Zinc Deficiency Blunts the Effectiveness of Antidepressants in the Olfactory Bulbectomy Model of Depression in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Olfactory Bulbectomy—Surgical Procedure
2.3. Zinc Deficiency Diet
2.4. Drug Administration
- Sham + ZnA-rats were similarly treated as OB, but with bulbs left intact and fed a zinc adequate diet (ZnA) of 50 mg Zn/kg.
- OB + ZnA-rats were subjected to bilateral olfactory bulbectomy and fed a zinc adequate diet (ZnA) of 50 mg Zn/kg.
- Sham + ZnD-rats were similarly treated as OB, but with bulbs left intact and fed a zinc-deficient diet (ZnD) of 3 mg Zn/kg.
- OB + ZnD-rats were subjected to bilateral olfactory bulbectomy and fed a zinc-deficient diet (ZnD) of 3 mg Zn/kg.

2.5. Open Field Test (OFT)
2.6. Forced Swim Test (FST)
2.7. Sucrose Intake Test (SIT)
2.8. Tissue and Blood Collection
2.9. Analysis of Zinc
2.10. Zinpyr-1 Staining
2.11. Timm Staining
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. Behavioral Studies
3.1.1. The Effect of OB, ZnD, and OB + ZnD Model on the Behavior of Rats
3.1.2. The Effect of Antidepressants and Zn Supplementation on Rats Subjected to the OB + ZnD Model
3.1.3. The Effects of OB, ZnD, and OB + ZnD Model on Serum Zinc Levels
3.1.4. The Effects of Antidepressants and Zn Supplementation on Serum Zinc Levels in Rats Subjected to the OB + ZnD Model
3.1.5. The Effects of Antidepressants and Zn Supplementation on Intracellular Zinc Concentration in Rats Subjected to the OB + ZnD Model
3.1.6. The Effects of Antidepressants and Zn Supplementation on Brain Synaptic Zinc Levels in Rats Subjected to the OB + ZnD Model
3.1.7. The Effects of OB, ZnD, and OB + ZnD Models on the Levels of ZnT1 and ZnT3 in the PFC and Hp
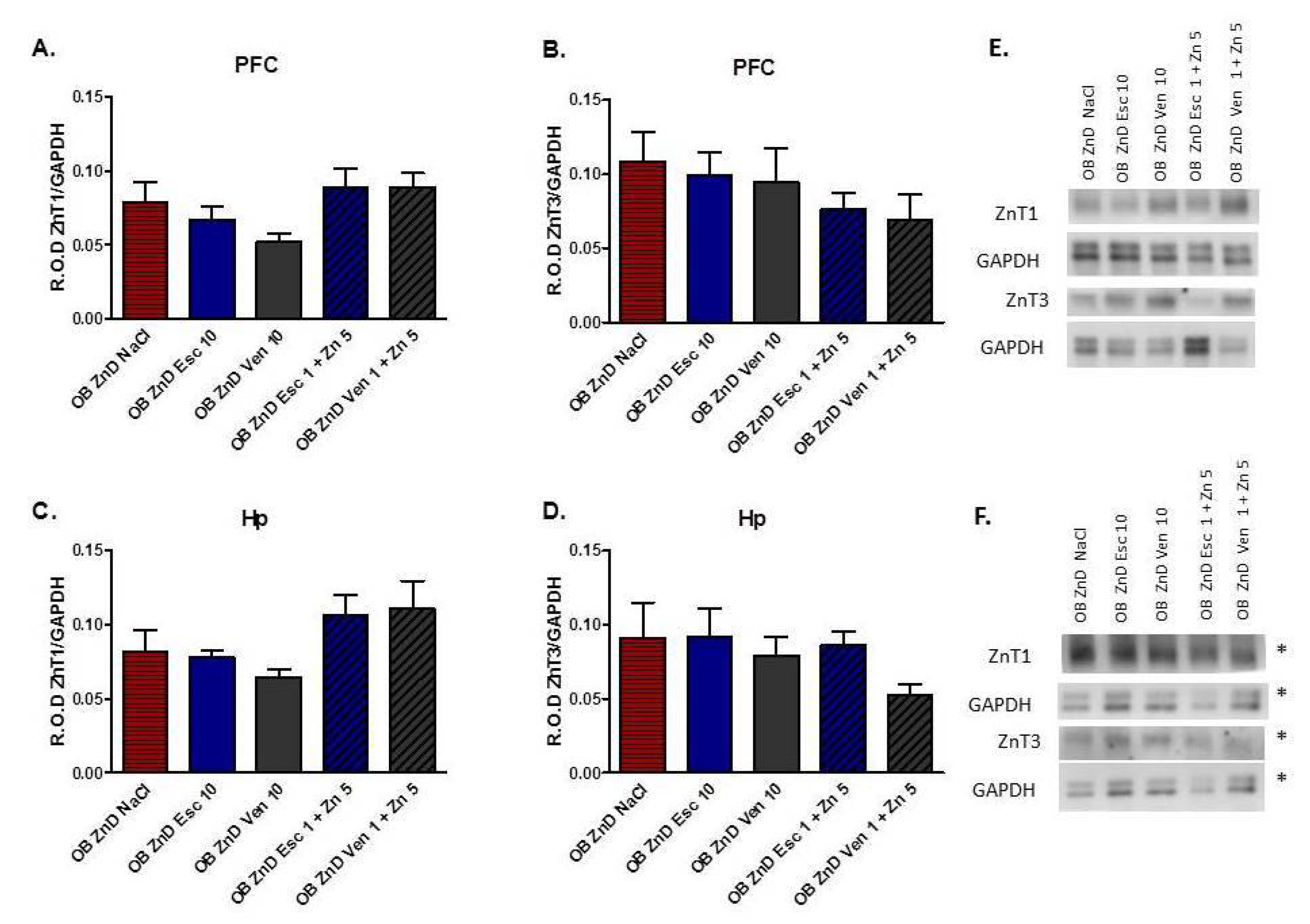
3.1.8. The Effect of Antidepressants and Zn Supplementation on the ZnT1 and ZnT3 Protein Levels in Rats Subjected to the OB + ZnD Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busfield, J. Challenging claims that mental illness has been increasing and mental well-being declining. Soc. Sci. Med. 2012, 75, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, B.H. Depression as a disease of modernity: Explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souery, D.; Oswald, P.; Massat, I.; Bailer, U.; Bollen, J.; Demyttenaere, K.; Kasper, S.; Lecrubier, Y.; Montgomery, S.; Serretti, A.; et al. Clinical factors associated with treatment resistance in major depressive disorder: Results from a European multicenter study. J. Clin. Psychiatry 2007, 68, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Burrows, G.D.; Norman, T.R.; Judd, F.K. Definition and differential diagnosis of treatment-resistant depression. Int. Clin. Psychopharmacol. 1994, 9, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Souery, D.; Amsterdam, J.; de Montigny, C.; Lecrubier, Y.; Montgomery, S.; Lipp, O.; Racagni, G.; Zohar, J.; Mendlewicz, J. Treatment resistant depression: Methodological overview and operational criteria. Eur. Neuropsychopharmacol. 1999, 9, 83–91. [Google Scholar] [CrossRef]
- Nemeroff, C.B. Prevalence and management of treatment-resistant depression. J. Clin. Psychiatry 2007, 68, 17. [Google Scholar]
- Willner, P.; Belzung, C. Treatment-resistant depression: Are animal models of depression fit for purpose? Psychopharmacology 2015, 232, 3473–3495. [Google Scholar] [CrossRef]
- Price, J.L.; Drevets, W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012, 16, 61–71. [Google Scholar] [CrossRef]
- Kelly, J.; Wrynn, A.; Leonard, B. The olfactory bulbectomized rat as a model of depression: An update. Pharmacol. Ther. 1997, 74, 299–316. [Google Scholar] [CrossRef]
- Song, C.; Leonard, B.E. The olfactory bulbectomised rat as a model of depression. Neurosci. Biobehav. Rev. 2005, 29, 627–647. [Google Scholar] [CrossRef]
- Jacka, F.N.; Maes, M.; Pasco, J.A.; Williams, L.; Berk, M. Nutrient intakes and the common mental disorders in women. J. Affect. Disord. 2012, 141, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Vashum, K.P.; McEvoy, M.; Milton, A.H.; McElduff, P.; Hure, A.; Byles, J.; Attia, J. Dietary zinc is associated with a lower incidence of depression: Findings from two Australian cohorts. J. Affect. Disord. 2014, 166, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Siwek, M.; Szewczyk, B.; Dudek, D.; Styczeń, K.; Sowa-Kućma, M.; Młyniec, K.; Siwek, A.; Witkowski, L.; Pochwat, B.; Nowak, G. Zinc as a marker of affective disorders. Pharmacol. Rep. 2013, 65, 1512–1518. [Google Scholar] [CrossRef]
- Siwek, M.; Dudek, D.; Schlegel-Zawadzka, M.; Morawska, A.; Piekoszewski, W.; Opoka, W.; Zięba, A.; Pilc, A.; Popik, P.; Nowak, G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J. Affect. Disord. 2010, 126, 447–452. [Google Scholar] [CrossRef]
- Młyniec, K.; Davies, C.L.; Budziszewska, B.; Opoka, W.; Reczyński, W.; Sowa-Kućma, M.; Doboszewska, U.; Pilc, A.; Nowak, G. Time course of zinc deprivation-induced alterations of mice behavior in the forced swim test. Pharmacol. Rep. 2012, 64, 567–575. [Google Scholar] [CrossRef]
- Młyniec, K.; Nowak, G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. 2012, 64, 249–255. [Google Scholar] [CrossRef]
- Doboszewska, U.; Szewczyk, B.; Sowa-Kucma, M.; Mlyniec, K.; Rafało, A.; Ostachowicz, B.; Lankosz, M.; Nowak, G. Antidepressant activity of fluoxetine in the zinc deficiency model in rats involves the NMDA receptor complex. Behav. Brain Res. 2015, 287, 323–330. [Google Scholar] [CrossRef]
- Nollet, M. Models of Depression: Unpredictable Chronic Mild Stress in Mice. Curr. Protoc. 2021, 1, e208. [Google Scholar] [CrossRef]
- Willner, P.; Gruca, P.; Lason, M.; Tota-Glowczyk, K.; Litwa, E.; Niemczyk, M.; Papp, M. Validation of chronic mild stress in the Wistar-Kyoto rat as an animal model of treatment-resistant depression. Behav. Pharmacol. 2019, 30, 239–250. [Google Scholar] [CrossRef]
- Eagle, A.; Mazei-Robison, M.; Robison, A. Sucrose Preference Test to Measure Stress-induced Anhedonia. Bio-Protocol 2016, 6, e1822. [Google Scholar] [CrossRef] [Green Version]
- Lumia, A.R.; Teicher, M.H.; Salchli, F.; Ayers, E.; Possidente, B. Olfactory bulbectomy as a model for agitated hyposerotonergic depression. Brain Res. 1992, 587, 181–185. [Google Scholar] [CrossRef]
- Leonard, B.E.; Tuite, M. Leonard, Anatomical, physiological and behavioral aspects of olfactory bulbectomy in the rat. Int. Rev. Neurobiol. 1981, 22, 1981. [Google Scholar]
- Pochwat, B.; Szewczyk, B.; Kotarska, K.; Rafało-Ulińska, A.; Siwiec, M.; Sowa, J.E.; Tokarski, K.; Siwek, A.; Bouron, A.; Friedland, K.; et al. Hyperforin Potentiates Antidepressant-Like Activity of Lanicemine in Mice. Front. Mol. Neurosci. 2018, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Doboszewska, U.; Sowa-Kućma, M.; Młyniec, K.; Pochwat, B.; Hołuj, M.; Ostachowicz, B.; Pilc, A.; Nowak, G.; Szewczyk, B. Zinc deficiency in rats is associated with up-regulation of hippocampal NMDA receptor. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2015, 56, 254–263. [Google Scholar] [CrossRef]
- Nowak, G.; Szewczyk, B.; Sadlik, K.; Piekoszewski, W.; Trela, F.; Florek, E.; Pilc, A. Reduced potency of zinc to interact with NMDA receptors in hippocampal tissue of suicide victims. Pol. J. Pharmacol. 2003, 55, 455–459. [Google Scholar]
- Grabrucker, A.M. A role for synaptic zinc in ProSAP/Shank PSD scaffold malformation in autism spectrum disorders. Dev. Neurobiol. 2014, 74, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Hagmeyer, S.; Romão, M.A.; Cristóvão, J.S.; Vilella, A.; Zoli, M.; Gomes, C.M.; Grabrucker, A.M. Distribution and Relative Abundance of S100 Proteins in the Brain of the APP23 Alzheimer’s Disease Model Mice. Front. Neurosci. 2019, 13, 640. [Google Scholar] [CrossRef]
- Sloviter, R.S. A simplified timm stain procedure compatible with formaldehyde fixation and routine paraffin embedding of rat brain. Brain Res. Bull. 1982, 8, 771–774. [Google Scholar] [CrossRef]
- Rafalo, A.; Zadrozna, M.; Nowak, B.; Kotarska, K.; Wiatrowska, K.; Pochwat, B.; Sowa-Kucma, M.; Misztak, P.; Nowak, G.; Szewczyk, B. The level of the zinc homeostasis regulating proteins in the brain of rats subjected to olfactory bulbectomy model of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 72, 36–48. [Google Scholar] [CrossRef]
- Pochwat, B.; Sowa-Kucma, M.; Kotarska, K.; Misztak, P.; Nowak, G.; Szewczyk, B. Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology 2015, 232, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Tamano, H.; Kan, F.; Kawamura, M.; Oku, N.; Takeda, A. Behavior in the forced swim test and neurochemical changes in the hippocampus in young rats after 2-week zinc deprivation. Neurochem. Int. 2009, 55, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Tassabehji, N.M.; Corniola, R.S.; Alshingiti, A.; Levenson, C.W. Zinc deficiency induces depression-like symptoms in adult rats. Physiol. Behav. 2008, 95, 365–369. [Google Scholar] [CrossRef]
- Whittle, N.; Lubec, G.; Singewald, N. Zinc deficiency induces enhanced depression-like behaviour and altered limbic activation reversed by antidepressant treatment in mice. Amino Acids 2009, 36, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Korotzer, A.; Su, G. Efficacy Comparison of Escitalopram and Citalopram in the Treatment of Major Depressive Disorder: Pooled Analysis of Placebo-Controlled Trials. CNS Spectr. 2002, 7, 40–44. [Google Scholar] [CrossRef]
- Lepola, U.M.; Loft, H.; Reines, E.H. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int. Clin. Psychopharmacol. 2003, 18, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.K.; Bhatt, S.; Jindal, A.; Gautam, B. Effect of combination of ketanserin and escitalopram on behavioral anomalies after olfactory bulbectomy: Prediction of quick onset of antidepressant action. Indian J. Pharmacol. 2014, 46, 639–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breuer, M.E.; Groenink, L.; Oosting, R.S.; Westenberg, H.G.; Olivier, B. Long-Term Behavioral Changes After Cessation of Chronic Antidepressant Treatment in Olfactory Bulbectomized Rats. Biol. Psychiatry 2007, 61, 990–995. [Google Scholar] [CrossRef]
- Poretti, M.B.; Sawant, R.S.; Rask-Andersen, M.; de Cuneo, M.F.; Schiöth, H.B.; Perez, M.F.; Carlini, V.P. Reduced vasopressin receptors activation mediates the anti-depressant effects of fluoxetine and venlafaxine in bulbectomy model of depression. Psychopharmacology 2016, 233, 1077–1086. [Google Scholar] [CrossRef]
- McGrath, C.; Norman, T.R. The effect of venlafaxine treatment on the behavioural and neurochemical changes in the olfactory bulbectomised rat. Psychopharmacology 1998, 136, 394. [Google Scholar] [CrossRef]
- Ranjbar, E.; Kasaei, M.S.; Mohammad-Shirazi, M.; Nasrollahzadeh, J.; Rashidkhani, B.; Shams, J.; Mostafavi, S.-A.; Mohammadi, M.R. Effects of Zinc Supplementation in Patients with Major Depression: A Randomized Clinical Trial. Iran. J. Psychiatry 2013, 8, 73–79. [Google Scholar] [PubMed]
- Szewczyk, B. Zinc homeostasis and neurodegenerative disorders. Front. Aging Neurosci. 2013, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.P.; Machado, D.G.; Bettio, L.E.; Capra, J.C.; Rodrigues, A.L.S. Interaction of zinc with antidepressants in the tail suspension test. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Kroczka, B.; Branski, P.; Palucha, A.; Pilc, A.; Nowak, G. Antidepressant-like properties of zinc in rodent forced swim test. Brain Res. Bull. 2001, 55, 297–300. [Google Scholar] [CrossRef]
- Nowak, G.; Szewczyk, B.; Wieronska, J.M.; Branski, P.; Palucha, A.; Pilc, A.; Sadlik, K.; Piekoszewski, W. Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res. Bull. 2003, 61, 159–164. [Google Scholar] [CrossRef]
- Sowa-Kucma, M.; Legutko, B.; Szewczyk, B.; Novak, K.; Znojek, P.; Poleszak, E.; Papp, M.; Pilc, A.; Nowak, G. Antidepressant-like activity of zinc: Further behavioral and molecular evidence. J. Neural Transm. 2008, 115, 1621–1628. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, K.; Klenk-Majewska, B.; Danilczuk, Z.; Wróbel, A.; Łupina, T.; Ossowska, G. Influence of zinc supplementation on imipramine effect in a chronic unpredictable stress (CUS) model in rats. Pharmacol. Rep. 2007, 59, 46–52. [Google Scholar]
- Roozbeh, J.; Sharifian, M.; Ghanizadeh, A.; Sahraian, A.; Sagheb, M.M.; Shabani, S.; Jahromi, A.H.; Kashfi, M.; Afshariani, R. Association of Zinc Deficiency and Depression in the Patients with End-stage Renal Disease on Hemodialysis. J. Ren. Nutr. 2011, 21, 184–187. [Google Scholar] [CrossRef]
- Nowak, G.; Ziȩba, A.; Dudek, D.; Krośniak, M.; Szymaczek, M.; Schlegel-Zawadzka, M. Serum trace elements in animal models and human depression. Part I. Zinc. Hum. Psychopharmacol. 1999, 14, 83–86. [Google Scholar] [CrossRef]
- Nowak, G.; Schlegel-Zawadzka, M. Alterations in Serum and Brain Trace Element Levels After Antidepressant Treatment. Biol. Trace Elem. Res. 1998, 73, 37–46. [Google Scholar] [CrossRef]
- Młyniec, K.; Budziszewska, B.; Reczyński, W.; Doboszewska, U.; Pilc, A.; Nowak, G. Zinc deficiency alters responsiveness to antidepressant drugs in mice. Pharmacol. Rep. 2013, 65, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Cieślik, K.; Sowa-Kućma, M.; Ossowska, G.; Legutko, B.; Wolak, M.; Opoka, W.; Nowak, G. Chronic unpredictable stress-induced reduction in the hippocampal brain-derived neurotrophic factor (BDNF) gene expression is antagonized by zinc treatment. Pharmacol. Rep. 2011, 63, 537–543. [Google Scholar] [CrossRef]
- Wróbel, A.; Serefko, A.; Wlaź, P.; Poleszak, E. The effect of imipramine, ketamine, and zinc in the mouse model of depression. Metab. Brain Dis. 2015, 30, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Rafało-Ulińska, A.; Poleszak, E.; Szopa, A.; Serefko, A.; Rogowska, M.; Sowa, I.; Wójciak, M.; Muszyńska, B.; Krakowska, A.; Gdula-Argasińska, J.; et al. Imipramine Influences Body Distribution of Supplemental Zinc Which May Enhance Antidepressant Action. Nutrients 2020, 12, 2529. [Google Scholar] [CrossRef] [PubMed]
- Sowa-Kućma, M.; Kowalska, M.; Szlósarczyk, M.; Gołembiowska, K.; Opoka, W.; Baś, B.; Pilc, A.; Nowak, G. Chronic treatment with zinc and antidepressants induces enhancement of presynaptic/extracellular zinc concentration in the rat prefrontal cortex. Amino Acids 2011, 40, 249–258. [Google Scholar] [CrossRef] [Green Version]
- Chrobak, A.A.; Siuda, K.; Tereszko, A.; Siwek, M.; Dudek, D. Zaburzenia psychiczne a struktura i funkcje móżdżku—Przeģlad najnowszych bádan. Psychiatria 2014, 11, 15–22. [Google Scholar]
- Leroi, I.; O’Hearn, E.; Marsh, L.; Lyketsos, C.G.; Rosenblatt, A.; Ross, C.A.; Brandt, J.; Margolis, R.L. Psychopathology in Patients with Degenerative Cerebellar Diseases: A Comparison to Huntington’s Disease. Am. J. Psychiatry 2002, 159, 1306–1314. [Google Scholar] [CrossRef]
- Schutter, D.J.L.G.; Van Honk, J. The cerebellum on the rise in human emotion. Cerebellum 2005, 4, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Schmahmann, J.D.; Weilburg, J.B.; Sherman, J.C. The neuropsychiatry of the cerebellum—Insights from the clinic. Cerebellum 2007, 6, 254–267. [Google Scholar] [CrossRef]
- Sandyk, R. Zinc Deficiency and Cerebellar Disease. Int. J. Neurosci. 1991, 60, 21–26. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Stoltenberg, M.; Huang, L.; Danscher, G.; Dahlström, A.; Shi, Y.; Li, J.-Y. Abundant expression of zinc transporters in Bergman glia of mouse cerebellum. Brain Res. Bull. 2005, 64, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nakamura, M.; Fujii, H.; Tamano, H. Synaptic Zn2+ homeostasis and its significance. Metallomics 2013, 5, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Minami, A.; Takefuta, S.; Tochigi, M.; Oku, N. Zinc homeostasis in the brain of adult rats fed zinc-deficient diet. J. Neurosci. Res. 2001, 63, 447–452. [Google Scholar] [CrossRef]
- Takeda, A.; Tamano, H.; Ogawa, T.; Takada, S.; Ando, M.; Oku, N.; Watanabe, M. Significance of serum glucocorticoid and chelatable zinc in depression and cognition in zinc deficiency. Behav. Brain Res. 2012, 226, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lyubartseva, G.; Smith, J.L.; Markesbery, W.R.; Lovell, M.A. Alterations of Zinc Transporter Proteins ZnT-1, ZnT-4 and ZnT-6 in Preclinical Alzheimer’s Disease Brain. Brain Pathol. 2010, 193, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Beyer, N.; Coulson, D.T.; Heggarty, S.; Ravid, R.; Hellemans, J.; Irvine, G.B.; Johnston, J.A. Zinc Transporter mRNA Levels in Alzheimer’s Disease Postmortem Brain. J. Alzheimers Dis. 2012, 29, 863–873. [Google Scholar] [CrossRef]
- Bosomworth, H.J.; Adlard, P.A.; Ford, D.; Valentine, R.A. Altered Expression of ZnT10 in Alzheimer’s Disease Brain. PLoS ONE 2013, 8, e65475. [Google Scholar] [CrossRef] [Green Version]
- Whitfield, D.R.; Vallortigara, J.; Alghamdi, A.; Hortobágyi, T.; Ballard, C.; Thomas, A.J.; O’Brien, J.T.; Aarsland, D.; Francis, P.T. Depression and Synaptic Zinc Regulation in Alzheimer Disease, Dementia with Lewy Bodies, and Parkinson Disease Dementia. Am. J. Geriatr. Psychiatry 2015, 23, 141–148. [Google Scholar] [CrossRef]
- Rafalo-Ulinska, A.; Piotrowska, J.; Kryczyk, A.; Opoka, W.; Sowa-Kucma, M.; Misztak, P.; Rajkowska, G.; Stockmeier, C.A.; Datka, W.; Nowak, G.; et al. Zinc transporters protein level in postmortem brain of depressed subjects and suicide victims. J. Psychiatr. Res. 2016, 83, 220–229. [Google Scholar] [CrossRef] [Green Version]
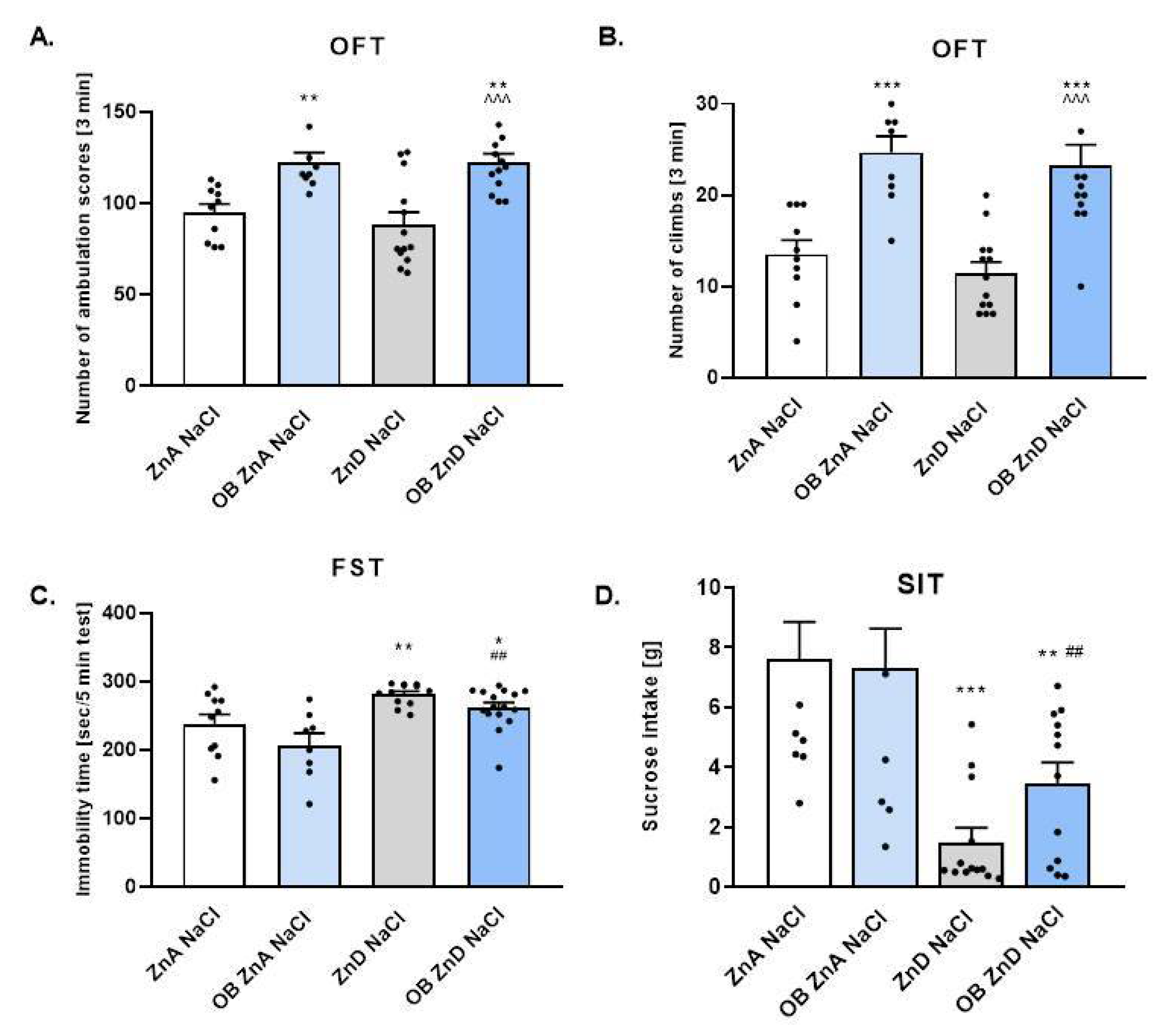
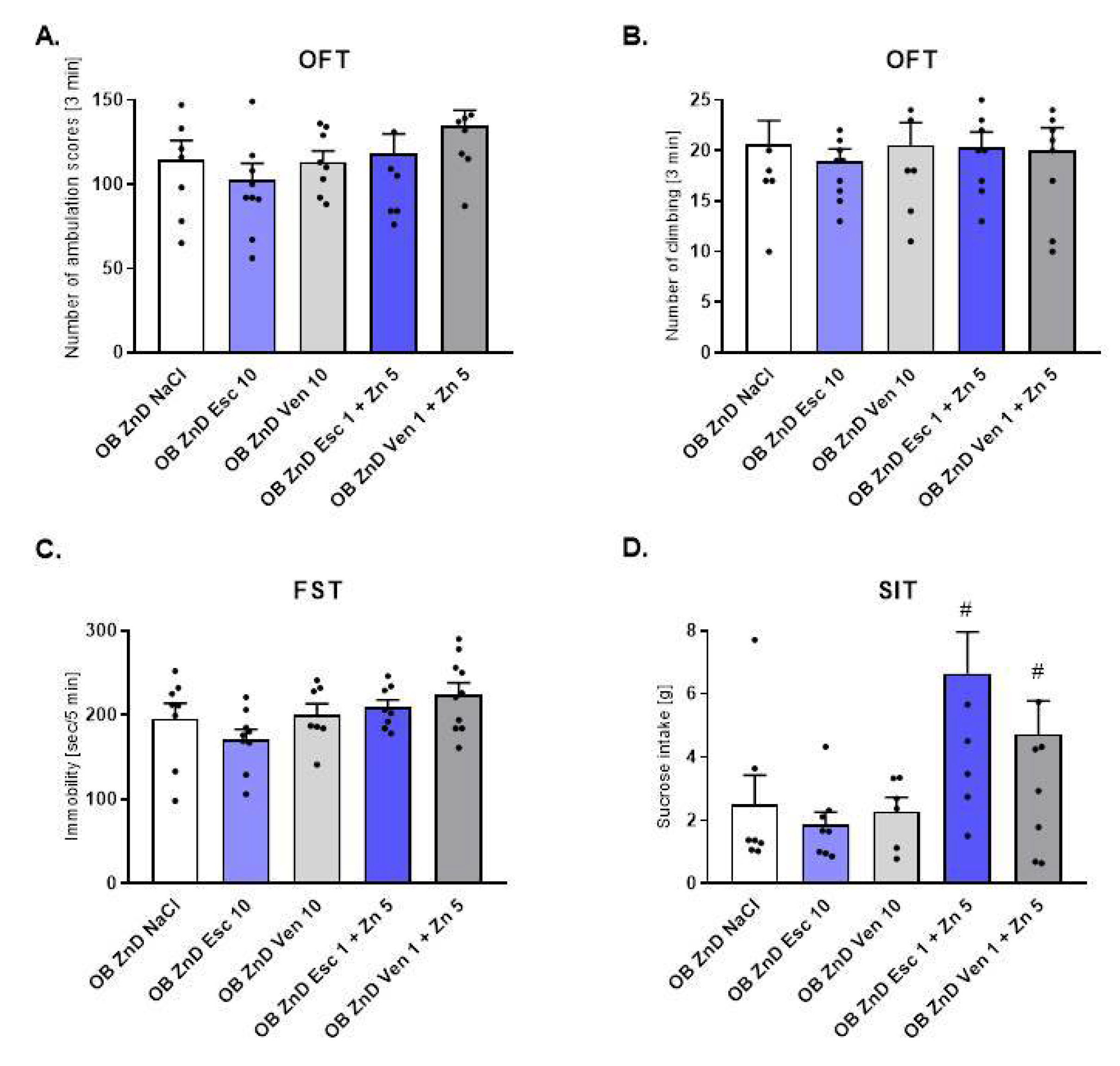
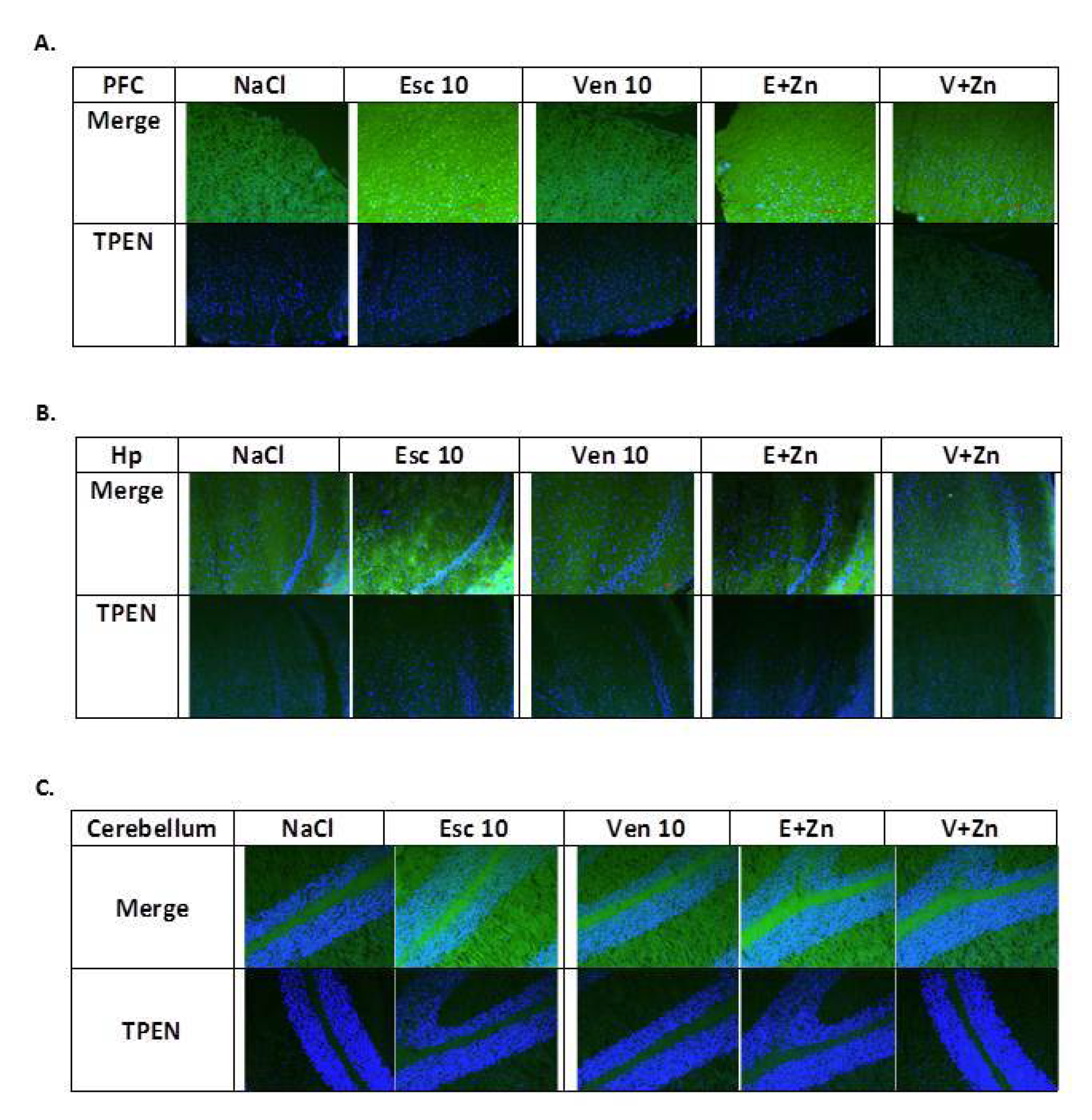
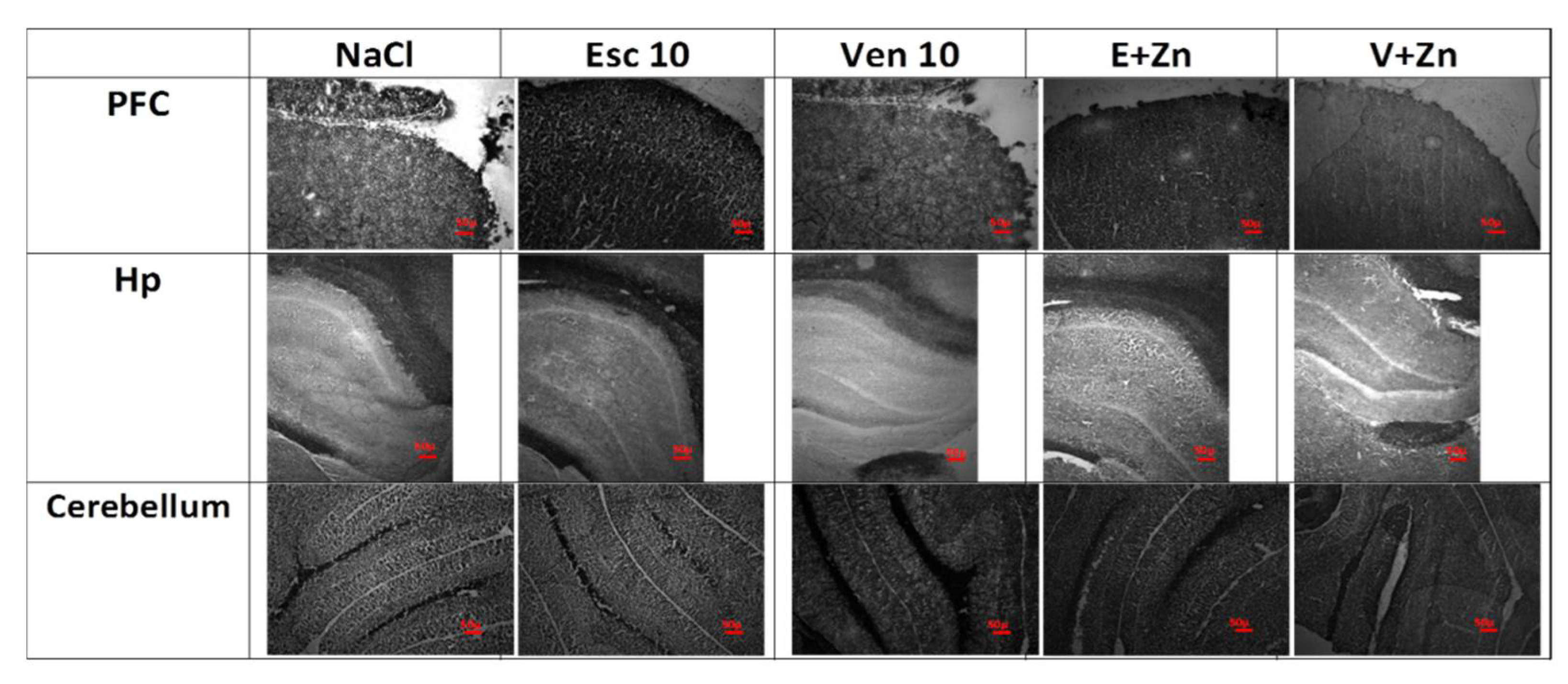

| Treatment | Mean ± SEM |
|---|---|
| ZnA NaCl | 0.3580 ± 0.082 |
| ZnA OB NaCl | 0.4945 ± 0.130 |
| ZnD NaCl | 0.1497 ± 0.027 * |
| ZnD OB NaCl | 0.0803 ± 0.014 ## |
| Treatment | Mean ± SEM |
|---|---|
| OB ZnD NaCl | 0.336 ± 0.064 |
| OB ZnD Esc 10 | 0.348 ± 0.077 |
| OB ZnD Ven 10 | 0.265 ± 0.126 |
| OB ZnD Esc 1 + Zn 5 | 0.578 ± 0.099 *** |
| OB ZnD Ven 1 + Zn 5 | 0.517 ± 0.084 ** |
| Brain Structure | Treatment | Mean ± SEM |
|---|---|---|
| OB ZnD NaCl | 54.056 ± 8.514 | |
| OB ZnD Esc 10 | 111.57 ± 9.431 * | |
| PFC | OB ZnD Ven 10 | 85.41 ± 3.895 |
| OB ZnD Esc 1 + Zn 5 | 110.87 ± 8.837 * | |
| OB ZnD Ven 1 + Zn 5 | 81.18 ± 6.010 | |
| OB ZnD NaCl | 107.2 ± 11.234 | |
| OB ZnD Esc 10 | 98.12 ± 8.271 | |
| Hp | OB ZnD Ven 10 | 88.66 ± 8.505 |
| OB ZnD Esc 1 + Zn 5 | 87.38 ± 8.023 | |
| OB ZnD Ven 1 + Zn 5 | 90.04 ± 9.042 | |
| OB ZnD NaCl | 59.58 ± 5.401 | |
| OB ZnD Esc 10 | 119.66 ± 8.068 ** | |
| Cerebellum | OB ZnD Ven 10 | 114.0 ± 8.521 * |
| OB ZnD Esc 1 + Zn 5 | 119.75 ± 10.276 * | |
| OB ZnD Ven 1 + Zn 5 | 79.16 ± 10.510 |
| Brain Structure | Treatment | Mean ± SEM |
|---|---|---|
| OB ZnD NaCl | 155.66 ± 7.48 | |
| OB ZnD Esc 10 | 201.50 ± 5.05 ** | |
| PFC | OB ZnD Ven 10 | 197.44 ± 8.27 * |
| OB ZnD Esc 1 + Zn 5 | 195.25 ± 7.68 ** | |
| OB ZnD Ven 1 + Zn 5 | 183.66 ± 5.30 * | |
| OB ZnD NaCl | 157.70 ± 13.11 | |
| OB ZnD Esc 10 | 161.66 ± 13.19 | |
| Hp | OB ZnD Ven 10 | 153.25 ± 11.08 |
| OB ZnD Esc 1 + Zn 5 | 174.03 ± 13.92 | |
| OB ZnD Ven 1 + Zn 5 | 150.27 ± 13.32 | |
| OB ZnD NaCl | 155.0 ± 9.78 | |
| OB ZnD Esc 10 | 177.83 ± 6.83 | |
| Cerebellum | OB ZnD Ven 10 | 192.80 ± 6.32 ** |
| OB ZnD Esc 1 + Zn 5 | 204.22 ± 2.61 ** | |
| OB ZnD Ven 1 + Zn 5 | 215.58 ± 8.76 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafało-Ulińska, A.; Pochwat, B.; Misztak, P.; Bugno, R.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B.; Poleszak, E.; Nowak, G.; Szewczyk, B. Zinc Deficiency Blunts the Effectiveness of Antidepressants in the Olfactory Bulbectomy Model of Depression in Rats. Nutrients 2022, 14, 2746. https://doi.org/10.3390/nu14132746
Rafało-Ulińska A, Pochwat B, Misztak P, Bugno R, Kryczyk-Poprawa A, Opoka W, Muszyńska B, Poleszak E, Nowak G, Szewczyk B. Zinc Deficiency Blunts the Effectiveness of Antidepressants in the Olfactory Bulbectomy Model of Depression in Rats. Nutrients. 2022; 14(13):2746. https://doi.org/10.3390/nu14132746
Chicago/Turabian StyleRafało-Ulińska, Anna, Bartłomiej Pochwat, Paulina Misztak, Ryszard Bugno, Agata Kryczyk-Poprawa, Włodzimierz Opoka, Bożena Muszyńska, Ewa Poleszak, Gabriel Nowak, and Bernadeta Szewczyk. 2022. "Zinc Deficiency Blunts the Effectiveness of Antidepressants in the Olfactory Bulbectomy Model of Depression in Rats" Nutrients 14, no. 13: 2746. https://doi.org/10.3390/nu14132746
APA StyleRafało-Ulińska, A., Pochwat, B., Misztak, P., Bugno, R., Kryczyk-Poprawa, A., Opoka, W., Muszyńska, B., Poleszak, E., Nowak, G., & Szewczyk, B. (2022). Zinc Deficiency Blunts the Effectiveness of Antidepressants in the Olfactory Bulbectomy Model of Depression in Rats. Nutrients, 14(13), 2746. https://doi.org/10.3390/nu14132746









