Development and Validation of an Insulin Resistance Model for a Population with Chronic Kidney Disease Using a Machine Learning Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source: National Health and Nutrition Examination Survey (NHANES)
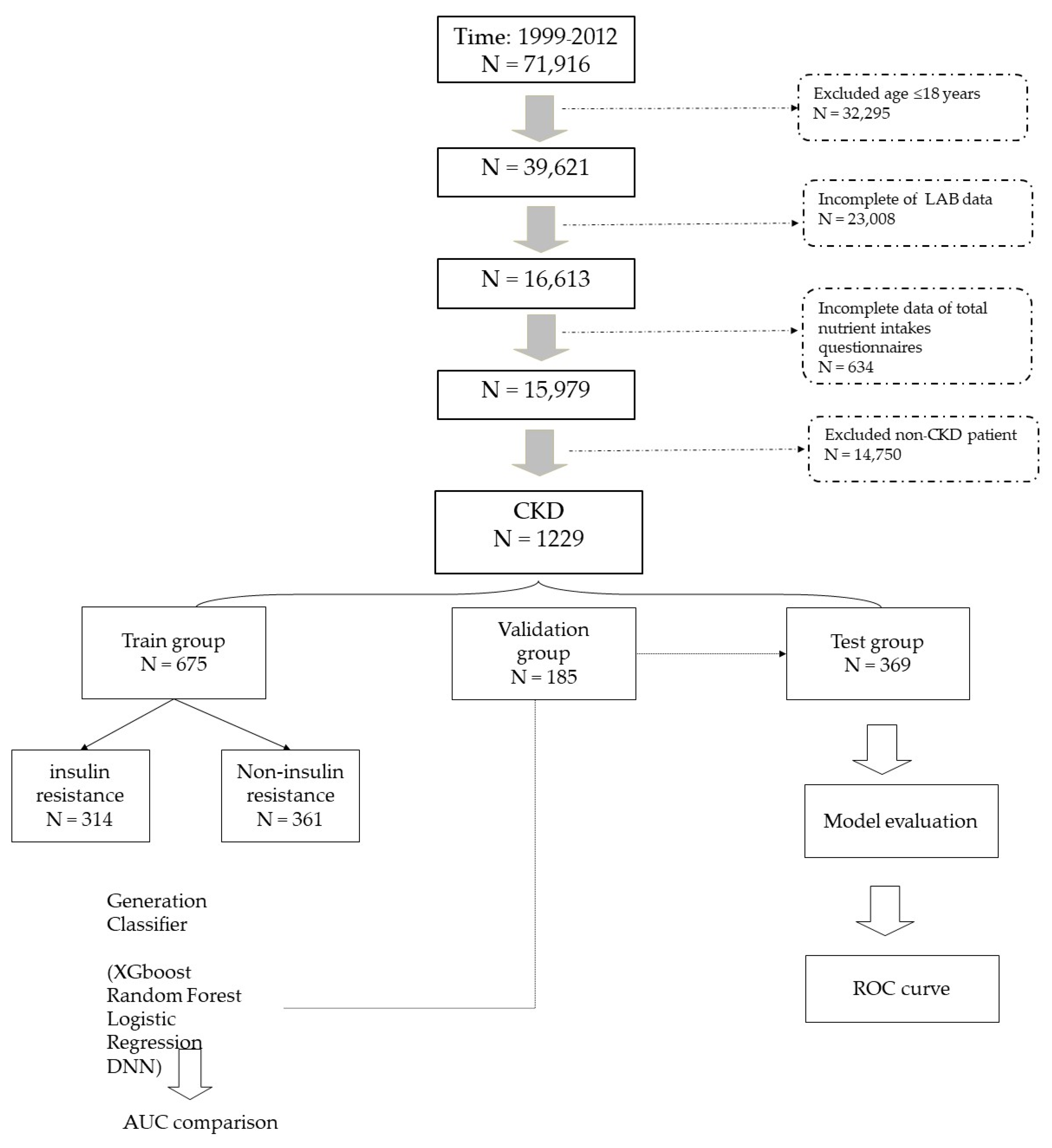
2.2. Definition of Target Population and Data Collection
2.3. Definition of Insulin Resistance (IR)
2.4. Model Building Process
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants with Chronic Kidney Disease According to HOMA-IR
3.2. Model Comparisons in IR prediction for CKD patients
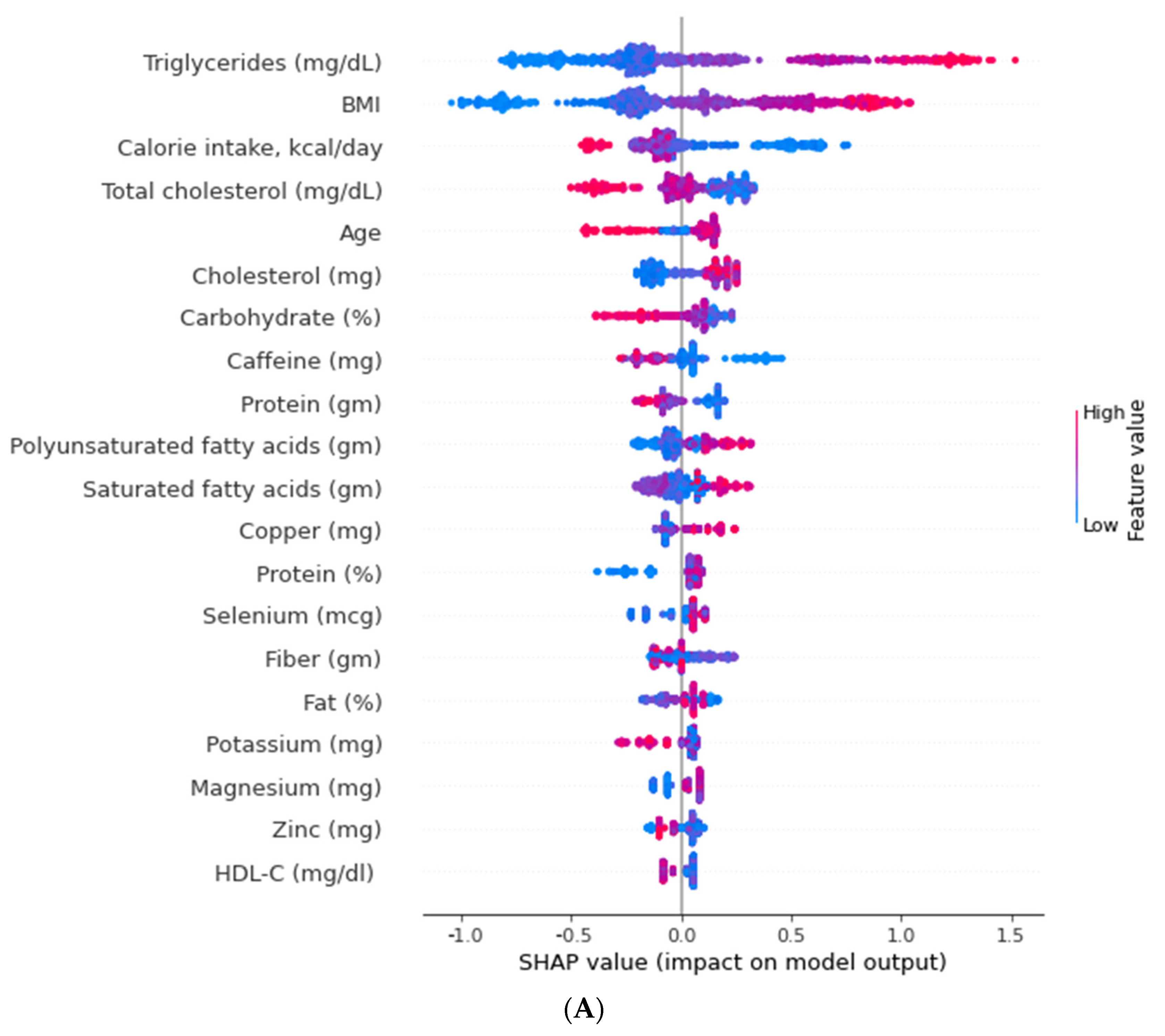
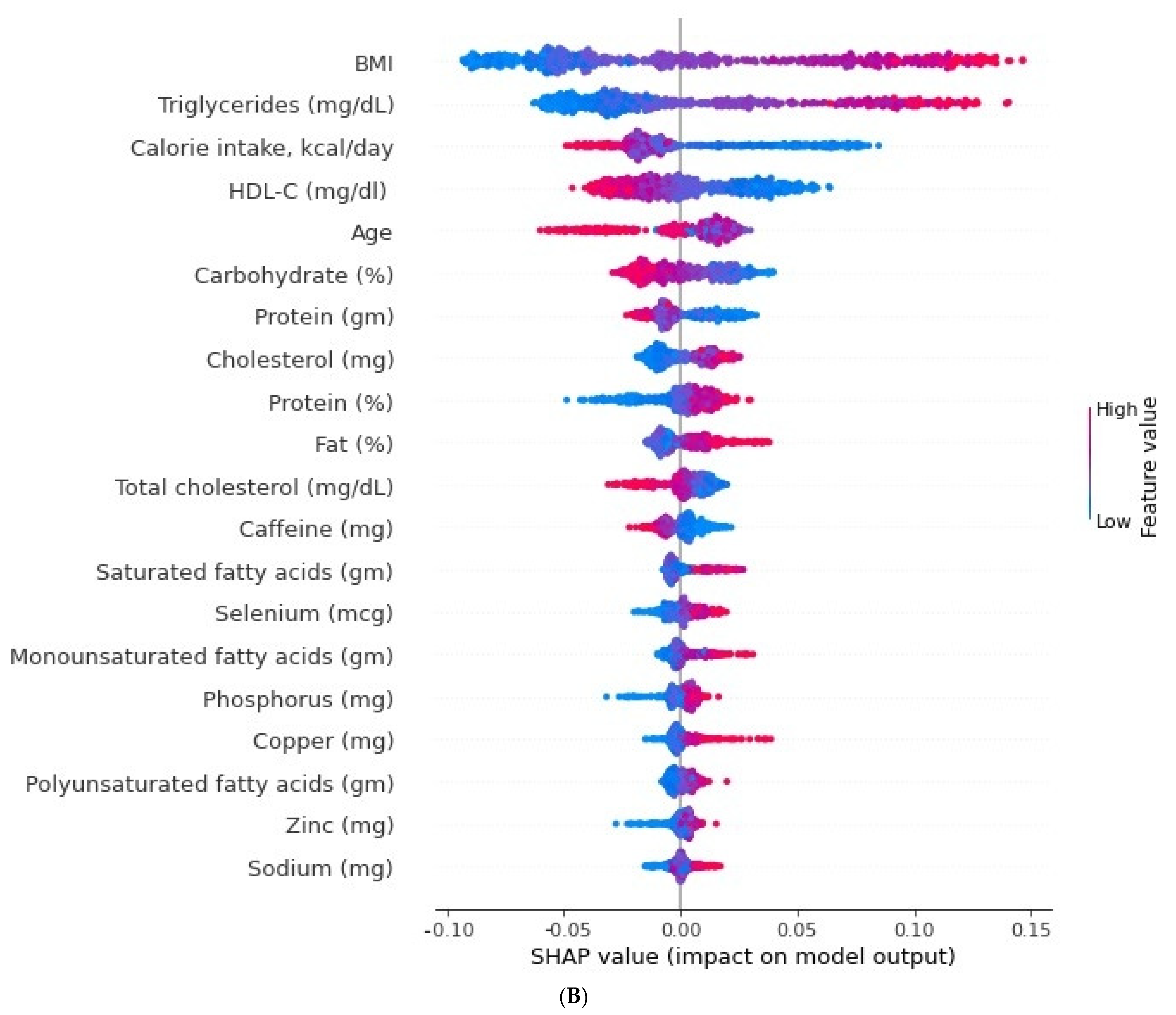
3.3. Relative Importance of Parameters in XGBoost and Random Forest (RF) Algorithms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, C.P.; Cheng, T.Y.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. In Renal Fibrosis: Mechanisms and Therapies; Advances in experimental medicine and biology; Springer: Singapore, 2019; Volume 1165, pp. 3–15. [Google Scholar] [CrossRef]
- Jha, V.; Wang, A.Y.; Wang, H. The impact of CKD identification in large countries: The burden of illness. Nephrol. Dial. Transplant. 2012, 27 (Suppl. 3), iii32–iii38. [Google Scholar] [CrossRef]
- Moller, D.E.; Flier, J.S. Insulin resistance--mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Semple, R.K.; Savage, D.B.; Cochran, E.K.; Gorden, P.; O’Rahilly, S. Genetic syndromes of severe insulin resistance. Endocr. Rev. 2011, 32, 498–514. [Google Scholar] [CrossRef] [Green Version]
- Fliser, D.; Pacini, G.; Engelleiter, R.; Kautzky-Willer, A.; Prager, R.; Franek, E.; Ritz, E. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998, 53, 1343–1347. [Google Scholar] [CrossRef] [Green Version]
- Spoto, B.; Pisano, A.; Zoccali, C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Ren. Physiol. 2016, 311, F1087–F1108. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A.; Alvestrand, A.; Smith, D.; Hendler, R.; Hendler, E.; Wahren, J. Insulin resistance in uremia. J. Clin. Investig. 1981, 67, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Folsom, A.R.; Eckfeldt, J.H.; Weitzman, S.; Ma, J.; Chambless, L.E.; Barnes, R.W.; Cram, K.B.; Hutchinson, R.G. Relation of carotid artery wall thickness to diabetes mellitus, fasting glucose and insulin, body size, and physical activity. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke 1994, 25, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, G.; O’Leary, D.H.; Zaccaro, D.; Haffner, S.; Rewers, M.; Hamman, R.; Selby, J.V.; Saad, M.F.; Savage, P.; Bergman, R. Insulin sensitivity and atherosclerosis. The Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Circulation 1996, 93, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, X.; Arnlöv, J.; Cederholm, T.; Stenvinkel, P.; Lindholm, B.; Risérus, U.; Carrero, J.J. Clinical correlates of insulin sensitivity and its association with mortality among men with CKD stages 3 and 4. Clin. J. Am. Soc. Nephrol. 2014, 9, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Shinohara, K.; Shoji, T.; Emoto, M.; Tahara, H.; Koyama, H.; Ishimura, E.; Miki, T.; Tabata, T.; Nishizawa, Y. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J. Am. Soc. Nephrol. 2002, 13, 1894–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, I.H.; Mehrotra, R. Insulin resistance in chronic kidney disease: A step closer to effective evaluation and treatment. Kidney Int. 2014, 86, 243–245. [Google Scholar] [CrossRef] [Green Version]
- Leikis, M.J.; McKenna, M.J.; Petersen, A.C.; Kent, A.B.; Murphy, K.T.; Leppik, J.A.; Gong, X.; McMahon, L.P. Exercise performance falls over time in patients with chronic kidney disease despite maintenance of hemoglobin concentration. Clin. J. Am. Soc. Nephrol. 2006, 1, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaartinen, K.; Syrjänen, J.; Pörsti, I.; Harmoinen, A.; Pasternack, A.; Huhtala, H.; Niemelä, O.; Mustonen, J. Insulin resistance and the progression of IgA glomerulonephritis. Nephrol. Dial. Transplant. 2007, 22, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Tokudome, G.; Hara, Y.; Sugano, N.; Endo, S.; Suetsugu, Y.; Kuriyama, S.; Hosoya, T. Insulin resistance is a risk factor for the progression of chronic kidney disease. Clin. Nephrol. 2009, 71, 643–651. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. 2005–2006, N. Smoking-Cigarette Use Data Documentation, Codebook, and Frequencies; CDC: Atlanta, GA, USA, 2020.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey 2009-2010 Data Documentation, Codebook, and Frequencies; CDC: Atlanta, GA, USA, 2012.
- Hsu, C.Y.; Yang, W.; Go, A.S.; Parikh, R.V.; Feldman, H.I. Analysis of Estimated and Measured Glomerular Filtration Rates and the CKD-EPI Equation Race Coefficient in the Chronic Renal Insufficiency Cohort Study. JAMA Netw. Open 2021, 4, e2117080. [Google Scholar] [CrossRef]
- Fowler, J.R.; Tucker, L.A.; Bailey, B.W.; LeCheminant, J.D. Physical Activity and Insulin Resistance in 6,500 NHANES Adults: The Role of Abdominal Obesity. J. Obes. 2020, 2020, 3848256. [Google Scholar] [CrossRef] [Green Version]
- Haffner, S.M.; Kennedy, E.; Gonzalez, C.; Stern, M.P.; Miettinen, H. A prospective analysis of the HOMA model. The Mexico City Diabetes Study. Diabetes Care 1996, 19, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Hayashi, T.; Sato, K.K.; Ogihara, T.; Kuzuya, N.; Anai, M.; Tsukuda, K.; Boyko, E.J.; Fujimoto, W.Y.; Kikuchi, M. Fasting tests of insulin secretion and sensitivity predict future prediabetes in Japanese with normal glucose tolerance. J. Diabetes Investig. 2010, 1, 191–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. 1999–2000, N. Plasma Fasting Glucose, Serum C-Peptide & Insulin Data Documentation, Codebook, and Frequencies; CDC: Atlanta, GA, USA, 2020.
- Centers for Disease Control and Prevention. 2005–2006, N. Plasma Fasting Glucose & Insulin Data Documentation, Codebook, and Frequencies; CDC: Atlanta, GA, USA, 2020.
- Centers for Disease Control and Prevention. 2001–2002, N. Plasma Fasting Glucose, Serum C-Peptide & Insulin Data Documentation, Codebook, and Frequencies; CDC: Atlanta, GA, USA, 2020.
- Centers for Disease Control and Prevention. 2003–2004, N. Plasma Fasting Glucose, Serum C-Peptide & Insulin Data Documentation, Codebook, and Frequencies; CDC: Atlanta, GA, USA, 2020.
- Centers for Disease Control and Prevention. 1999–2000, N. Questionnaire Data; CDC: Atlanta, GA, USA, 2020. [Google Scholar]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sit, D.; Kadiroglu, A.K.; Yilmaz, M.E.; Kara, I.H.; Isikoglu, B. The prevalence of insulin resistance and its relationship between anemia, secondary hyperparathyroidism, inflammation, and cardiac parameters in chronic hemodialysis patients. Ren. Fail. 2005, 27, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Sit, D.; Kadiroglu, A.K.; Kayabasi, H.; Yilmaz, M.E. The prevalence of insulin resistance in nondiabetic nonobese patients with chronic kidney disease. Adv. Ther. 2006, 23, 988–998. [Google Scholar] [CrossRef]
- Akalın, N.; Köroğlu, M.; Harmankaya, Ö.; Akay, H.; Kumbasar, B. Comparison of insulin resistance in the various stages of chronic kidney disease and inflammation. Ren. Fail. 2015, 37, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Casp. J. Intern. Med. 2013, 4, 627–635. [Google Scholar]
- Park, D.; Jeong, E.; Kim, H.; Pyun, H.W.; Kim, H.; Choi, Y.J.; Kim, Y.; Jin, S.; Hong, D.; Lee, D.W.; et al. Machine Learning-Based Three-Month Outcome Prediction in Acute Ischemic Stroke: A Single Cerebrovascular-Specialty Hospital Study in South Korea. Diagnostics 2021, 11, 1909. [Google Scholar] [CrossRef]
- Sun, L.; Tang, L.; Shao, G.; Qiu, Q.; Lan, T.; Shao, J. A Machine Learning-Based Classification System for Urban Built-Up Areas Using Multiple Classifiers and Data Sources. Remote Sens. 2020, 12, 91. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, S.M.; Lee, S. A Unified Approach to Interpreting Model Predictions. In Proceedings of the 31st Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017. [Google Scholar]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey: Analytic Guidelines, 2011-2014 and 2015-2016; CDC: Atlanta, GA, USA, 2018.
- Chen, J.; Muntner, P.; Hamm, L.L.; Jones, D.W.; Batuman, V.; Fonseca, V.; Whelton, P.K.; He, J. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann. Intern. Med. 2004, 140, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Ishida, K.; Sairenchi, T.; Takahashi, H.; Ohba, S.; Shiigai, T.; Narita, M.; Koyama, A. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007, 71, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, Z.; Washco, V.; Morse, S.; Reisin, E. The impacts of obesity on the cardiovascular and renal systems: Cascade of events and therapeutic approaches. Curr. Hypertens. Rep. 2015, 17, 7. [Google Scholar] [CrossRef]
- Pulakat, L.; DeMarco, V.G.; Whaley-Connell, A.; Sowers, J.R. The Impact of Overnutrition on Insulin Metabolic Signaling in the Heart and the Kidney. Cardiorenal Med. 2011, 1, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aroor, A.R.; McKarns, S.; Demarco, V.G.; Jia, G.; Sowers, J.R. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metab. Clin. Exp. 2013, 62, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Bottiglieri, T.; McCullough, P.A. The Central Role of Endothelial Dysfunction in Cardiorenal Syndrome. Cardiorenal Med. 2017, 7, 104–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landau, M.; Kurella-Tamura, M.; Shlipak, M.G.; Kanaya, A.; Strotmeyer, E.; Koster, A.; Satterfield, S.; Simsonick, E.M.; Goodpaster, B.; Newman, A.B.; et al. Correlates of insulin resistance in older individuals with and without kidney disease. Nephrol. Dial. Transplant. 2011, 26, 2814–2819. [Google Scholar] [CrossRef]
- Becker, B.; Kronenberg, F.; Kielstein, J.T.; Haller, H.; Morath, C.; Ritz, E.; Fliser, D. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: The mild and moderate kidney disease study. J. Am. Soc. Nephrol. 2005, 16, 1091–1098. [Google Scholar] [CrossRef]
- Mykkänen, L.; Zaccaro, D.J.; Wagenknecht, L.E.; Robbins, D.C.; Gabriel, M.; Haffner, S.M. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: The insulin resistance atherosclerosis study. Diabetes 1998, 47, 793–800. [Google Scholar] [CrossRef]
- Chen, J.; Muntner, P.; Hamm, L.L.; Fonseca, V.; Batuman, V.; Whelton, P.K.; He, J. Insulin resistance and risk of chronic kidney disease in nondiabetic US adults. J. Am. Soc. Nephrol. 2003, 14, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Schrauben, S.J.; Jepson, C.; Hsu, J.Y.; Wilson, F.P.; Zhang, X.; Lash, J.P.; Robinson, B.M.; Townsend, R.R.; Chen, J.; Fogelfeld, L.; et al. Insulin resistance and chronic kidney disease progression, cardiovascular events, and death: Findings from the chronic renal insufficiency cohort study. BMC Nephrol. 2019, 20, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of Minerals and Trace Elements in Diabetes and Insulin Resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Ellahham, S. Artificial Intelligence: The Future for Diabetes Care. Am. J. Med. 2020, 133, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Ogami, C.; Tsuji, Y.; Seki, H.; Kawano, H.; To, H.; Matsumoto, Y.; Hosono, H. An artificial neural network-pharmacokinetic model and its interpretation using Shapley additive explanations. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 760–768. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Wang, H.; Luo, J.C.; Luo, M.H.; Liu, L.P.; Yu, S.J.; Liu, K.; Zhang, Y.J.; Sun, P.; Tu, G.W.; et al. Development and Validation of a Machine-Learning Model for Prediction of Extubation Failure in Intensive Care Units. Front. Med. 2021, 8, 676343. [Google Scholar] [CrossRef]
- Roder, J.; Maguire, L.; Georgantas, R., 3rd; Roder, H. Explaining multivariate molecular diagnostic tests via Shapley values. BMC Med. Inform. Decis. Mak. 2021, 21, 211. [Google Scholar] [CrossRef]
- Song, X.; Liu, M.; Waitman, L.R.; Patel, A.; Simpson, S.Q. Clinical factors associated with rapid treatment of sepsis. PLoS ONE 2021, 16, e0250923. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Dounousi, E.; Siamopoulos, K.C. Dyslipidemia in chronic kidney disease: An approach to pathogenesis and treatment. Am. J. Nephrol. 2008, 28, 958–973. [Google Scholar] [CrossRef]
- Samuelsson, O.; Attman, P.O.; Knight-Gibson, C.; Larsson, R.; Mulec, H.; Weiss, L.; Alaupovic, P. Complex apolipoprotein B-containing lipoprotein particles are associated with a higher rate of progression of human chronic renal insufficiency. J. Am. Soc. Nephrol. 1998, 9, 1482–1488. [Google Scholar] [CrossRef]
- Hadjadj, S.; Duly-Bouhanick, B.; Bekherraz, A.; BrIdoux, F.; Gallois, Y.; Mauco, G.; Ebran, J.; Marre, M. Serum triglycerides are a predictive factor for the development and the progression of renal and retinal complications in patients with type 1 diabetes. Diabetes Metab. 2004, 30, 43–51. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, M.T.; Sung, C.C.; Hung, K.C.; Wu, C.C.; Lo, L.; Lu, K.C. Insulin resistance in patients with chronic kidney disease. J. Biomed. Biotechnol. 2012, 2012, 691369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boden, G.; Lebed, B.; Schatz, M.; Homko, C.; Lemieux, S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes 2001, 50, 1612–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chueire, V.B.; Muscelli, E. Effect of free fatty acids on insulin secretion, insulin sensitivity and incretin effect—A narrative review. Arch. Endocrinol. Metab. 2021, 65, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kalbacher, E.; Koppe, L.; Zarrouki, B.; Pillon, N.J.; Fouque, D.; Soulage, C.O. Human uremic plasma and not urea induces exuberant secretion of leptin in 3T3-L1 adipocytes. J. Ren. Nutr. 2011, 21, 72–75. [Google Scholar] [CrossRef]
- Guarnieri, G.; Zanetti, M.; Vinci, P.; Cattin, M.R.; Barazzoni, R. Insulin resistance in chronic uremia. J. Ren. Nutr. 2009, 19, 20–24. [Google Scholar] [CrossRef]
- Tan, P.Y.; Soma Roy, M. Dietary copper and selenium are associated with insulin resistance in overweight and obese Malaysian adults. Nutr. Res. 2021, 93, 38–47. [Google Scholar] [CrossRef]
- Bjørklund, G.; Dadar, M.; Pivina, L.; Doşa, M.D.; Semenova, Y.; Aaseth, J. The Role of Zinc and Copper in Insulin Resistance and Diabetes Mellitus. Curr. Med. Chem. 2020, 27, 6643–6657. [Google Scholar] [CrossRef]
- Wei, J.; Zeng, C.; Gong, Q.Y.; Yang, H.B.; Li, X.X.; Lei, G.H.; Yang, T.B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Bleys, J.; Navas-Acien, A.; Guallar, E. Serum selenium and diabetes in U.S. adults. Diabetes Care 2007, 30, 829–834. [Google Scholar] [CrossRef] [Green Version]
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Stranges, S.; Sieri, S.; Vinceti, M.; Grioni, S.; Guallar, E.; Laclaustra, M.; Muti, P.; Berrino, F.; Krogh, V. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health 2010, 10, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.W.; Chang, H.H.; Yang, K.C.; Kuo, C.S.; Lee, L.T.; Huang, K.C. High serum selenium levels are associated with increased risk for diabetes mellitus independent of central obesity and insulin resistance. BMJ Open Diabetes Res. Care 2016, 4, e000253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, M.P.; Ribeiro, M.J.; Sacramento, J.F.; Conde, S.V. Chronic caffeine intake reverses age-induced insulin resistance in the rat: Effect on skeletal muscle Glut4 transporters and AMPK activity. Age 2013, 35, 1755–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, T.C.; Liu, C.P.; Cheng, W.H.; Chen, B.R.; Lu, P.J.; Cheng, P.W.; Ho, W.Y.; Sun, G.C.; Liou, J.C.; Tseng, C.J. Caffeine intake improves fructose-induced hypertension and insulin resistance by enhancing central insulin signaling. Hypertension 2014, 63, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Conde, S.V.; Nunes da Silva, T.; Gonzalez, C.; Mota Carmo, M.; Monteiro, E.C.; Guarino, M.P. Chronic caffeine intake decreases circulating catecholamines and prevents diet-induced insulin resistance and hypertension in rats. Br. J. Nutr. 2012, 107, 86–95. [Google Scholar] [CrossRef]
- Lee, S.; Min, J.Y.; Min, K.B. Caffeine and Caffeine Metabolites in Relation to Insulin Resistance and Beta Cell Function in U.S. Adults. Nutrients 2020, 12, 1783. [Google Scholar] [CrossRef]



| Overall | HOMA IR ≤ 3 | HOMA IR > 3 | p-Value | |
|---|---|---|---|---|
| Epidemiology | ||||
| Case number, n | 1229 | 657 | 572 | |
| HOMA-IR | 4.73(4.21–5.25) | 1.76(1.68–1.84) | 8.44(7.5–9.38) | <0.0001 |
| Age (year/o) | 71.51(70.7–72.33) | 72.12(70.91–73.32) | 70.76(69.79–71.74) | <0.0001 |
| Male, n (%) | 573(40.99) | 290(38.75) | 283(43.79) | 0.0577 |
| Ethnicity, n (%) | ||||
| Non-Hispanic white | 824(82.62) | 475(85.02) | 349(79.63) | 0.0112 |
| Non-Hispanic black | 214(9.24) | 95(7.2) | 119(11.8) | |
| Mexican American/others | 191(8.13) | 87(7.78) | 104(8.57) | |
| Body mass index (kg/m2) | 29.04(28.61–29.47) | 26.72(26.21–27.23) | 31.94(31.24–32.63) | <0.0001 |
| Laboratory data | ||||
| Glycohemoglobin (%) | 5.96(5.88–6.03) | 5.68(5.62–5.74) | 6.3(6.18–6.42) | <0.0001 |
| Fasting plasma glucose (mg/dl) | 109.75(107.05–112.46) | 96.88(95.2–98.56) | 125.8(121.11–130.49) | <0.0001 |
| HDL cholesterol (mg/dL) | 53.06(52.06–54.06) | 57.07(55.59–58.54) | 48.07(46.57–49.57) | <0.0001 |
| Total cholesterol (mg/dL) | 193.47(190.44–196.51) | 196.97(192.15–201.79) | 189.11(184.4–193.83) | <0.0001 |
| Triglycerides (mg/dL) | 149.91(143.58–156.24) | 125.34(120.23–130.44) | 180.55(169.83–191.26) | <0.0001 |
| Daily food intake | ||||
| Macronutrients | ||||
| Daily energy intake (kcal)/kg | 21.82(21.19–22.46) | 23.65(22.64–24.66) | 19.54(18.7–20.37) | <0.0001 |
| Daily carbohydrate intake ratio (%) | 50.15(49.29–51.01) | 51.25(50.26–52.25) | 48.77(47.55–49.98) | <0.0001 |
| Daily protein intake (gm)/kg | 0.85(0.82–0.89) | 0.9(0.85–0.94) | 0.8(0.75–0.84) | <0.0001 |
| Daily protein intake ratio (%) | 16.06(15.62–16.49) | 15.68(15.18–16.19) | 16.52(15.89–17.16) | <0.0001 |
| Daily total fat intake ratio (%) | 33.8(33.18–34.41) | 33.06(32.27–33.86) | 34.71(33.75–35.67) | <0.0001 |
| Total saturated fatty acids (gm) | 20.98(20.15–21.81) | 20.13(18.94–21.32) | 22.04(20.64–23.44) | <0.0001 |
| Total monounsaturated fatty acids (gm) | 23.2(22.27–24.12) | 22.25(20.88–23.61) | 24.38(22.8–25.95) | <0.0001 |
| Total polyunsaturated fatty acids (gm) | 13.92(13.36–14.49) | 13.62(12.78–14.45) | 14.31(13.29–15.33) | <0.0001 |
| Daily cholesterol intake (mg) | 241.11(227.22–255) | 219.72(203.13–236.32) | 267.78(244.03–291.53) | <0.0001 |
| Micronutrients | ||||
| Daily fiber intake (gm) | 13.82(13.22–14.42) | 14.06(13.18–14.94) | 13.52(12.67–14.37) | <0.0001 |
| Total folate (mcg) | 340.79(328.33–353.26) | 347.07(328.95–365.19) | 332.97(314.62–351.31) | <0.0001 |
| Vitamin B6 (mg) | 1.69(1.62–1.76) | 1.7(1.6–1.81) | 1.67(1.55–1.79) | <0.0001 |
| Vitamin B12 (mcg) | 4.66(4.34–4.98) | 4.6(4.15–5.05) | 4.74(4.24–5.23) | <0.0001 |
| Vitamin C (mg) | 78.24(72.81–83.67) | 78.18(71.14–85.22) | 78.31(69.35–87.26) | <0.0001 |
| Calcium (mg) | 760.8(731.17–790.44) | 745.21(704.7–785.73) | 780.24(731.9–828.58) | <0.0001 |
| Phosphorus (mg) | 1083.49(1050.23–1116.74) | 1055.72(1008.52–1102.91) | 1118.1(1059.64–1176.57) | <0.0001 |
| Magnesium (mg) | 238.5(230.36–246.64) | 239.31(227.53–251.09) | 237.49(225.38–249.61) | <0.0001 |
| Iron (mg) | 13.57(13.03–14.1) | 13.68(12.93–14.44) | 13.42(12.62–14.21) | <0.0001 |
| Zinc (mg) | 9.99(9.49–10.49) | 9.79(9.08–10.49) | 10.25(9.58–10.92) | <0.0001 |
| Copper (mg) | 1.08(1.03–1.13) | 1.06(1–1.13) | 1.1(1.03–1.17) | <0.0001 |
| Sodium (mg) | 2782.85(2687.8–2877.9) | 2670.51(2541.28–2799.74) | 2922.9(2765.66–3080.13) | <0.0001 |
| Potassium (mg) | 2366.12(2298.49–2433.75) | 2339.97(2242.93–2437.01) | 2398.72(2271.46–2525.97) | <0.0001 |
| Selenium (mcg) | 88.69(85.61–91.78) | 87.21(83.2–91.22) | 90.55(85.22–95.88) | <0.0001 |
| Caffeine (mg) | 144.84(131.87–157.81) | 140.78(125.71–155.85) | 149.9(131.22–168.58) | <0.0001 |
| Theobromine (mg) | 30.68(26.29–35.07) | 31.15(25.51–36.78) | 30.09(24.48–35.71) | <0.0001 |
| Alcohol (gm) | 4.05(3.12–4.99) | 5.13(3.73–6.52) | 2.72(1.64–3.8) | <0.0001 |
| Smoking, n (%) | 606(49.67) | 322(50.46) | 284(48.68) | 0.6232 |
| Cardiovascular disease, n (%) | 188(16.48) | 92(14.96) | 96(18.37) | 0.2434 |
| Hypertension, n (%) | 882(70.82) | 423(62.88) | 459(80.72) | <0.0001 |
| Diabetes mellitus, n (%) | 425(30.81) | 126(16.51) | 299(48.63) | <0.0001 |
| 29 Features | XGboost | Random Forest | Logistic Regression | Deep Neural Network |
|---|---|---|---|---|
| Validation | ||||
| AUC of ROC | 0.8 | 0.83 | 0.81 | 0.82 |
| Accuracy | 0.72 | 0.77 | 0.72 | 0.75 |
| Sensitivity | 0.72 | 0.72 | 0.67 | 0.74 |
| Specificity | 0.72 | 0.81 | 0.77 | 0.75 |
| PPV | 0.69 | 0.77 | 0.72 | 0.72 |
| NPV | 0.75 | 0.77 | 0.73 | 0.77 |
| Test | ||||
| AUC of ROC | 0.78 | 0.77 | 0.76 | 0.76 |
| Accuracy | 0.7 | 0.7 | 0.68 | 0.69 |
| Sensitivity | 0.66 | 0.6 | 0.63 | 0.66 |
| Specificity | 0.73 | 0.78 | 0.73 | 0.72 |
| PPV | 0.69 | 0.7 | 0.67 | 0.67 |
| NPV | 0.71 | 0.69 | 0.69 | 0.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-L.; Liu, W.-J.; Tsai, S.-F. Development and Validation of an Insulin Resistance Model for a Population with Chronic Kidney Disease Using a Machine Learning Approach. Nutrients 2022, 14, 2832. https://doi.org/10.3390/nu14142832
Lee C-L, Liu W-J, Tsai S-F. Development and Validation of an Insulin Resistance Model for a Population with Chronic Kidney Disease Using a Machine Learning Approach. Nutrients. 2022; 14(14):2832. https://doi.org/10.3390/nu14142832
Chicago/Turabian StyleLee, Chia-Lin, Wei-Ju Liu, and Shang-Feng Tsai. 2022. "Development and Validation of an Insulin Resistance Model for a Population with Chronic Kidney Disease Using a Machine Learning Approach" Nutrients 14, no. 14: 2832. https://doi.org/10.3390/nu14142832
APA StyleLee, C.-L., Liu, W.-J., & Tsai, S.-F. (2022). Development and Validation of an Insulin Resistance Model for a Population with Chronic Kidney Disease Using a Machine Learning Approach. Nutrients, 14(14), 2832. https://doi.org/10.3390/nu14142832






