Secondary Metabolites in the Dendrobium heterocarpum Methanolic Extract and Their Impacts on Viability and Lipid Storage of 3T3-L1 Pre-Adipocytes
Abstract
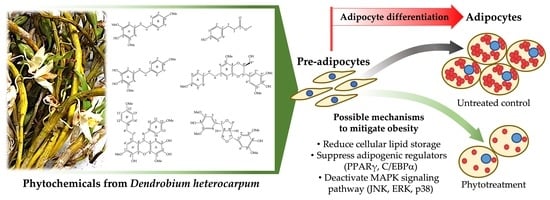
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction, Fractionation, and Phytochemical Elucidation
2.3. Cell Culture and Adipocyte Differentiation
2.4. Cytotoxicity Assessments
2.5. Quantification of Cellular Lipid Content
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
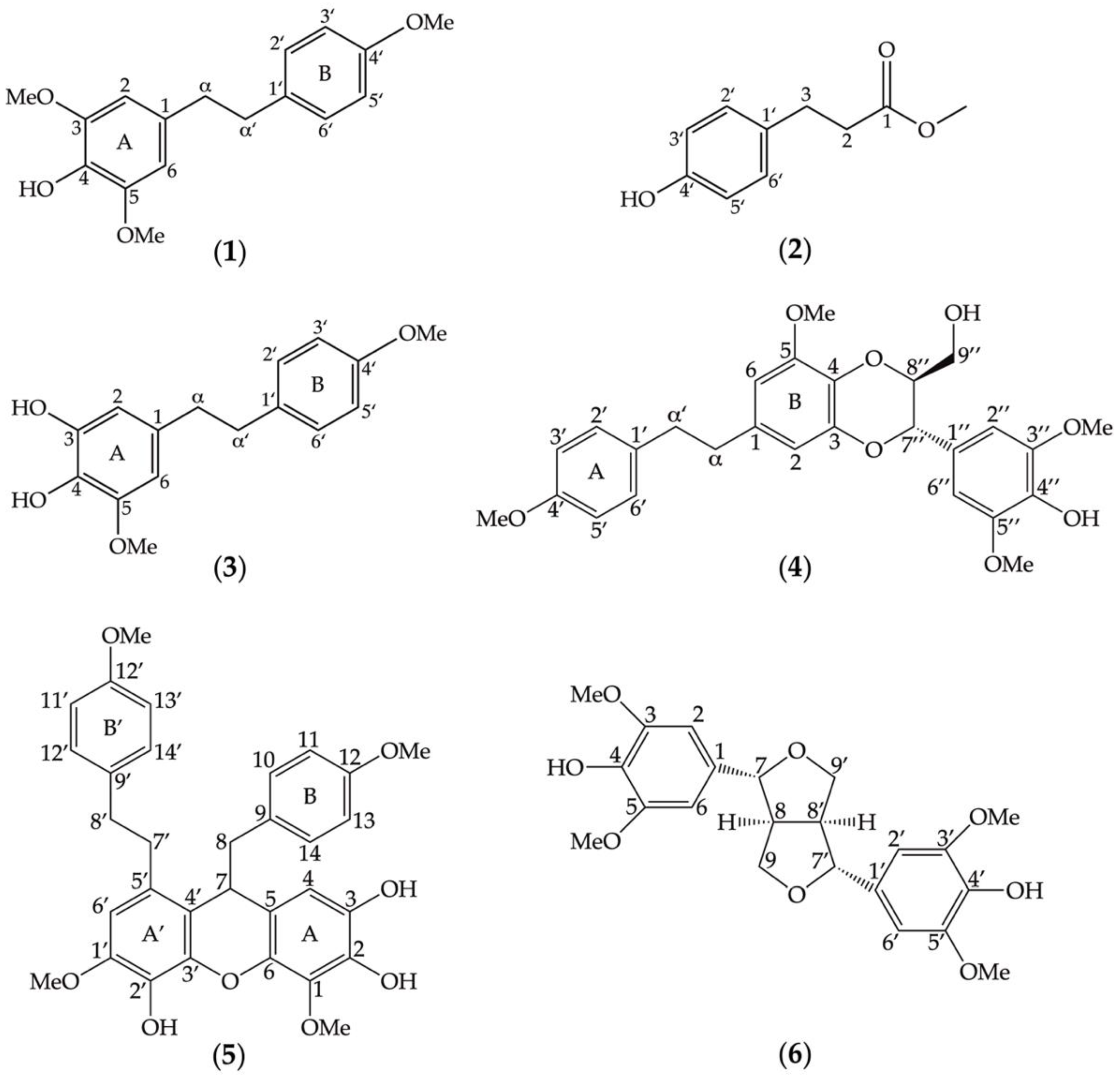
3.1. Secondary Metabolites in the Methanolic Extracts of D. heterocarpum
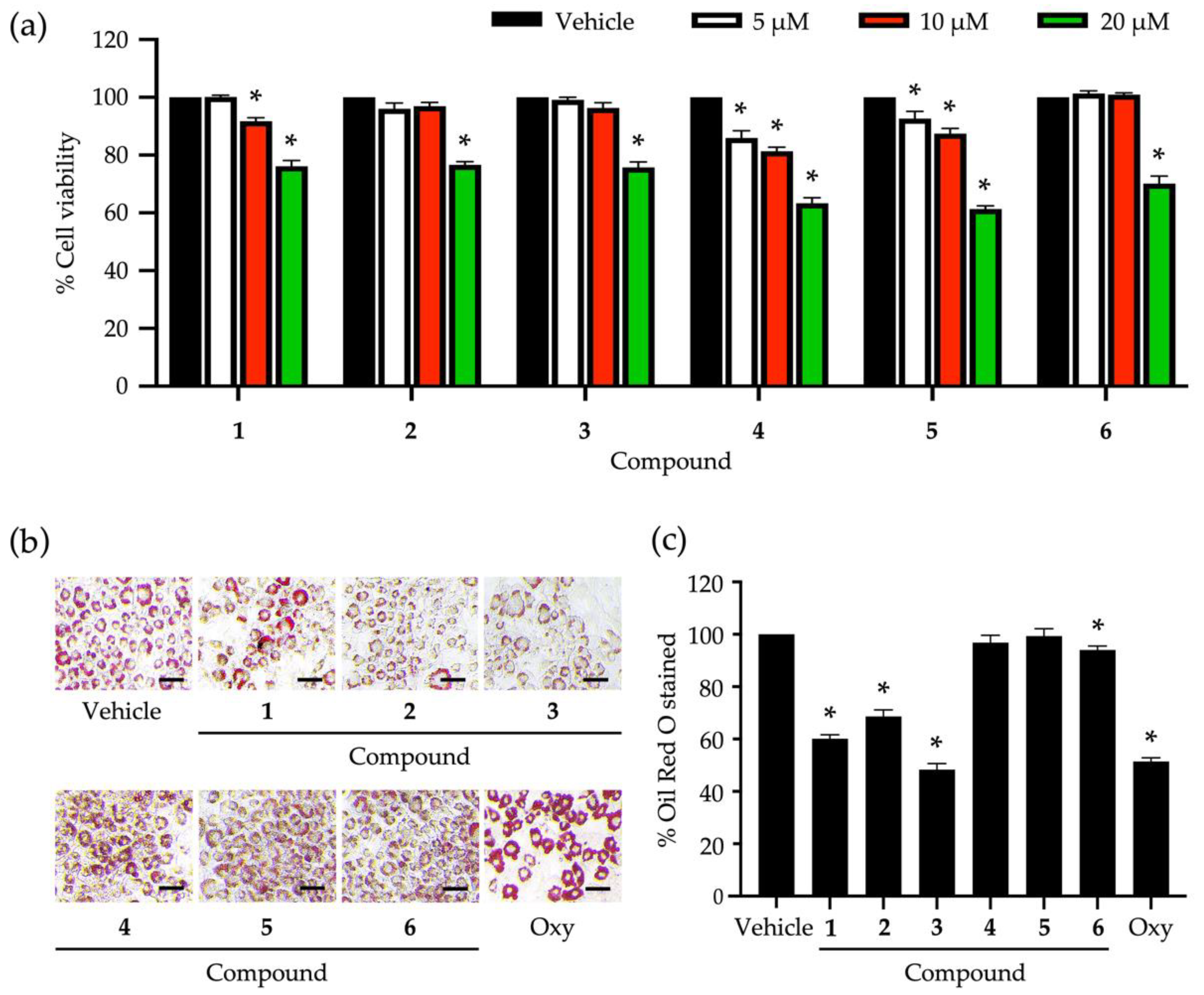
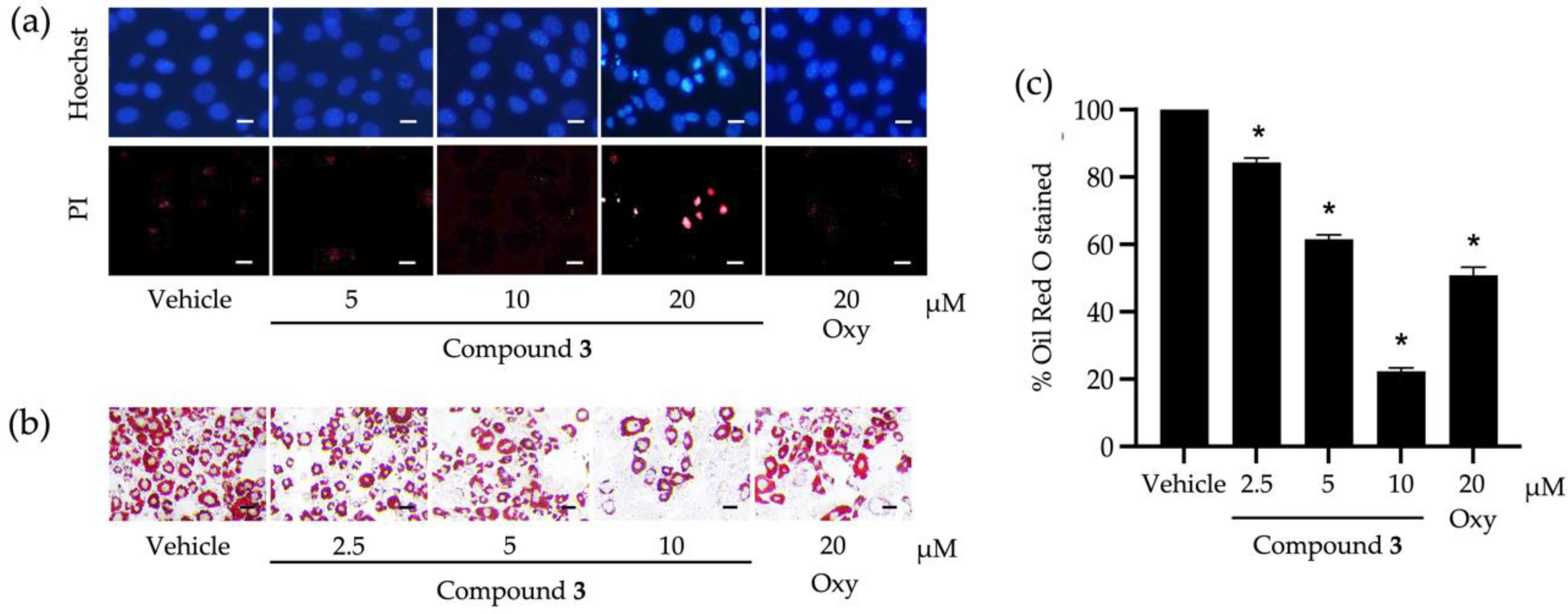
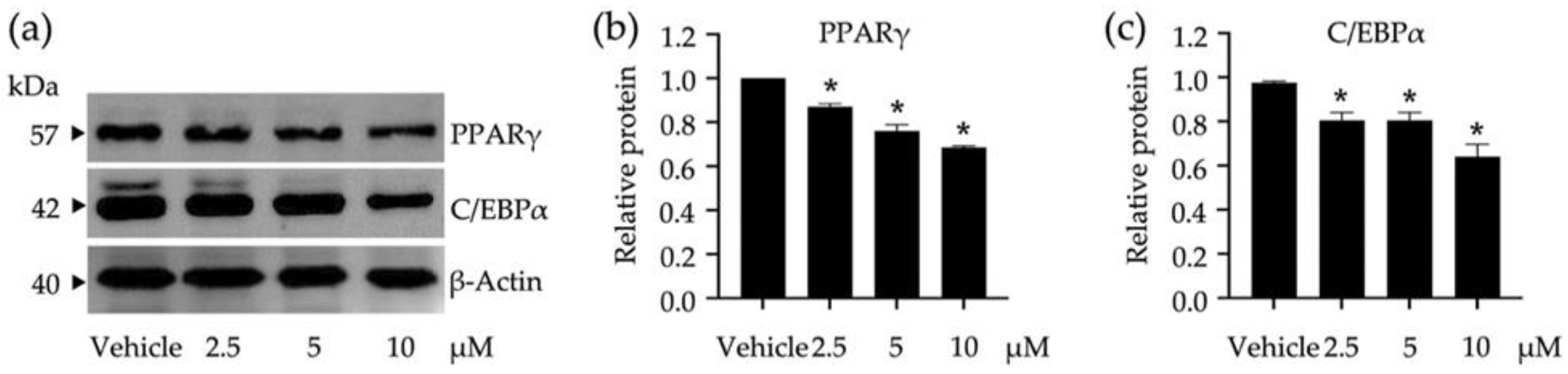
3.2. Roles of Identified Phytochemicals in the Growth and Development of Adipocytic Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xi, B.; Yang, L.; Sun, J.; Zhao, M.; Bovet, P. Trends in the prevalence of overweight, obesity, and abdominal obesity among Chinese adults between 1993 and 2015. Int. J. Obes. 2021, 45, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.H. Deaths attributable to obesity. JAMA. 2005, 293, 1918–1919. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Rhee, D.-K.; Kim, B.-O.; Pyo, S. Inhibitory effect of sinigrin on adipocyte differentiation in 3T3-L1 cells: Involvement of AMPK and MAPK pathways. Biomed. Pharmacother. 2018, 102, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Jakab, J.; Miškić, B.; Miškić, S.; Juranić, B.; Ćosić, V.; Schwarz, D.; Včev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Cowherd, R.M.; Lyle, R.E.; McGehee, R.E., Jr. Molecular regulation of adipocyte differentiation. Semin. Cell Dev. Biol. 1999, 10, 3–10. [Google Scholar] [CrossRef]
- Rayalam, S.; Yang, J.-E.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Prusty, D.; Park, B.-H.; Davis, K.E.; Farmer, R.S. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Lee, H.S.; Han, H.-K.; Choi, C.-I. Saikosaponin A and D inhibit adipogenesis via the AMPK and MAPK signaling pathways in 3T3-L1 adipocytes. Int. J. Mol. Sci. 2021, 22, 11409. [Google Scholar] [CrossRef] [PubMed]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.T.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Lao, W.; Tan, Y.; Jin, X.; Xiao, L.; Kim, J.J.Y.; Qu, X. Comparison of cytotoxicity and the anti-adipogenic effect of green tea polyphenols with epigallocatechin-3-gallate in 3T3-L1 preadipocytes. Am. J. Chin. Med. 2015, 43, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.; Ng, T.B.; Yao, R.M.; Shi, J.; Xu, K.; Cho Wing Sze, S.; Zhang, K.Y. Evaluation of chemical constituents and important mechanism of pharmacological biology in Dendrobium plants. Evid.-Based Compl. Alt. 2015, 2015, 841752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiaohua, J.; Singchi, C.; Yibo, L. Taxonomic revision of Dendrobium moniliforme complex (Orchidaceae). Sci. Hortic. 2009, 120, 143–145. [Google Scholar] [CrossRef]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory activities and glucose uptake stimulatory effect of phenolic compounds from Dendrobium formosum. Rev. Bras. Farmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Limpanit, R.; Chuanasa, T.; Likhitwitayawuid, K.; Jongbunpresert, V.; Sritularak, B. α-Glucosidase inhibitors from Dendrobium tortile. Rec. Nat. Prod. 2016, 10, 609. [Google Scholar]
- Liu, Y.; Yang, L.; Zhang, Y.; Liu, X.; Wu, Z.; Gilbert, R.G.; Deng, B.; Wang, K. Dendrobium officinale polysaccharide ameliorates diabetic hepatic glucose metabolism via glucagon-mediated signaling pathways and modifying liver-glycogen structure. J. Ethnopharmacol. 2020, 248, 112308. [Google Scholar] [CrossRef]
- Lu, Y.; Kuang, M.; Hu, G.-P.; Wu, R.-B.; Wang, J.; Liu, L.; Lin, Y.-C. Loddigesiinols G–J: α-Glucosidase inhibitors from Dendrobium loddigesii. Molecules 2014, 19, 8544–8555. [Google Scholar] [CrossRef] [Green Version]
- Maitreesophone, P.; Khine, H.E.E.; Quiel Lasam Nealiga, J.; Kongkatitham, V.; Panuthai, P.; Chaotham, C.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and pancreatic lipase inhibitory effects and anti-adipogenic activity of dendrofalconerol B, a bisbibenzyl from Dendrobium harveyanum. S. Afr. J. Bot. 2022, 146, 187–195. [Google Scholar] [CrossRef]
- Na Ranong, S.; Likhitwitayawuid, K.; Mekboonsonglarp, W.; Sritularak, B. New dihydrophenanthrenes from Dendrobium infundibulum. Nat. Prod. Res. 2019, 33, 420–426. [Google Scholar] [CrossRef] [PubMed]
- San, H.T.; Boonsnongcheep, P.; Putalun, W.; Mekboonsonglarp, W.; Sritularak, B.; Likhitwitayawuid, K. α-Glucosidase inhibitory and glucose uptake stimulatory effects of phenolic compounds from Dendrobium christyanum. Nat. Prod. Commun. 2020, 15, 1934578X20913453. [Google Scholar]
- Sarakulwattana, C.; Mekboonsonglarp, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2020, 34, 1694–1701. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, F.; Yang, M.; Zhang, J.; Chen, J.; Zhan, R.; Li, L.; Chen, Y. Isolation of α-glucosidase inhibitors including a new flavonol glycoside from Dendrobium devonianum. Nat. Prod. Res. 2014, 28, 1900–1905. [Google Scholar] [CrossRef]
- Thant, M.T.; Chatsupun, N.; Mekboonsonglarp, W.; Sritularak, B.; Likhitwitayawuid, K. New fluorene derivatives from Dendrobium gibsonii and their α-glucosidase inhibitory activity. Molecules 2020, 25, 4931. [Google Scholar] [CrossRef] [PubMed]
- Thant, M.T.; Khine, H.E.E.; Quiel Lasam Nealiga, J.; Chatsumpun, N.; Chaotham, C.; Sritularak, B.; Likhitwitayawuid, K. α-Glucosidase inhibitory activity and anti-adipogenic effect of compounds from Dendrobium delacourii. Molecules 2022, 27, 1156. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Huang, M.; Lo, K.; Chen, W.-L.; He, Y.-Y.; Xu, Y.; Zheng, H.; Hu, H.; Wang, J. Anti-diabetic effect of a shihunine-rich extract of Dendrobium loddigesii on 3T3-L1 cells and db/db mice by up-regulating AMPK–GLUT4–PPARα. Molecules 2019, 24, 2673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaddhanaphuti, N.B. A Field Guide to the Wild Orchids of Thailand, 4th ed.; Silkworm Books: Chiang Mai, Thailand, 2005; p. 117. [Google Scholar]
- Tan, H.Y.; Iris, M.Y.; Li, E.T.; Wang, M. Inhibitory effects of oxyresveratrol and cyanomaclurin on adipogenesis of 3T3-L1 cells. J. Funct. Foods 2015, 15, 207–216. [Google Scholar] [CrossRef]
- Majumder, P.L.; Guha, S.; Sen, P. Bibenzyl derivatives from the orchid Dendrobium amoenum. Phytochemistry 1999, 52, 1365–1369. [Google Scholar] [CrossRef]
- Fang, Y.-S.; Yang, M.-H.; Cai, L.; Wang, J.-P.; Yin, T.-P.; Yu, J.; Ding, Z.-T. New phenylpropanoids from Bulbophyllum retusiusculum. Arch. Pharm. Res. 2018, 41, 1074–1081. [Google Scholar] [CrossRef]
- Zhi-Ming, B.; Zheng-Tao, W.; Luo-Shan, X. Chemical constituents of Dendrobium moniliforme. Acta Bot. Sin. 2004, 46, 124–126. [Google Scholar]
- Li, Y.; Wang, C.-L.; Guo, S.-X.; Yang, J.-S.; Xiao, P.-G. Two new compounds from Dendrobium candidum. Chem. Pharm. Bull. 2008, 56, 1477–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sritularak, B.; Likhitwitayawuid, K. New bisbibenzyls from Dendrobium falconeri. Helv. Chim. Acta 2009, 92, 740–744. [Google Scholar] [CrossRef]
- Sritularak, B.; Duangrak, N.; Likhitwitayawuid, K. A new bibenzyl from Dendrobium secundum. Z. Naturforsch. C 2011, 66, 205–208. [Google Scholar] [CrossRef]
- Qu, J.; Tan, S.; Xie, X.; Wu, W.; Zhu, H.; Li, H.; Liao, X.; Wang, J.; Zhou, Z.-A.; Huang, S. Dendrobium Officinale polysaccharide attenuates insulin resistance and abnormal lipid metabolism in obese mice. Front. Pharmacol. 2021, 12, 659626. [Google Scholar] [CrossRef]
- Zakir, H.A.; Subbarao, G.V.; Pearse, S.J.; Gopalakrishnan, S.; Ito, O.; Ishikawa, T.; Kawano, N.; Nakahara, K.; Yoshihashi, T.; Ono, H.; et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol. 2008, 180, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Ren, G.; Deng, W.Z.; Xie, Y.F.; Wu, C.H.; Li, W.Y.; Xiao, C.Y.; Chen, Y.L. Bibenzyl derivatives from leaves of Dendrobium officinale. Nat. Prod. Commun. 2020, 15, 1934578X20908678. [Google Scholar] [CrossRef] [Green Version]
- Putri, H.E.; Nutho, B.; Rungrotmongkol, T.; Sritularak, B.; Vinayanuwattikun, C.; Chanvorachote, P. Bibenzyl analogue DS-1 inhibits MDM2-mediated p53 degradation and sensitizes apoptosis in lung cancer cells. Phytomedicine 2021, 85, 153534. [Google Scholar] [CrossRef]
- Putri, H.E.; Sritularak, B.; Chanvorachote, P. DS-1 inhibits migration and invasion of non-small-cell lung cancer cells through suppression of epithelial to mesenchymal transition and integrin β1/FAK signaling. Anticancer Res. 2021, 41, 2913–2923. [Google Scholar] [CrossRef]
- Mittraphab, A.; Muangnoi, C.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. A new bibenzyl-phenanthrene derivative from Dendrobium signatum and its cytotoxic activity. Nat. Prod. Commun. 2016, 11, 1934578X1601100526. [Google Scholar] [CrossRef] [Green Version]
- Pengpaeng, P.; Sritularak, B.; Chanvorachote, P. Dendrofalconerol A sensitizes anoikis and inhibits migration in lung cancer cells. J. Nat. Med. 2015, 69, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Pengpaeng, P.; Sritularak, B.; Chanvorachote, P. Dendrofalconerol A suppresses migrating cancer cells via EMT and integrin proteins. Anticancer Res. 2015, 35, 201–205. [Google Scholar] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial activity of simple coumarins: Structural requirements for biological activity. Z. Naturforsch. C 1999, 54, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, F.; Qi, J.; Song, X.C.; Hu, Z.F.; Zhu, D.N.; Yu, B.Y. Homoisoflavonoids from the fibrous roots of Polygonatum odoratum with glucose uptake-stimulatory activity in 3T3-L1 adipocytes. J. Nat. Prod. 2010, 73, 548–552. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Xu, B.; Zeng, G.; Tan, J.; He, X.; Hu, C.; Zhou, Y. Chemical constituents of Morus alba L. and their inhibitory effect on 3T3-L1 preadipocyte proliferation and differentiation. Fitoterapia 2014, 98, 222–227. [Google Scholar] [CrossRef]
- Nishina, A.; Ukiya, M.; Fukatsu, M.; Koketsu, M.; Ninomiya, M.; Sato, D.; Yamamoto, J.; Kobayashi-Hattori, K.; Okubo, T.; Tokuoka, H.; et al. Effects of various 5, 7-dihydroxyflavone analogs on adipogenesis in 3T3-L1 cells. Biol. Pharm. Bull. 2015, 38, 1794–1800. [Google Scholar] [CrossRef] [Green Version]
- Bryan, R.F.; Fallon, L. Crystal structure of syringaresinol. J. Chem. Soc. Perkin Trans. 1976, 2, 341–345. [Google Scholar] [CrossRef]
- Chen, Y.G.; Yu, H.; Lian, X. Isolation of stilbenoids and lignans from Dendrobium hongdie. Trop. J. Pharm. Res. 2015, 14, 2055–2059. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.M.; Chen, J.J.; Yu, H.; Zhao, Y.X.; Zhou, J. Two novel bibenzyls from Dendrobium trigonopus. J. Asian Nat. Prod. Res. 2008, 10, 647–651. [Google Scholar] [CrossRef]
- Zhou, J. Chemical constituents of Dendrobium officinale. Chin. Tradit. Herb. Drugs 2015, 24, 1292–1295. [Google Scholar]
- Lv, H.W.; Li, Y.X.; Luo, M.; Qi, J.M.; Fu, Z.F.; Zhang, H.J.; Guo, Y.Q.; Chu, C.; Li, H.B.; Yan, J.Z. Two new nor-lignans from Selaginella pulvinata (Hook. & Grev.) Maxim and their antihyperglycemic activities. Nat. Prod. Res. 2021, 36, 279–286. [Google Scholar]
- Poulos, S.P.; Dodson, M.V.; Hausman, G.J. Cell line models for differentiation: Preadipocytes and adipocytes. Exp. Biol. Med. 2010, 235, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.; McGee, S.L. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte 2015, 4, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.R.; Barrick, C.; Kim, K.A.; Lindner, J.; Blondeau, B.; Fujimoto, Y.; Shiota, M.; Kesterson, R.A.; Kahn, B.B.; Magnuson, M.A. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2005, 102, 6207–6212. [Google Scholar] [CrossRef] [Green Version]
- Darlington, G.J.; Wang, N.; Hanson, R.W. C/EBPα: A critical regulator of genes governing integrative metabolic processes. Curr. Opin. Genet. Dev. 1995, 5, 565–570. [Google Scholar] [CrossRef]
- Tarantino, G.; Caputi, A. JNKs, insulin resistance and inflammation: A possible link between NAFLD and coronary artery disease. World J. Gastroenterol. 2011, 17, 3785–3794. [Google Scholar] [CrossRef]
- Kusuyama, J.; Komorizono, A.; Bandow, K.; Ohnishi, T.; Matsuguchi, T. CXCL3 positively regulates adipogenic differentiation. J. Lipid Res. 2016, 57, 1806–1820. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Su, Q.; Bai, L.; Li, M.; Liu, J.; Liu, X.; Zhang, C.; Jiang, Z.; He, J.; Shi, J.; et al. Recent research progress on natural small molecule bibenzyls and its derivatives in Dendrobium species. Eur. J. Med. Chem. 2020, 204, 112530. [Google Scholar] [CrossRef]
- Maekawa, T.; Jin, W.; Ishii, S. The role of ATF-2 family transcription factors in adipocyte differentiation: Antiobesity effects of p38 inhibitors. Mol. Cell. Biol. 2010, 30, 613–625. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warinhomhoun, S.; Khine, H.E.E.; Sritularak, B.; Likhitwitayawuid, K.; Miyamoto, T.; Tanaka, C.; Punsawad, C.; Punpreuk, Y.; Sungthong, R.; Chaotham, C. Secondary Metabolites in the Dendrobium heterocarpum Methanolic Extract and Their Impacts on Viability and Lipid Storage of 3T3-L1 Pre-Adipocytes. Nutrients 2022, 14, 2886. https://doi.org/10.3390/nu14142886
Warinhomhoun S, Khine HEE, Sritularak B, Likhitwitayawuid K, Miyamoto T, Tanaka C, Punsawad C, Punpreuk Y, Sungthong R, Chaotham C. Secondary Metabolites in the Dendrobium heterocarpum Methanolic Extract and Their Impacts on Viability and Lipid Storage of 3T3-L1 Pre-Adipocytes. Nutrients. 2022; 14(14):2886. https://doi.org/10.3390/nu14142886
Chicago/Turabian StyleWarinhomhoun, Sakan, Hnin Ei Ei Khine, Boonchoo Sritularak, Kittisak Likhitwitayawuid, Tomofumi Miyamoto, Chiaki Tanaka, Chuchard Punsawad, Yanyong Punpreuk, Rungroch Sungthong, and Chatchai Chaotham. 2022. "Secondary Metabolites in the Dendrobium heterocarpum Methanolic Extract and Their Impacts on Viability and Lipid Storage of 3T3-L1 Pre-Adipocytes" Nutrients 14, no. 14: 2886. https://doi.org/10.3390/nu14142886