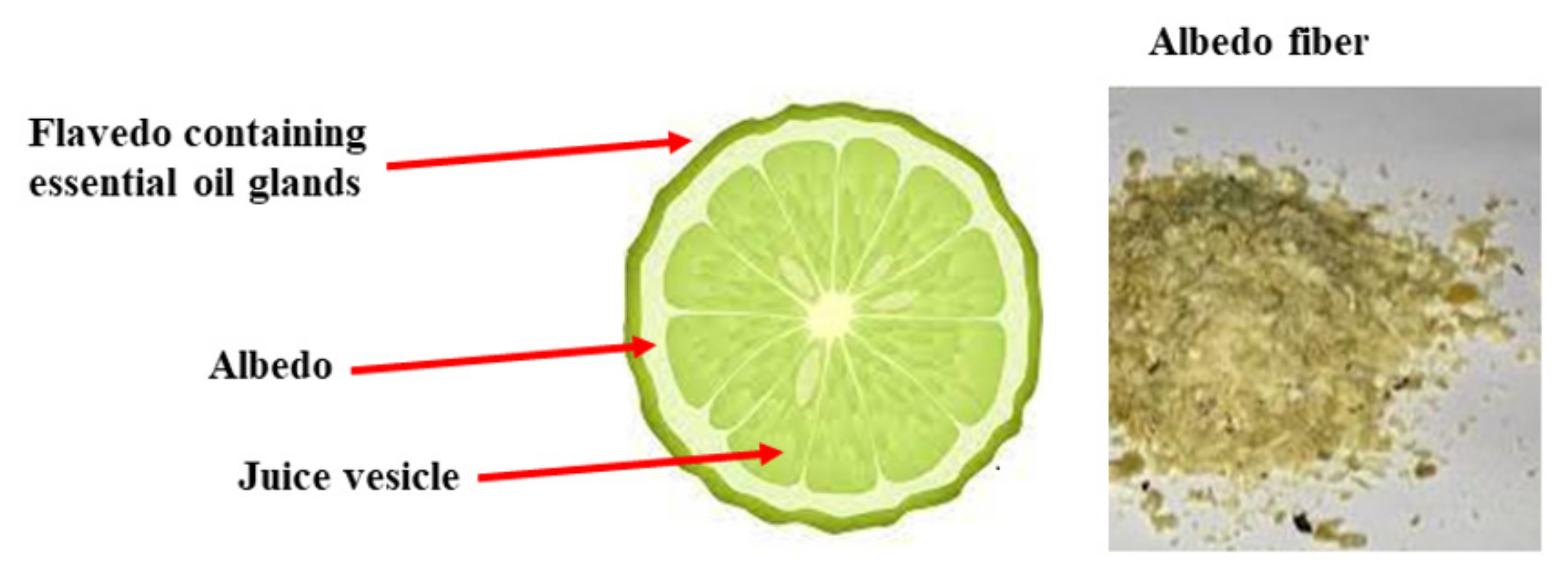
4.2. Involvement of Citrus bergamia in the Intestinal Microbiota
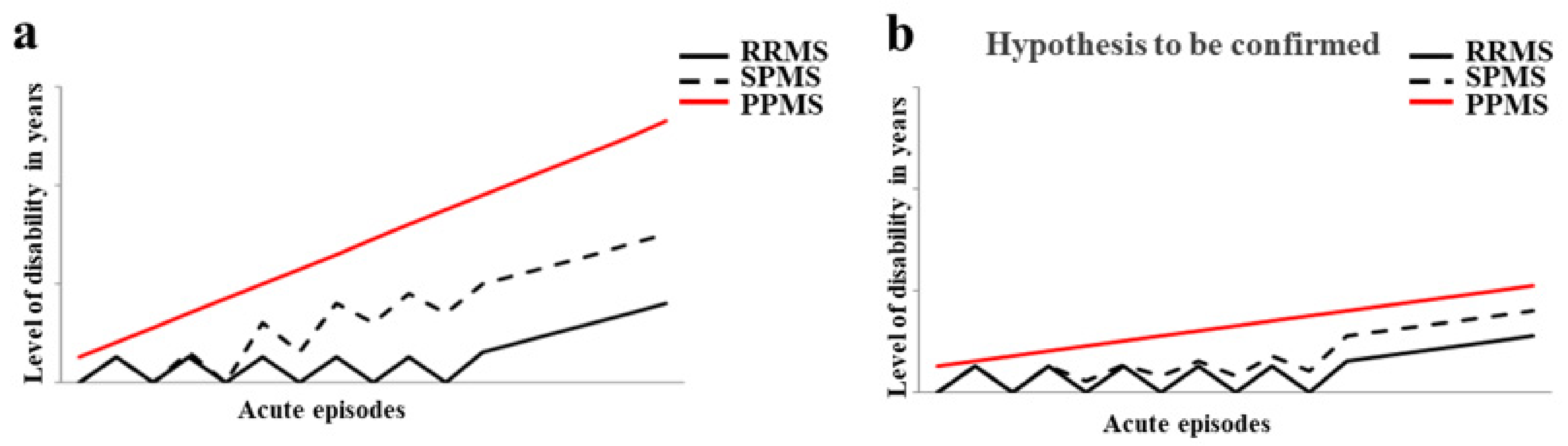
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Díaz, C.; Zarco, L.A.; Rivera, D.M. Highly active multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2019, 30, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Doshi, A.; Chataway, J. Multiple sclerosis, a treatable disease. Clin. Med. 2016, 16 (Suppl. S6), s53–s59. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, P.; Rossano, R. Nutrition Facts in Multiple Sclerosis. ASN Neuro 2015, 7, 1759091414568185. [Google Scholar] [CrossRef] [Green Version]
- Kamińska, J.; Koper, O.M.; Piechal, K.; Kemona, H. Multiple sclerosis—Etiology and diagnostic potential. Postepy Hig. Med. Dosw. 2017, 71, 551–563. [Google Scholar] [CrossRef]
- Solomon, A.J. Diagnosis, Differential Diagnosis, and Misdiagnosis of Multiple Sclerosis. Continuum 2019, 25, 611–635. [Google Scholar] [CrossRef]
- Naseri, A.; Nasiri, E.; Sahraian, M.A.; Daneshvar, S.; Talebi, M. Clinical Features of Late-Onset Multiple Sclerosis: A Systematic Review and Meta-analysis. Mult. Scler. Relat. Disord. 2021, 50, 102816. [Google Scholar] [CrossRef]
- Brola, W.; Steinborn, B. Pediatric multiple sclerosis—Current status of epidemiology, diagnosis and treatment. Neurol. Neurochir. Pol. 2020, 54, 508–517. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Simpson, A.; Mowry, E.; Newsome, S.D. Early Aggressive Treatment Approaches for Multiple Sclerosis. Curr. Treat. Options Neurol. 2021, 23, 19. [Google Scholar] [CrossRef]
- Ziemssen, T.; Derfuss, T.; de Stefano, N.; Giovannoni, G.; Palavra, F.; Tomic, D.; Vollmer, T.; Schippling, S. Optimizing treatment success in multiple sclerosis. J. Neurol. 2016, 263, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Bronwlee, W.S.; Swanton, J.K.; Altmann, D.R.; Ciccarelli, O.; Miller, D.H. Earlier and more frequent diagnosis of multiple sclerosis using the McDonald criteria. J. Neurol. Neurosurg. Psychiatry 2015, 86, 584–585. [Google Scholar] [CrossRef] [Green Version]
- Baldassari, L.E.; Fox, R.J. Therapeutic Advances and Challenges in the Treatment of Progressive Multiple Sclerosis. Drugs 2018, 78, 1549–1566. [Google Scholar] [CrossRef]
- Hemond, C.C.; Bakshi, R. Magnetic Resonance Imaging in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028969. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [Green Version]
- Ochoa-Repáraz, J.; Kirby, T.O.; Kasper, L.H. The Gut Microbiome and Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029017. [Google Scholar] [CrossRef]
- Ghareghani, M.; Reiter, R.J.; Zibara, K.; Farhadi, N. Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway. Front. Immunol. 2018, 9, 2484. [Google Scholar] [CrossRef]
- Bagur, M.J.; Murcia, M.A.; Jiménez-Monreal, A.M.; Tur, J.A.; Bibiloni, M.M.; Alonso, G.L.; Martínez-Tomé, M. Influence of Diet in Multiple Sclerosis: A Systematic Review. Adv. Nutr. 2017, 8, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Zhang, Z.; Cheng, L.; Zhang, X.; Liu, Y.; Zhang, R.; Weng, P.; Wu, Z. Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota. Food Res. Int. 2021, 149, 110675. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Constantin, D. Molecular Mechanisms of Muscle Fatigue. Int. J. Mol. Sci. 2021, 22, 11587. [Google Scholar] [CrossRef] [PubMed]
- Wahls, T.L.; Chenard, C.A.; Snetselaar, L.G. Review of Two Popular Eating Plans within the Multiple Sclerosis Community: Low Saturated Fat and Modified Paleolithic. Nutrients 2019, 11, 352. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, A.; Salvati, S. Exogenous lipids in myelination and myelination. Kaohsiung J. Med. Sci. 1997, 13, 19–29. [Google Scholar]
- Ruiz-Núñez, B. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Yadav, V.; Bourdette, D. Complementary and alternative medicine: Is there a role in multiple sclerosis? Curr. Neurol. Neurosci. Rep. 2006, 6, 259–267. [Google Scholar] [CrossRef]
- Oppedisano, F.; Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Macrì, R.; et al. The Potential for Natural Antioxidant Supplementation in the Early Stages of Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 2618. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Scicchitano, M.; Scarano, F.; Bosco, F.; Oppedisano, F.; Macrì, R.; et al. Environmental and Nutritional “Stressors” and Oligodendrocyte Dysfunction: Role of Mitochondrial and Endoplasmatic Reticulum Impairment. Biomedicines 2020, 8, 553. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, S.; Taylor, B.; Ponsonby, A.L.; Lucas, R.M.; Dwyer, T. An adverse lipid profile and increased levels of adiposity significantly predict clinical course after a first demyelinating event. J. Neurol. Neurosurg. Psychiatry 2017, 88, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Uher, T.; Fellows, K.; Horakova, D.; Zivadinov, R.; Vaneckova, M.; Sobisek, L. Serum lipid profile changes predict neurodegeneration in interferon-beta1a-treated multiple sclerosis patients. J. Lipid Res. 2017, 58, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Haghikia, A.; Jorg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015, 43, 817–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.M.; Mar, S.; Hart, J.; et al. Contribution of dietary intake to relapse rate in early paediatric multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28–33. [Google Scholar] [CrossRef]
- Neate, S.L.; Donald, A.; Jelinek, G.A.; Nag, N. Experiences of and attitudes to lifestyle modification for the management of multiple sclerosis: A qualitative analysis of free-text survey data. Health Expect. 2022, 25, 214–222. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Von Geldern, G.; Mowry, E.M. The influence of nutritional factors on the prognosis of multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 678–869. [Google Scholar] [CrossRef]
- De Carvalho, T.S. Calorie restriction or dietary restriction: How far they can protect the brain against neurodegenerative diseases? Neural Regen. Res. 2022, 17, 1640–1644. [Google Scholar] [CrossRef]
- Parks, N.E.; Jackson-Tarlton, C.S.; Verdad, R.; Johnston, B.C. Dietary interventions for multiple sclerosis-related outcomes. Cochrane Database Syst. Rev. 2020, 5, CD004192. [Google Scholar]
- Hidalgo, M.A.; Carretta, M.D.; Burgos, R.A. Long Chain Fatty Acids as Modulators of Immune Cells Function: Contribution of FFA1 and FFA4 Receptors. Front. Physiol. 2021, 12, 668330. [Google Scholar] [CrossRef]
- Tan, G.A.; Furber, K.L.; Thangaraj, M.P.; Sobchishin, L.; Doucette, J.R.; Nazarali, A.J. Organotypic Cultures from the Adult CNS: A Novel Model to Study Demyelination and Remyelination Ex Vivo. Cell. Mol. Neurobiol. 2018, 38, 317–328. [Google Scholar] [CrossRef]
- Penkert, H.; Bertrand, A.; Tiwari, V.; Breimann, S.; Müller, S.A.; Jordan, P.M.; Gerl, M.J.; Klose, C.; Cantuti-Castelvetri, L.; Bosch-Queralt, M.; et al. Proteomic and lipidomic profiling of demyelinating lesions identifies fatty acids as modulators in lesion recovery. Cell Rep. 2021, 37, 109898. [Google Scholar] [CrossRef]
- Masullo, L.; Papas, M.A.; Cotugna, N.; Baker, S.; Mahoney, L.; Trabulsi, J. Complementary and alternative medicine use and nutrient intake among individuals with multiple sclerosis in the United States. J. Community Health 2015, 40, 153–160. [Google Scholar] [CrossRef]
- Silbermann, E.; Senders, A.; Wooliscroft, L.; Rice, J.; Cameron, M.; Waslo, C.; Orban, A.; Chase, E.; Yadav, V.; Bourdette, D.; et al. Cross-sectional survey of complementary and alternative medicine used in Oregon and Southwest Washington to treat multiple sclerosis: A 17-Year update. Mult. Scler. Relat. Disord. 2020, 41, 102041. [Google Scholar] [CrossRef] [PubMed]
- Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadgkiss, E.J.; Jelinek, G.A.; Weiland, T.J.; Pereira, N.G.; Marck, C.H.; van der Meer, D.M. The association of diet with quality of life, disability, and relapse rate in an international sample of people with multiple sclerosis. Nutr. Neurosci. 2015, 18, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Noormohammadi, M.; Ghorbani, Z.; Naser Moghadasi, A.; Saeedirad, Z.; Shahemi, S.; Ghanaatgar, M.; Rezaeimanesh, N.; Hekmatdoost, A.; Ghaemi, A.; Razeghi Jahromi, S. MIND Diet Adherence Might be Associated with a Reduced Odds of Multiple Sclerosis: Results from a Case-Control Study. Neurol. Ther. 2022, 11, 397–412. [Google Scholar] [CrossRef]
- Sharifi, M.H.; Keshani, P.; Salehi, A.; Jaladat, A.M.; Mirzaei, Z.; Nikseresht, A. Association between multiple sclerosis and dietary patterns based on the traditional concept of food nature: A case-control study in Iran. BMC Neurol. 2021, 21, 453. [Google Scholar] [CrossRef]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.; Levasseur, V.; Cross, A.H.; Piccio, L. An overview of the current state of evidence for the role of specific diets in multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 36, 101393. [Google Scholar] [CrossRef]
- Fitzgerald, K.C.; Tyry, T.; Salter, A.; Cofield, S.S.; Cutter, G.; Fox, R.; Marrie, R.A. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology 2018, 90, e1–e11. [Google Scholar] [CrossRef]
- Gu, Y.; Brickman, A.M.; Stern, Y.; Habeck, C.G.; Razlighi, Q.R.; Luchsinger, J.A.; Manly, J.J.; Schupf, N.; Mayeux, R.; Scarmeas, N. Mediterranean diet and brain structure in a multiethnic elderly cohort. Neurology 2015, 85, 1744–1751. [Google Scholar] [CrossRef] [Green Version]
- García-Casares, N.; Gallego Fuentes, P.; Barbancho, M.Á.; López-Gigosos, R.; García-Rodríguez, A.; Gutiérrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. [Google Scholar] [CrossRef]
- Yadav, V.; Marracci, G.; Kim, E.; Spain, R.; Cameron, M.; Overs, S.; Riddehough, A.; Li, D.K.; McDougall, J.; Lovera, J.; et al. Low-fat, plant-based diet in multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2016, 9, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisht, B.; Darling, W.G.; Grossmann, R.E.; Shivapour, E.T.; Lutgendorf, S.K.; Snetselaar, L.G.; Hall, M.J.; Zimmerman, M.B.; Wahls, T.L. A Multimodal Intervention for Patients with Secondary Progressive Multiple Sclerosis: Feasibility and Effect on Fatigue. J. Altern. Complement. Med. 2014, 20, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Feige, J.; Moser, T.; Bieler, L.; Schwenker, K.; Hauer, L.; Sellner, J. Vitamin D Supplementation in Multiple Sclerosis: A Critical Analysis of Potentials and Threats. Nutrients 2020, 12, 783. [Google Scholar] [CrossRef] [Green Version]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef] [Green Version]
- Smolders, J.; Torkildsen, O.; Camu, W.; Holmoy, T. An update on vitamin D and disease activity in Multiple sclerosis. CNS Drugs 2019, 33, 1187–1199. [Google Scholar] [CrossRef] [Green Version]
- Ascherio, A.; Munger, K.L. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention-An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [Green Version]
- Mititelu, R.R.; Albu, C.V.; Bacanoiu, M.V.; Padureanu, V.; Padureanu, R.; Olaru, G.; Buga, A.M.; Balasoiu, M. Homocysteine as a Predictor Tool in Multiple Sclerosis. Discoveries 2021, 9, e135. [Google Scholar] [CrossRef]
- Saboor-Yaraghi, A.A.; Harirchian, M.H.; Mohammadzadeh Honarvar, N.; Bitarafan, S.; Abdolahi, M.; Siassi, F.; Salehi, E.; Sahraian, M.A.; Eshraghian, M.R.; Roostaei, T.; et al. The effect of vitamin A supplementation on FoxP3 and TGF-b gene expression in avonex-treated multiple sclerosis patients. J. Mol. Neurosci. 2015, 56, 608–612. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Städler, B. Artificial Biology: Molecular Design and Cell Mimicry. Small 2020, 16, e2003442. [Google Scholar] [CrossRef]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, K.S.; Sang, Y.; Straus, S.K. Viral Proteins with PxxP and PY Motifs May Play a Role in Multiple Sclerosis. Viruses 2022, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Rigante, D.; Esposito, S. Infections and Systemic Lupus Erythematosus: Binding or Sparring Partners? Int. J. Mol. Sci. 2015, 16, 17331–17343. [Google Scholar] [CrossRef] [Green Version]
- Libbey, J.E.; Fujinami, R.S. Viral mouse models used to study multiple sclerosis: Past and present. Arch. Virol. 2021, 166, 1015–1033. [Google Scholar] [CrossRef]
- Steinman, L.; Utz, P.J.; Robinson, W.H. Suppression of autoimmunity via microbial mimics of altered peptide ligands. Curr. Top. Microbiol. Immunol. 2005, 296, 55–63. [Google Scholar]
- Butcher, P.J. Milk consumption and multiple sclerosis: An etiological hypothesis. Med. Hypotheses 1986, 19, 169–178. [Google Scholar] [CrossRef]
- Malosse, D.; Perron, H.; Sasco, A.; Seigneurin, J.M. Correlation between milk and dairy product consumption and multiple sclerosis prevalence: A worldwide study. Neuroepidemiology 1992, 11, 304–312. [Google Scholar] [CrossRef]
- Winer, S.; Astsaturov, I.; Cheung, R.K.; Schrade, K.; Gunaratnam, L.; Wood, D.D.; Moscarello, M.A.; O’Connor, P.; McKerlie, C.; Becker, D.J.; et al. T Cells of Multiple Sclerosis Patients Target a Common Environmental Peptide that Causes Encephalitis in Mice. J. Immunol. 2001, 166, 4751–4756. [Google Scholar] [CrossRef] [Green Version]
- Magyari, M. Role of socio-economic and reproductive factors in the risk of multiple sclerosis. Acta Neurol. Scand. 2015, 132, 20–23. [Google Scholar] [CrossRef] [Green Version]
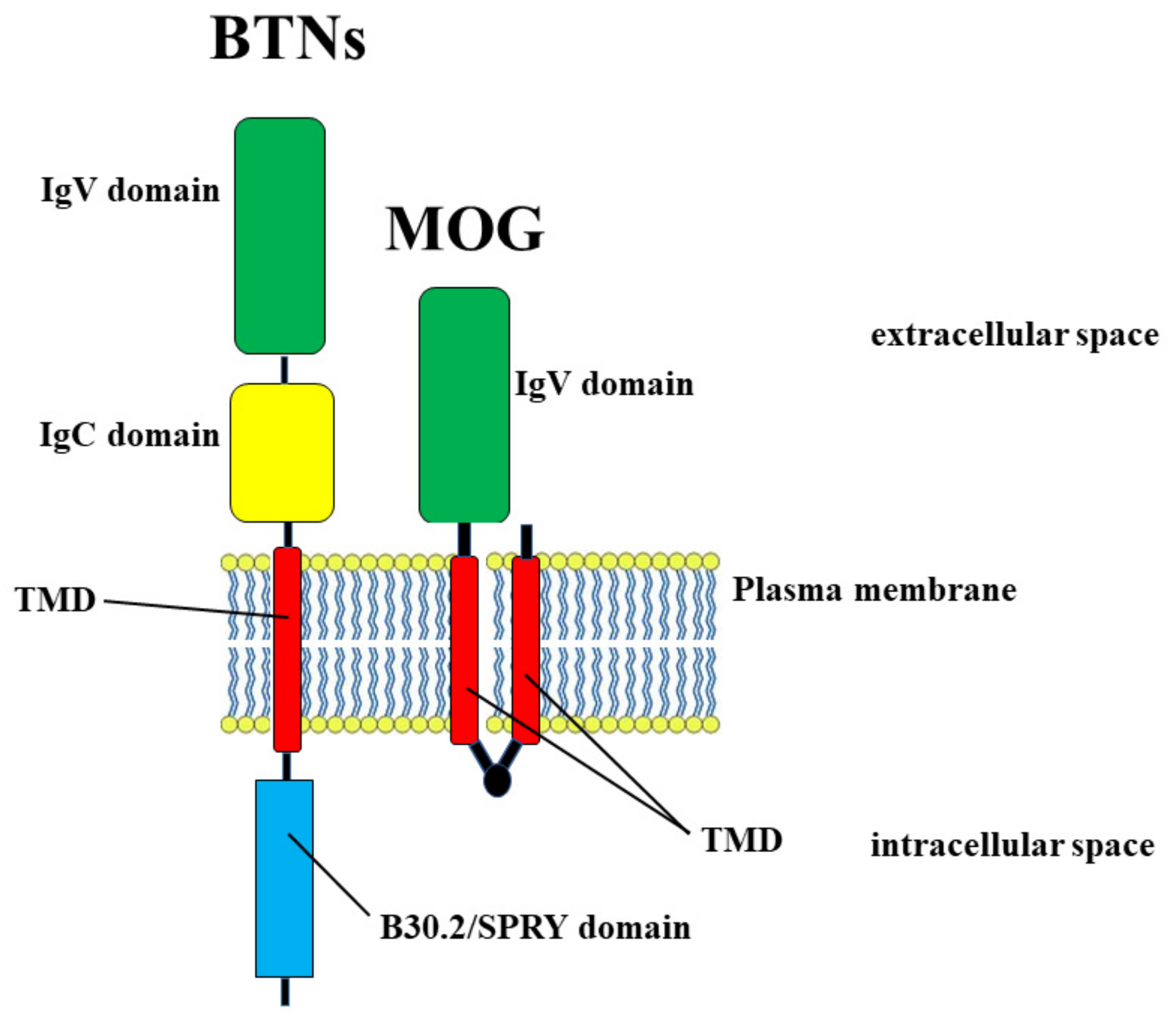
- Redwan, E.M.; Al-Hejin, A.M.; Almehdar, H.A.; Elsaway, A.M.; Uversky, V.N. Prediction of Disordered Regions and Their Roles in the Anti-Pathogenic and Immunomodulatory Functions of Butyrophilins. Molecules 2018, 23, 328. [Google Scholar] [CrossRef] [Green Version]
- Stefferl, A.; Schubart, A.; Storch, M.; Amini, A.; Mather, I.; Lassmann, H.; Linington, H. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J. Immunol. 2000, 165, 2859–2865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guggenmos, J.; Schubart, A.S.; Ogg, S.; Andersson, M.; Olsson, T.; Mather, I.H.; Linington, C. Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. J. Immunol. 2004, 172, 661–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlach, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human Milk Oligosaccharides and Bacterial Profile Modulate Infant Body Composit.ion during Exclusive Breastfeeding. Int. J. Mol. Sci. 2022, 23, 2865. [Google Scholar] [CrossRef] [PubMed]
- Vantourout, P.; Laing, A.; Woodward, M.J.; Zlatareva, I.; Apolonia, L.; Jones, A.W.; Snijders, A.P.; Malim, M.H.; Hayday, A.C. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing γδ T cell biology. Proc. Natl. Acad. Sci. USA 2018, 115, 1039–1044. [Google Scholar] [CrossRef] [Green Version]
- Wynford-Thomas, D.A.; Reith, W.; Trowsdale, J. Regulation of Immunity by Butyrophilins. Annu. Rev. Immunol. 2016, 34, 151–172. [Google Scholar]
- Chunder, R.; Weier, A.; Mäurer, H.; Luber, N.; Enders, M.; Luber, G.; Heider, T.; Spitzer, A.; Tacke, S.; Becker-Gotot, J.; et al. Antibody cross-reactivity between casein and myelin-associated glycoprotein results in central nervous system demyelination. Proc. Natl. Acad. Sci. USA 2022, 119, e2117034119. [Google Scholar] [CrossRef]
- Mañá, P.; Goodyear, M.; Bernard, C.; Tomioka, R.; Freire-Garabal, M.; Liñares, D. Tolerance induction by molecular mimicry: Prevention and suppression of experimental autoimmune encephalomyelitis with the milk protein butyrophilin. Int. Immunol. 2004, 16, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Mosca, F.; Giannì, M.L. Human milk: Composition and health benefits. Pediatr. Med. Chir. 2017, 39, 155. [Google Scholar] [CrossRef] [Green Version]
- Na, S.; Na, L.; Xinwang, D.; Haitao, N. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017, 4, 14. [Google Scholar]
- Kim, S.; Jazwinski, S.M. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology 2018, 64, 513–520. [Google Scholar] [CrossRef]
- Davenport, E.R.; Mizrahi-Man, O.; Michelini, K.; Barreiro, L.B.; Ober, C.; Gilad, Y. Seasonal Variation in Human Gut Microbiome Composition. PLoS ONE 2014, 9, e90731. [Google Scholar] [CrossRef]
- Iebba, V.; Totino, V.; Gagliardi, A.; Santangelo, F.; Cacciotti, F.; Trancassini, M. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016, 39, 1–12. [Google Scholar]
- Ron, R.; Fragman-Sapir, O.; Kadmon, R. Dispersal increases ecological selection by increasing effective community size. Proc. Natl. Acad. Sci. USA 2018, 115, 11280–11285. [Google Scholar] [CrossRef] [Green Version]
- Obrenovich, M.E.M. Leaky Gut, Leaky Brain? Microorganisms 2018, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef]
- Belkaid, Y.; Harrison, O.J. Homeostatic immunity and the microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.W.; Clemente, J.C.; Peter, I.; Loos, R.J.F. The prenatal gut microbiome: Are we colonized with bacteria in utero? Pediatr. Obes. 2017, 12, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Brahm, P.; Valdés, V. Benefits of breastfeeding and risks associated with not breastfeeding. Rev. Chil. Pediatr. 2017, 88, 15–21. [Google Scholar]
- Vieira Borba, V.; Sharif, K.; Shoenfeld, Y. Breastfeeding and autoimmunity: Programing health from the beginning. Am. J. Reprod. Immunol. 2017, 79, e12778. [Google Scholar] [CrossRef]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s Milk: A Purposeful Contribution to the Development of the Infant Microbiota and Immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayres, S.; Visentin, L. Breastfeeding: Uncovering barriers and offering solutions. Curr. Opin. Pediatr. 2018, 30, 591–596. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Petra, A.I.; Panagiotidou, S.; Hatziagelaki, E.; Stewart, J.M.; Conti, P.; Theoharides, T.C. Gut-Microbiota-Brain Axis and Its Effect on Neuropsychiatric Disorders with Suspected Immune Dysregulation. Clin. Ther. 2015, 37, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.I.; Li, L.; Azmal, S.A.; Yao, L. Differential expression of toll-like receptor 13 and ribosomal protein L29 in inflammatory lung and brain. J. Biol. Regul. Homeost. Agents 2019, 33, 1023–1040. [Google Scholar]
- Julio-Pieper, M.; Bravo, J.A.; Aliaga, E.; Gotteland, M. Review article: Intestinal barrier dysfunction and central nervous system disorders—A controversial association. Aliment. Pharmacol. Ther. 2014, 40, 1187–1201. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Dupont, H.L. New Approaches for Bacteriotherapy: Prebiotics, New-Generation Probiotics, and Synbiotics. Clin. Infect. Dis. 2015, 60, S108–S121. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883. [Google Scholar] [CrossRef]
- Giuffrè, M.; Moretti, R.; Campisciano, G.; da Silveira, A.B.M.; Monda, V.M.; Comar, M.; Di Bella, S.; Antonello, R.M.; Luzzati, R.; Crocè, L.S. You Talking to Me? Says the Enteric Nervous System (ENS) to the Microbe. How Intestinal Microbes Interact with the ENS. J. Clin. Med. 2020, 9, 3705. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stilling, R.M.; Stanton, C. Collective unconscious: How gut microbes shape human behavior. J. Psychiatr. Res. 2015, 63, 1–9. [Google Scholar] [CrossRef] [Green Version]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014, 5, 146. [Google Scholar] [CrossRef]
- Wu, H.J.; Ivanov, I.I.; Hattori, K.; Shima, T.; Umesaki, Y.; Littman, D.R.; Benoist, C.; Mathis, D. Gut-residing segmented filamentous bacteria can drive autoimmune arthritis via T helper 17 cells. Immunity 2010, 32, 815–827. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Microbes and Health Sackler Colloquium: Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108, 4615–4622. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef] [Green Version]
- Kriegel, M.A.; Sefik, E.; Hill, J.A.; Wu, H.J.; Benoist, C.; Mathis, D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 2011, 108, 11548–11553. [Google Scholar] [CrossRef] [Green Version]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Hart, J.; Roalstad, S.; Graves, J.; Spencer, C.M.; Lynch, S.V.; Zamvil, S.S.; Waubant, E. Associations between the gut microbiota and host immune markers in pediatric multiple sclerosis and controls. BMC Neurol. 2016, 16, 182. [Google Scholar] [CrossRef] [Green Version]
- Tsunoda, I. Lymphatic system and gut microbiota affect immunopathology of neuroinflammatory diseases, including multiple sclerosis, neuromyelitis optica and Alzheimer’s disease. Clin. Exp. Neuroimmunol. 2017, 8, 177–179. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, J.; Liu, H.; He, F.; Chen, A.; Yang, H.; Pi, B. Meta-analysis of FOXP3 gene rs3761548 and rs2232365 polymorphism and multiple sclerosis susceptibility. Medicine 2019, 98, e17224. [Google Scholar] [CrossRef]
- Khan, U.; Ghazanfar, H.T. Lymphocytes and Autoimmunity. Int. Rev. Cell Mol. Biol. 2018, 341, 125–168. [Google Scholar]
- Farrokhi, V.; Nemati, R.; Nichols, F.C.; Yao, X.; Anstadt, E. Bacterial lipodipeptide, Lipid 654, is a microbiome-associated biomarker for multiple sclerosis. Clin. Transl. Immunol. 2013, 2, e8. [Google Scholar] [CrossRef]
- Browne, R.W.; Jakimovski, D.; Ziliotto, N.; Kuhle, J.; Bernardi, F.; Weinstock-Guttman, B.; Zivadinov, R.; Ramanathan, M. High-density lipoprotein cholesterol is associated with multiple sclerosis fatigue: A fatigue-metabolism nexus? J. Clin. Lipidol. 2019, 13, 654–663. [Google Scholar] [CrossRef]
- Wagley, S.; Bokori-Brown, M.; Morcrette, H.; Malaspina, A.; D’Arcy, C. Evidence of Clostridium perfringens epsilon toxin associated with multiple sclerosis. Mult. Scler. 2019, 25, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Savva, C.G.; Clark, A.R.; Naylor, C.E.; Popoff, M.R.; Moss, D.S. The pore structure of Clostridium perfringens epsilon toxin. Nat. Commun. 2019, 10, 2641. [Google Scholar] [CrossRef]
- Anwar, Z.; Regan, S.B.; Linden, J. Enrichment and Detection of Clostridium perfringens Toxinotypes in Retail Food Samples. J. Vis. Exp. 2019, 152, e59931. [Google Scholar] [CrossRef] [Green Version]
- Abdurasulova, I.N.; Tarasova, E.A.; Nikiforova, I.G.; Il’ves, A.G.; Ivashkova, V.; Matsulevich, A.V.; Tatarinov, A.E.; Shangina, L.V.; Ermolenko, E.I.; Suvorov, A.N. The intestinal microbiota composition in patients with multiple sclerosis receiving different disease-modifying therapies DMT. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 2018, 118, 62–69. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, K.J. The intestinal microbiota and its role in human health and disease. Med. Investig. 2016, 63, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Liu, Z.; Zhu, C.; Mou, H.; Kong, Q. Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 2019, 59, S130–S152. [Google Scholar] [CrossRef]
- McRorie, J.W. Psyllium is not fermented in the human gut. Neurogastroenterol. Motil. 2015, 27, 1681–2682. [Google Scholar] [CrossRef]
- Jalanka, J.; Major, G.; Murray, K.; Singh, G.; Nowak, A.; Kurtz, C.; Silos-Santiago, I.; Johnston, J.M.; Spiller, R. The Effect of Psyllium Husk on Intestinal Microbiota in Constipated Patients and Healthy Controls. Int. J. Mol. Sci. 2019, 20, 433. [Google Scholar] [CrossRef] [Green Version]
- Holscher, H.D.; Caporaso, J.G.; Hooda, S.; Brulc, J.M.; Fahey, G.C.J.; Swanson, K.S.; Fahey, G.C., Jr.; Swanson, K.S. Fiber supplementation influences phylogenetic structure and functional capacity of the human intestinal microbiome: Follow-up of a randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 55–64. [Google Scholar] [CrossRef]
- Holscher, H.D.; Bauer, L.L.; Vishnupriya, G.; Pelkman, C.L.; Fahey, G.C.; Swanson, K.S.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Bosscher, D.; Breynaert, A.; Pieters, L.; Hermans, N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J. Physiol. Pharmacol. 2009, 60, 5–11. [Google Scholar] [PubMed]
- Sharma, P.; Kharkwal, A.C.; Kharkwal, H.; Abdin, M.Z.; Varma, A. A Review on pharmacological properties of Aloe vera. Int. J. Pharm. Sci. Rev. Res. 2014, 29, 31–37. [Google Scholar]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Tradit. Complement. Med. 2014, 5, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Kaplan, D.N.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid.-Based Complement. Alternat. Med. 2021, 2021, 2488804. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef] [Green Version]
- Nisticò, S.; Ehrlich, J.; Gliozzi, M.; Maiuolo, J.; del Duca, E.; Muscoli, C.; Mollace, V. Telomere and telomerase modulation by bergamot polyphenolic fraction in experimental photoageing in human keratinocytes. J. Biol. Regul. Homeost. Agents 2015, 29, 723–728. [Google Scholar]
- Celia, C.; Trapasso, E.; Locatelli, M.; Navarra, M.; Ventura, C.A.; Wolfram, J.; Carafa, M.; Morittu, V.M.; Britti, D.; di Marzio, L.; et al. Anticancer activity of liposomal bergamot essential oil (BEO) on human neuroblastoma cells. Colloids Surf. B Biointerfaces 2013, 112, 548–553. [Google Scholar] [CrossRef]
- Lauro, F.; Ilari, S.; Giancotti, L.A.; Morabito, C.; Malafoglia, V.; Gliozzi, M.; Palma, E.; Salvemini, D.; Muscoli, C. The protective role of bergamot polyphenolic fraction on several animal models of pain. PharmaNutrition 2016, 4, S35–S40. [Google Scholar] [CrossRef]
- Bagetta, G.; Morrone, L.A.; Rombolà, L.; Amantea, D.; Russo, R.; Berliocchi, L.; Sakurada, S.; Sakurada, T.; Rotiroti, D.; Corasaniti, M.T. Neuropharmacology of the essential oil of bergamot. Fitoterapia 2010, 81, 453–461. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L.; and C. bergamia Risso and Poit. peel essential oils. J. Food Sci. 2012, 77, H40–H46. [Google Scholar] [CrossRef]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef]
- Li, W.Q.; Kuriyama, S.; Li, Q.; Nagai, M.; Hozawa, A.; Nishino, Y.; Tsuji, I. Citrus consumption and cancer incidence: The Ohsaki cohort study. Int. J. Cancer 2010, 127, 1913–1922. [Google Scholar] [CrossRef]
- Xu, J.J.; Wu, X.; Li, M.M.; Li, G.Q.; Yang, Y.T.; Luo, H.J.; Huang, W.H.; Chung, H.Y.; Ye, W.C.; Wang, G.C.; et al. Antiviral Activity of Polymethoxylated Flavones from “Guangchenpi”, the Edible and Medicinal Pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef]
- Tripoli, E.; la Guardia, M.; Giammanco, S.; di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, C.; Gratteri, S.; et al. Antioxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J. Mol. Cell. Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef] [Green Version]
- Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Mollace, R.; Bosco, F.; Scarano, F.; Scicchitano, M.; Maretta, A.; Palma, E.; et al. Lipid-lowering effect of bergamot polyphenolic fraction: Role of pancreatic cholesterol ester hydrolase. J. Biol. Regul. Homeost. Agents 2017, 31, 1087–1093. [Google Scholar]
- Mollace, V.; Scicchitano, M.; Paone, S.; Casale, F.; Calandruccio, C.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Nucera, S.; et al. Hypoglycemic and Hypolipemic Effects of a New Lecithin Formulation of Bergamot Polyphenolic Fraction: A Double Blind, Randomized, Placebo-Controlled Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 136–143. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Taghizadeh-Alisaraei, A.; Hosseini, S.H.; Ghobadian, B.; Motevali, A. Biofuel production from citrus wastes: A feasibility study in Iran. Renew. Sustain. Energy Rev. 2017, 69, 1100–1112. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Cautela, D.; Vella, F.M.; Laratta, B. The effect of processing methods on phyto-chemical composition in bergamot juice. Foods 2019, 8, 474. [Google Scholar] [CrossRef] [Green Version]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid composition of citrus juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [Green Version]
- Di Donna, L.; de Luca, G.; Mazzotti, F.; Napoli, A.; Salerno, R.; Taverna, D. Stat-in-like principles of bergamot fruit (Citrus bergamia): Isolation of 3-hydrox-ymethylglutaryl flavonoid glycosides. J. Nat. Prod. 2009, 72, 1352–1354. [Google Scholar] [CrossRef]
- Firrman, J.; Liu, L.; Argoty, G.A.; Zhang, L.; Tomasula, P.; Wang, M. Analysis of temporal changes in growth and gene expression for commensal gut microbes in response to the polyphenol naringenin. Microbiol. Insights 2018, 11, 1178636118775100. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: Controlled clinical trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Mazza, E.; Ferro, Y.; Gliozzi, M.; Nucera, S.; Paone, S.; Aversa, I.; Pujia, R.; Marafioti, G.; Musolino, V.; et al. A new breakfast brioche containing bergamot fiber prevents insulin and glucose increase in healthy volunteers: A pilot study. Minerva Endocrinol. 2021, 46, 214–225. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Gokhale, J.S.; Mulla, M.Z.; Patil, S. A comprehensive overview of functional and rheological properties of aloe vera and its application in foods. J. Food Sci. Technol. 2021, 58, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; Wilches-Torres, A.; Castaño, J.A.G. Extraction and Physicochemical Characterization of Dried Powder Mucilage from Opuntia ficus-indica Cladodes and Aloe Vera Leaves: A Comparative Study. Polymers 2021, 13, 1689. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Pi, F.; Cheng, Y.; Guo, Y.; Qian, H. Extraction, Purification, Structural Characteristics, Biological Activities and Pharmacological Applications of Acemannan, a Polysaccharide from Aloe vera: A Review. Molecules 2019, 24, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jangra, A.; Sharma, G.; Sihag, S.; Chhokar, V. The dark side of miracle plant-Aloe vera: A review. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef] [PubMed]
- Minjares-Fuentes, R.; Femenia, A.; Comas-Serra, F.; Rodríguez-González, V.M. Compositional and Structural Features of the Main Bioactive Polysaccharides Present in the Aloe vera Plant. J. AOAC Int. 2018, 101, 1711–1719. [Google Scholar] [CrossRef]
- Tanner, S.A.; Lacroix, C.; Del’Homme, C.; Jans, C.; Zihler Berner, A.; Bernalier-Donadille, A.; Chassard, C. Effect of Bifidobacterium thermophilum RBL67 and fructo-oligosaccharides on the gut microbiota in Göttingen minipigs. Br. J. Nutr. 2015, 114, 746–755. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Tornero-Martínez, A.; Cruz-Ortiz, R.; Jaramillo-Flores, M.E.; Osorio-Díaz, P.; Ávila-Reyes, S.V.; Alvarado-Jasso, G.M.; Mora-Escobedo, R. In vitro Fermentation of Polysaccharides from Aloe vera and the Evaluation of Antioxidant Activity and Production of Short Chain Fatty Acids. Molecules 2019, 4, 3605. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Du, P.; Cheng, Y.; Guo, Y.; Hu, B.; Yao, W.; Qian, H. Study on fecal fermentation characteristics of aloe polysaccharides in vitro and their predictive modeling. Carbohydr. Polym. 2021, 256, 117571. [Google Scholar] [CrossRef]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. 2014, 6, 525–531. [Google Scholar] [CrossRef]
- Shi, X.D.; Yin, J.Y.; Cui, S.W.; Wang, Q.; Wang, S.Y.; Nie, S.P. Comparative study on glucomannans with different structural characteristics: Functional properties and intestinal production of short chain fatty acids. Int. J. Biol. Macromol. 2020, 164, 826–835. [Google Scholar] [CrossRef]
- Wingerchuk, D.M.; Carter, J.L. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 2014, 89, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Alfredsson, L.; Olsson, T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a028944. [Google Scholar] [CrossRef] [Green Version]
- Roman, S.N.; Fitzgerald, K.C.; Beier, M.; Mowry, E.M. Safety and feasibility of various fasting-mimicking diets among people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 42, 102149. [Google Scholar] [CrossRef]
- Meyer, N.; Rinholm, J.E. Mitochondria in Myelinating Oligodendrocytes: Slow and Out of Breath? Metabolites 2021, 11, 359. [Google Scholar] [CrossRef]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Bosco, F.; et al. Effects of Bergamot Polyphenols on Mitochondrial Dysfunction and Sarcoplasmic Reticulum Stress in Diabetic Cardiomyopathy. Nutrients 2021, 13, 2476. [Google Scholar] [CrossRef]
- Beckett, J.M.; Bird, M.L.; Pittaway, J.K.; Ahuja, K.D. Diet and Multiple Sclerosis: Scoping Review of Web-Based Recommendations. Interact. J. Med. Res. 2019, 8, e10050. [Google Scholar] [CrossRef]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Scicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin Disturbances Produced by Sub-Toxic Concentration of Heavy Metals: The Role of Oligodendrocyte Dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef] [Green Version]
- Corasaniti, M.T.; Maiuolo, J.; Maida, S.; Fratto, V.; Navarra, M.; Russo, R.; Amantea, D.; Morrone, L.A.; Bagetta, G. Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro. Br. J. Pharmacol. 2007, 151, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical antioxidants as novel neuroprotective agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Macri, R.; Oppedisano, F.; Scarano, F.; Zito, M.C.; et al. The Effect of Ferula communis Extract in Escherichia coli Lipopolysaccharide-Induced Neuroinflammation in Cultured Neurons and Oligodendrocytes. Int. J. Mol. Sci. 2021, 22, 7910. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Carresi, C.; Scarano, F.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; et al. The “frail” brain blood barrier in neurodegenerative diseases: Role of early disruption of endothelial cell-to-cell connections. Int. J. Mol. Sci. 2018, 19, 2693. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Nucera, S.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Ruga, S.; et al. The Role of Endothelial Dysfunction in Peripheral Blood Nerve Barrier: Molecular Mechanisms and Pathophysiological Implications. Int. J. Mol. Sci. 2019, 20, 3022. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, M.; Berni Canani, R. Lactose Intolerance: Common Misunderstandings. Ann. Nutr. Metab. 2018, 73, 30–37. [Google Scholar] [CrossRef]
- Escribano, B.M.; Muñoz-Jurado, A.; Luque, E.; Conde, C.; Feijóo, M.; LaTorre, M.; Valdelvira, M.E.; Buendía, P.; Giraldo, A.I.; Caballero-Villarraso, J.; et al. Lactose and Casein Cause Changes on Biomarkers of Oxidative Damage and Dysbiosis in an Experimental Model of Multiple Sclerosis. CNS Neurol. Disord. Drug Targets 2021, 21, 680–692. [Google Scholar] [CrossRef]
- Eichinger, A.; Neumaier, I.; Skerra, A. The extracellular region of bovine milk butyrophilin exhibits closer structural similarity to human myelin oligodendrocyte glycoprotein than to immunological BTN family receptors. Biol. Chem. 2021, 402, 1187–1202. [Google Scholar] [CrossRef]
- Beltrán, E.; Paunovic, M.; Gebert, D.; Cesur, E.; Jeitler, M.; Höftberger, R.; Malotka, J.; Mader, S.; Kawakami, N.; Meinl, E.; et al. Archeological neuroimmunology: Resurrection of a pathogenic immune response from a historical case sheds light on human autoimmune encephalomyelitis and multiple sclerosis. Acta Neuropathol. 2021, 141, 67–83. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Huh, S.Y.; Rifas-Shiman, S.L.; Zera, C.A.; Edwards, J.W.R.; Oken, E.; Weiss, S.T.; Gillman, M.W. Delivery by caesarean section and risk of obesity in preschool age children: A prospective cohort study. Arch. Dis. Child. 2012, 97, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggesbo, M.; Botten, G.; Stigum, H.; Nafstad, P.; Magnus, P. Is delivery by cesarean section a risk factor for food allergy? J. Allergy Clin. Immunol. 2003, 112, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Perez-Munoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Sontag, M.K.; Lozupone, C.A.; Eggesbø, M. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome 2017, 5, 113. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [Green Version]
- Backhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Bolte, E.E.; Moorshead, D.; Aagaard, K.M. Maternal and early life exposures and their potential to influence development of the microbiome. Genome Med. 2022, 14, 4. [Google Scholar] [CrossRef]
- Jeddi, Z.; Gryech, I.; Ghogho, M.; El Hammoumi, M.; Mahraoui, C. Machine Learning for Predicting the Risk for Childhood Asthma Using Prenatal, Perinatal, Postnatal and Environmental Factors. Healthcare 2021, 9, 1464. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Dalla Costa, G.; Romeo, M.; Esposito, F.; Sangalli, F.; Colombo, B.; Radaelli, M.; Moiola, L.; Comi, G.; Martinelli, V. Caesarean section and infant formula feeding are associated with an earlier age of onset of multiple sclerosis. Mult. Scler. Relat. Disord. 2019, 33, 75–77. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, e143774. [Google Scholar] [CrossRef]
- Muraro, P.A.; Pasquini, M.; Atkins, H.L.; Bowen, J.D.; Farge, D.; Fassas, A.; Freedman, M.S.; Georges, G.E.; Gualandi, F.; Hamerschlak, N.; et al. Long-term outcomes after autologous hematopoietic stem cell transplantation for multiple sclerosis. JAMA Neurol. 2017, 74, 459–469. [Google Scholar] [CrossRef]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Giovannoni, G.; Cutter, G.; Sormani, M.P.; Belachew, S.; Hyde, R.; Koendgen, H.; Knappertz, V.; Tomic, D.; Leppert, D.; Herndon, R.; et al. Is multiple sclerosis a length-dependent central axonopathy? The case for therapeutic lag and the asynchronous progressive MS hypotheses. Mult. Scler. Relat. Disord. 2017, 12, 70–78. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiuolo, J.; Musolino, V.; Gliozzi, M.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Macri, R.; et al. Involvement of the Intestinal Microbiota in the Appearance of Multiple Sclerosis: Aloe vera and Citrus bergamia as Potential Candidates for Intestinal Health. Nutrients 2022, 14, 2711. https://doi.org/10.3390/nu14132711
Maiuolo J, Musolino V, Gliozzi M, Carresi C, Scarano F, Nucera S, Scicchitano M, Oppedisano F, Bosco F, Macri R, et al. Involvement of the Intestinal Microbiota in the Appearance of Multiple Sclerosis: Aloe vera and Citrus bergamia as Potential Candidates for Intestinal Health. Nutrients. 2022; 14(13):2711. https://doi.org/10.3390/nu14132711
Chicago/Turabian StyleMaiuolo, Jessica, Vincenzo Musolino, Micaela Gliozzi, Cristina Carresi, Federica Scarano, Saverio Nucera, Miriam Scicchitano, Francesca Oppedisano, Francesca Bosco, Roberta Macri, and et al. 2022. "Involvement of the Intestinal Microbiota in the Appearance of Multiple Sclerosis: Aloe vera and Citrus bergamia as Potential Candidates for Intestinal Health" Nutrients 14, no. 13: 2711. https://doi.org/10.3390/nu14132711
APA StyleMaiuolo, J., Musolino, V., Gliozzi, M., Carresi, C., Scarano, F., Nucera, S., Scicchitano, M., Oppedisano, F., Bosco, F., Macri, R., Palma, E., Muscoli, C., & Mollace, V. (2022). Involvement of the Intestinal Microbiota in the Appearance of Multiple Sclerosis: Aloe vera and Citrus bergamia as Potential Candidates for Intestinal Health. Nutrients, 14(13), 2711. https://doi.org/10.3390/nu14132711