A Review of the Potential Consequences of Pearl Millet (Pennisetum glaucum) for Diabetes Mellitus and Other Biomedical Applications
Abstract
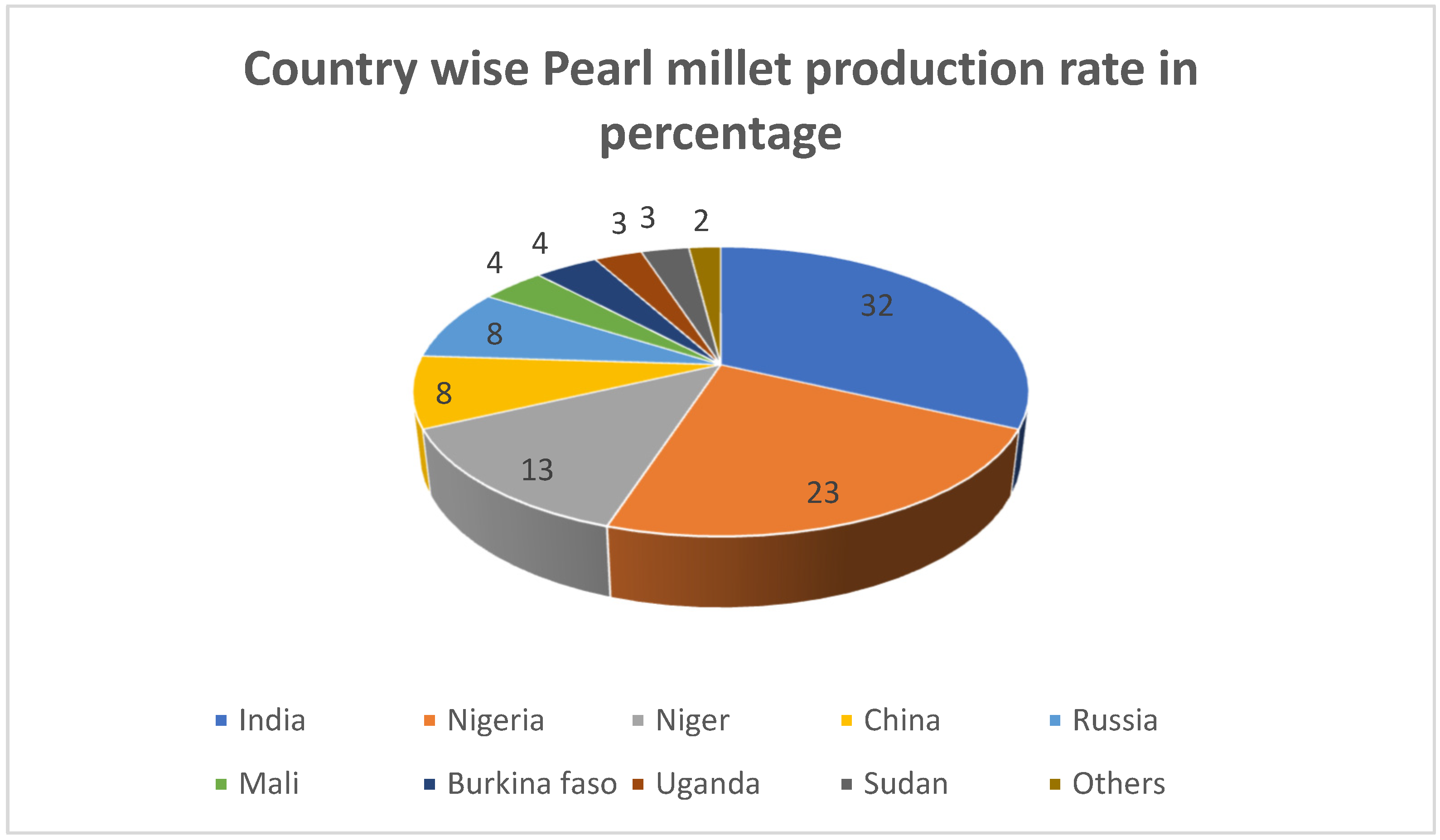
:1. Introduction
2. Millets for Diabetes Control
3. Pearl Millet and Its Nutritional Significance
4. Pearl Millet and Diabetes
5. Pearl Millet in the Human Disease Management System
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mechchate, H.; Es-safi, I.; Louba, A.; Alqahtani, A.S.; Nasr, F.A.; Noman, O.M.; Farooq, M.; Alharbi, M.S.; Alqahtani, A.; Bari, A.; et al. In Vitro Alpha-Amylase and Alpha-Glucosidase Inhibitory Activity and In Vivo Antidiabetic Activity of Withania frutescens L. Foliar Extract. Molecules 2021, 26, 293. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Q.; Qu, K.; Luo, Y.; Yin, D.; Ju, Y.; Tang, H. Predicting Diabetes Mellitus with Machine Learning Techniques. Front. Genet. 2018, 9, 515. [Google Scholar] [CrossRef] [PubMed]
- Nolan, C.J.; Prentki, M. Insulin Resistance and Insulin Hypersecretion in the Metabolic Syndrome and Type 2 Diabetes: Time for a Conceptual Framework Shift. Diabetes Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, J.; Liu, Y.; Sun, G. Interactions between and Shared Molecular Mechanisms of Diabetic Peripheral Neuropathy and Obstructive Sleep Apnea in Type 2 Diabetes Patients. J. Diabetes Res. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Sone, H.; Tanaka, S.; Tanaka, S.; Iimuro, S.; Oida, K.; Yamasaki, Y.; Oikawa, S.; Ishibashi, S.; Katayama, S.; Ohashi, Y.; et al. Serum Level of Triglycerides Is a Potent Risk Factor Comparable to LDL Cholesterol for Coronary Heart Disease in Japanese Patients with Type 2 Diabetes: Subanalysis of the Japan Diabetes Complications Study (JDCS). J. Clin. Endocrinol. Metab. 2011, 96, 3448–3456. [Google Scholar] [CrossRef]
- Bitzur, R.; Cohen, H.; Kamari, Y.; Shaish, A.; Harats, D. Triglycerides and HDL Cholesterol: Stars or Second Leads in Diabetes? Diabetes Care 2009, 32, S373–S377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop-Busui, R.; Boulton, A.J.M.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Oei, L.; Rivadeneira, F.; Zillikens, M.C.; Oei, E.H.G. Diabetes, Diabetic Complications, and Fracture Risk. Curr. Osteoporos. Rep. 2015, 13, 106–115. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard, P. Discrepancies in Bone Mineral Density and Fracture Risk in Patients with Type 1 and Type 2 Diabetes—A Meta-Analysis. Osteoporos. Int. 2007, 18, 427–444. [Google Scholar] [CrossRef]
- Saito, M.; Fujii, K.; Soshi, S.; Tanaka, T. Reductions in Degree of Mineralization and Enzymatic Collagen Cross-Links and Increases in Glycation-Induced Pentosidine in the Femoral Neck Cortex in Cases of Femoral Neck Fracture. Osteoporos. Int. 2006, 17, 986–995. [Google Scholar] [CrossRef]
- Ma, L.; Oei, L.; Jiang, L.; Estrada, K.; Chen, H.; Wang, Z.; Yu, Q.; Zillikens, M.C.; Gao, X.; Rivadeneira, F. Association between Bone Mineral Density and Type 2 Diabetes Mellitus: A Meta-Analysis of Observational Studies. Eur. J. Epidemiol. 2012, 27, 319–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oei, L.; Zillikens, M.C.; Dehghan, A.; Buitendijk, G.H.S.; Castano-Betancourt, M.C.; Estrada, K.; Stolk, L.; Oei, E.H.G.; van Meurs, J.B.J.; Janssen, J.A.M.J.L.; et al. High Bone Mineral Density and Fracture Risk in Type 2 Diabetes as Skeletal Complications of Inadequate Glucose Control: The Rotterdam Study. Diabetes Care 2013, 36, 1619–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, G.; Weiss, C.; Lehmann, G.; Niwa, T.; Stein, G.; Franke, S. Advanced Glycation End Product Modification of Bone Proteins and Bone Remodelling: Hypothesis and Preliminary Immunohistochemical Findings. Ann. Rheum. Dis. 2006, 65, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.R. Bone Cells and Bone Turnover in Diabetes Mellitus. Curr. Osteoporos. Rep. 2015, 13, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Halvatsiotis, P.; Short, K.R.; Bigelow, M.; Nair, K.S. Synthesis Rate of Muscle Proteins, Muscle Functions, and Amino Acid Kinetics in Type 2 Diabetes. Diabetes 2002, 51, 2395–2404. [Google Scholar] [CrossRef] [Green Version]
- Bassil, M.S.; Gougeon, R. Muscle Protein Anabolism in Type 2 Diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 83–88. [Google Scholar] [CrossRef]
- Moller, N.; Nair, K.S. Diabetes and Protein Metabolism. Diabetes 2008, 57, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Asif, M. The Prevention and Control the Type-2 Diabetes by Changing Lifestyle and Dietary Pattern. J. Educ. Health Promot. 2014, 3, 1. [Google Scholar] [CrossRef]
- Willett, W.; Manson, J.; Liu, S. Glycemic Index, Glycemic Load, and Risk of Type 2 Diabetes. Am. J. Clin. Nutr. 2002, 76, 274S–280S. [Google Scholar] [CrossRef] [Green Version]
- Amadoubr, I.; Gounga, M.E.; Le, G.-W. Millets: Nutritional Composition, Some Health Benefits and Processing—A Review. Emir. J. Food Agric. 2013, 25, 501. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.S.M.; Zhang, Q.; Chen, J.; Shen, Q. Millet Grains: Nutritional Quality, Processing, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2013, 12, 281–295. [Google Scholar] [CrossRef]
- Nambiar, V.S.; Sareen, N.; Shahu, T.; Desai, R.; Dhaduk, J.J.; Nambiar, S. Potential Functional Implications of Pearl Millet (Pennisetum glaucum) in Health and Disease. J. Appl. Pharm. Sci. 2011, 1, 62–67. [Google Scholar]
- Nishizawa, N.; Togawa, T.; Park, K.; Sato, D.; Miyakoshi, Y.; Inagaki, K.; Ohmori, N.; Ito, Y.; Nagasawa, T. Dietary Japanese Millet Protein Ameliorates Plasma Levels of Adiponectin, Glucose, and Lipids in Type 2 Diabetic Mice. Biosci. Biotechnol. Biochem. 2009, 73, 351–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitha, S.; Govindaraj, M.; Kane-Potaka, J. Balanced Amino Acid and Higher Micronutrients in Millets Complements Legumes for Improved Human Dietary Nutrition. Cereal Chem. 2020, 97, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Shobana, S.; Krishnaswamy, K.; Sudha, V.; Malleshi, N.G.; Anjana, R.M.; Palaniappan, L.; Mohan, V. Chapter one: Finger Millet (Ragi, Eleusine coracana L.): A Review of Its Nutritional Properties, Processing, and Plausible Health Benefits. Adv. Food Nutr. Res. 2013, 69, 1–39. [Google Scholar]
- Arora, P.D.; Fan, L.; Sodek, J.; Kapus, A.; McCulloch, C.A. Differential Binding to Dorsal and Ventral Cell Surfaces of Fibroblasts: Effect on Collagen Phagocytosis. Exp. Cell Res. 2003, 286, 366–380. [Google Scholar] [CrossRef]
- Satankar, M.; Kumar, U.; Patil, A.K.; Kautkar, S. Pearl Millet: A Fundamental Review on Underutilized Source of Nutrition. Multilogic Sci. 2020, 10, 1081–1084. [Google Scholar]
- Singh, N.; Singh, S.P.; Kumar, M.; Kanhiya, K.; Kumar, A. Nutri Cereal Pearlmillet: Way Forward. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 578–581. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdelaal, E.; Noamam, M. Antioxidant Activity and Nutrient Composition of Selected Cereals for Food Use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Emmambux, M.N. Products Containing Other Speciality Grains: Sorghum, the Millets and Pseudocereals. In Technology of Functional Cereal Products; Elsevier: Amsterdam, The Netherlands, 2008; pp. 281–335. [Google Scholar]
- Pattanashetti, S.K.; Upadhyaya, H.D.; Dwivedi, S.L.; Vetriventhan, M.; Reddy, K.N. Pearl Millet. In Genetic and Genomic Resources for Grain Cereals Improvement; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–289. [Google Scholar]
- Chapke, R.R.; Prabhakar, R.; Prasad, G.S.; Das, I.K.; Tonapi, V.A. Improved Millets Production Technologies and Their Impact; IIMR Publication: Hyderabad, India, 2018; pp. 1–88. [Google Scholar]
- Malik, S. Pearl Millet-Nutritional Value and Medicinal Uses. Int. J. Adv. Res. Innov. Ideas Educ. 2015, 1, 414–418. [Google Scholar]
- Dasa, F.; Nguyen, B. Relation among Proximate Compositions, Rheological Properties and Injera Making Quality of Millet Varieties. Adv. Crop Sci. Technol. 2020, 8, 1000453. [Google Scholar]
- Bhupender, S.K.; Rajneesh, B.; Baljeet, S.Y. Physicochemical, Functional, Thermal and Pasting Properties of Starches Isolated from Pearl Millet Cultivars. Int. Food Res. J. 2013, 20, 1555–1561. [Google Scholar]
- Giannoccaro, E.; Wang, Y.; Chen, P. Effects of Solvent, Temperature, Time, Solvent-to-Sample Ratio, Sample Size, and Defatting on the Extraction of Soluble Sugars in Soybean. J. Food Sci. 2006, 71, C59–C64. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Clegg, C.; Rooney, L.W. Effects of Parboiling and Decortication on the Nutritional Value of Sorghum (Sorghum bicolor L. Moench) and Pearl Millet (Pennisetum glaucum L.). J. Cereal Sci. 1994, 19, 83–89. [Google Scholar] [CrossRef]
- Mondal, D.; Awana, A.; Aggarwal, S.; Das, D.; Thomas, B.; Singh, S.P.; Satyavathi, C.T.; Sundaram, R.M.; Anand, A.; Singh, A.; et al. Microstructure, matrix interactions, and molecular structure are the key determinants of inherent glycemic potential in pearl millet (Pennisetum glaucum). Food Hydrocoll. 2022, 127, 107481. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Fermented and Malted Millet Products in Africa: Expedition from Traditional/Ethnic Foods to Industrial Value-Added Products. Crit. Rev. Food Sci. Nutr. 2018, 58, 463–474. [Google Scholar] [CrossRef]
- Jellum, M.D.; Powell, J.B. Fatty Acid Composition of Oil from Pearl Millet Seed. Agron. J. 1971, 63, 29–33. [Google Scholar] [CrossRef]
- Osagie, A.U.; Kates, M. Lipid Composition of Millet (Pennisetum americanum) Seeds. Lipids 1984, 19, 958–965. [Google Scholar] [CrossRef]
- Kamath, M.V.; Belavady, B. Unavailable Carbohydrates of Commonly Consumed Indian Foods. J. Sci. Food Agric. 1980, 31, 192–202. [Google Scholar] [CrossRef]
- Saini, S.; Saxena, S.; Samtiya, M.; Puniya, M.; Dhewa, T. Potential of Underutilized Millets as Nutri-Cereal: An Overview. J. Food Sci. Technol. 2021, 58, 4465–4477. [Google Scholar] [CrossRef] [PubMed]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential Use of Pearl Millet (Pennisetum glaucum (L.) R. Br. in Brazil: Food Security, Processing, Health Benefits and Nutritional Products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [CrossRef] [PubMed] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.v; Metz, G.L.; Alberti, K.G. Dietary Fibres, Fibre Analogues, and Glucose Tolerance: Importance of Viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef] [Green Version]
- Mani, U.v; Prabhu, B.M.; Damle, S.S.; Mani, I. Glycaemic Index of Some Commonly Consumed Foods in Western India. Asia. Pac. J. Clin. Nutr. 1993, 2, 111–114. [Google Scholar]
- Asp, N.G. Nutritional Classification and Analysis of Food Carbohydrates. Am. J. Clin. Nutr. 1994, 59, 679S–681S. [Google Scholar] [CrossRef]
- Geetha, K.; Yankanchi, G.M.; Hulamani, S.; Hiremath, N. Glycemic Index of Millet Based Food Mix and Its Effect on Pre-diabetic Subjects. J. Food Sci. Technol. 2020, 57, 2732–2738. [Google Scholar] [CrossRef]
- Shukla, K.; Narain, J.P.; Puri, P.; Gupta, A.; Bijlani, R.L.; Mahapatra, S.C.; Karmarkar, M.G. Glycaemic Response to Maize, Bajra and Barley. Indian J. Physiol. Pharmacol. 1991, 35, 249–254. [Google Scholar]
- Abdelgadir, M.; Abbas, M.; Järvi, A.; Elbagir, M.; Eltom, M.; Berne, C. Glycaemic and Insulin Responses of Six Traditional Sudanese Carbohydrate-Rich Meals in Subjects with Type 2 Diabetes Mellitus. Diabet. Med. 2005, 22, 213–217. [Google Scholar] [CrossRef]
- Collings, P.; Williams, C.; MacDonald, I. Effects of Cooking on Serum Glucose and Insulin Responses to Starch. Br. Med. J. (Clin. Res. Ed.) 1981, 282, 1032. [Google Scholar] [CrossRef] [Green Version]
- Sukar, K.A.O.; Abdalla, R.I.; Humeda, H.S.; Alameen, A.O.; Mubarak, E.I. Effect of Pearl Millet on Glycaemic Control and Lipid Profile in Streptozocin Induced Diabetic Wistar Rat Model. Asian J. Med. Health 2020, 18, 40–51. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, I.M.; Hsu, F.L.; Chen, C.F.; Cheng, J.T. Antihyperglycemic Action of Isoferulic Acid in Streptozotocin-Induced Diabetic Rats. Br. J. Pharmacol. 2000, 129, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salar, R.K.; Purewal, S.S. Phenolic Content, Antioxidant Potential and DNA Damage Protection of Pearl Millet (Pennisetum glaucum) Cultivars of North Indian Region. J. Food Meas. Charact. 2017, 11, 126–133. [Google Scholar] [CrossRef]
- Alzahrani, N.S.; Alshammari, G.M.; El-Ansary, A.; Yagoub, A.E.A.; Amina, M.; Saleh, A.; Yahya, M.A. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients 2022, 14, 1791. [Google Scholar] [CrossRef] [PubMed]
- Tomar, M.; Bhardwaj, R.; Kumar, M.; Singh, S.P.; Krishnan, V.; Kansal, R.; Verma, R.; Yadav, V.K.; Dahuja, A.; Ahlawat, S.P.; et al. Nutritional composition patterns and application of multivariate analysis to evaluate indigenous Pearl millet ((Pennisetum glaucum (L.) R. Br.) germplasm. J. Food Compost. Anal. 2021, 103, 104086. [Google Scholar] [CrossRef]
- Kangama, C.O. Pearl millet (Pennisetum glaucum) perspectives in Africa. Int. J. Sci. Res. Arch. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Patel, S. Cereal Bran Fortified-Functional Foods for Obesity and Diabetes Management: Triumphs, Hurdles and Possibilities. J. Funct. Foods 2015, 14, 255–269. [Google Scholar] [CrossRef]
- Nani, A.; Belarbi, M.; Ksouri-Megdiche, W.; Abdoul-Azize, S.; Benammar, C.; Ghiringhelli, F.; Hichami, A.; Khan, N.A. Effects of Polyphenols and Lipids from Pennisetum glaucum Grains on T-Cell Activation: Modulation of Ca2+ and ERK1/ERK2 Signaling. BMC Complement. Altern. Med. 2015, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- Hegde, P.S.; Rajasekaran, N.S.; Chandra, T.S. Effects of the Antioxidant Properties of Millet Species on Oxidative Stress and Glycemic Status in Alloxan-Induced Rats. Nutr. Res. 2005, 25, 1109–1120. [Google Scholar] [CrossRef]
- Krishnan, R.; Meera, M.S. Pearl Millet Minerals: Effect of Processing on Bioaccessibility. J. Food Sci. Technol. 2018, 55, 3362–3372. [Google Scholar] [CrossRef]
- Annor, G.A.; Tyl, C.; Marcone, M.; Ragaee, S.; Marti, A. Why Do Millets Have Slower Starch and Protein Digestibility than Other Cereals? Trends Food Sci. Technol. 2017, 66, 73–83. [Google Scholar] [CrossRef]
- Cao, H.; Chen, X. Structures Required of Flavonoids for Inhibiting Digestive Enzymes. Anticancer Agents Med. Chem. 2012, 12, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Kam, J.; Puranik, S.; Yadav, R.; Manwaring, H.R.; Pierre, S.; Srivastava, R.K.; Yadav, R.S. Dietary Interventions for Type 2 Diabetes: How Millet Comes to Help. Front. Plant Sci. 2016, 7, 1454. [Google Scholar] [CrossRef] [PubMed]
- Adéoti, K.; Kouhoundé, S.H.S.; Noumavo, P.A.; Baba-Moussa, F.; Toukourou, F. Nutritional value and physicochemical composition of pearl millet (Pennisetum glaucum) produced in Benin. J. Microbiol. Biotech. Food Sci. 2017, 7, 92–96. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Taylor, J.R.N.; Belton, P.S.; Beta, T.; Duodu, K.G. Increasing the Utilisation of Sorghum, Millets and Pseudocereals: Developments in the Science of Their Phenolic Phytochemicals, Biofortification and Protein Functionality. J. Cereal Sci. 2014, 59, 257–275. [Google Scholar] [CrossRef] [Green Version]
- Devi, P.; Vijayabharathi, R.; Sathyabama, S.; Malleshi, N.; Priyadarisini, V. Health Benefits of Finger Millet (Eleusine coracana L.) Polyphenols and Dietary Fiber: A Review. J. Food Sci. Technol. 2014, 51, 1021–1040. [Google Scholar] [CrossRef] [Green Version]
- Kajla, P.; Ambawat, S.; Singh, S.; Suman. Biofortification and Medicinal Value of Pearl Millet Flour. In Pearl Millet; CRC Press: Boca Raton, FL, USA, 2020; pp. 139–157. [Google Scholar]
- Ambati, K.; Sucharitha, K.V. Millets-Review on Nutritional Profiles and Health Benefits. Int. J. Recent Sci. Res. 2019, 10, 33943–33948. [Google Scholar]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Benton, D.; Young, H.A. Reducing Calorie Intake May Not Help You Lose Body Weight. Perspect. Psychol. Sci. 2017, 12, 703–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.R.N.; Emmambux, M.N. Gluten-Free Foods and Beverages from Millets. In Gluten-Free Cereal Products and Beverages; Elsevier: Amsterdam, The Netherlands, 2008; pp. 119–148. [Google Scholar]
- Hassan, Z.M.; Sebola, N.A.; Mabelebele, M. The Nutritional Use of Millet Grain for Food and Feed: A Review. Agric. Food Secur. 2021, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Verma, P.; Saha, S.; Singh, S.; Vinutha, T.; Kumar, R.R.; Kulshreshta, A.; Singh, S.P.; Sathyavathi, T.; Sachdev, A.; et al. Polyphenol-enriched extract from pearl millet (Pennisetum glaucum) inhibits key enzymes involved in post prandial hyper glycemia (α-amylase, α-glucosidase) and regulates hepatic glucose uptake. Biocatal. Agric. Biotechnol. 2022, in press. [Google Scholar] [CrossRef]
- Ribichini, E.; Stigliano, S.; Rossi, S.; Zaccari, P.; Sacchi, M.C.; Bruno, G.; Badiali, D.; Severi, C. Role of Fibre in Nutritional Management of Pancreatic Diseases. Nutrients 2019, 11, 2219. [Google Scholar] [CrossRef] [Green Version]


| Nutrients | Amount | |
|---|---|---|
| Basic Components | Proteins | 22 g |
| Water | 17.3 g | |
| Ash | 6.5 g | |
| Calories | Total Calories | 756 cal |
| Calories from Carbohydrates | 600 cal | |
| Calories from Fates | 71 cal | |
| Calories from Proteins | 85.3 cal | |
| Carbohydrates | Total Carbohydrates | 146 g |
| Dietary Fibre | 17 g | |
| Fatty acids | Total Fat | 8.4 g |
| Saturated Fat | 1.4 g | |
| Monounsaturated Fatty Acid | 1.5 g | |
| Polyunsaturated Fatty Acid | 4.3 g | |
| Omega-3 Fatty Acids | 236 mg | |
| Omega-6-Fatty Acids | 4 g | |
| Vitamins | Vitamin E | 100 µg |
| Vitamin K | 1.8 µg | |
| Thiamine | 842 µg | |
| Riboflavin | 580 µg | |
| Niacin | 9.4 mg | |
| Vitamin B6 | 768 µg | |
| Foliate | 170 µg | |
| Pantothenic Acid | 170 µg | |
| Minerals | Calcium | 16 mg |
| Iron | 6 mg | |
| Magnesium | 228 mg | |
| Phosphorus | 570 mg | |
| Potassium | 390 mg | |
| Sodium | 10 mg | |
| Zinc | 3.4 mg | |
| Copper | 1.5 mg | |
| Manganese | 3.3 mg | |
| Selenium | 5.4 µg | |
| Amino Acids (g/100 g protein) | Leucine | 10.7 |
| Isoleucine | 4.4 | |
| Valine | 4.9 | |
| Threonine | 4.0 | |
| Arginine | 4.6 | |
| Lysine | 3.1 | |
| Methionine | 1.1 | |
| Cisteine | 1.5 | |
| Tryptophan | 1.4 | |
| Glutamic Acid | 23.0 | |
| Alanine | 8.7 | |
| Proline | 5.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, J.; Umapathy, V.R.; Vengadassalapathy, S.; Hussain, S.F.J.; Rajagopal, P.; Jayaraman, S.; Veeraraghavan, V.P.; Palanisamy, C.P.; Gopinath, K. A Review of the Potential Consequences of Pearl Millet (Pennisetum glaucum) for Diabetes Mellitus and Other Biomedical Applications. Nutrients 2022, 14, 2932. https://doi.org/10.3390/nu14142932
Pei J, Umapathy VR, Vengadassalapathy S, Hussain SFJ, Rajagopal P, Jayaraman S, Veeraraghavan VP, Palanisamy CP, Gopinath K. A Review of the Potential Consequences of Pearl Millet (Pennisetum glaucum) for Diabetes Mellitus and Other Biomedical Applications. Nutrients. 2022; 14(14):2932. https://doi.org/10.3390/nu14142932
Chicago/Turabian StylePei, JinJin, Vidhya Rekha Umapathy, Srinivasan Vengadassalapathy, Shazia Fathima Jaffer Hussain, Ponnulakshmi Rajagopal, Selvaraj Jayaraman, Vishnu Priya Veeraraghavan, Chella Perumal Palanisamy, and Krishnasamy Gopinath. 2022. "A Review of the Potential Consequences of Pearl Millet (Pennisetum glaucum) for Diabetes Mellitus and Other Biomedical Applications" Nutrients 14, no. 14: 2932. https://doi.org/10.3390/nu14142932
APA StylePei, J., Umapathy, V. R., Vengadassalapathy, S., Hussain, S. F. J., Rajagopal, P., Jayaraman, S., Veeraraghavan, V. P., Palanisamy, C. P., & Gopinath, K. (2022). A Review of the Potential Consequences of Pearl Millet (Pennisetum glaucum) for Diabetes Mellitus and Other Biomedical Applications. Nutrients, 14(14), 2932. https://doi.org/10.3390/nu14142932








