Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides
Abstract
:1. Introduction
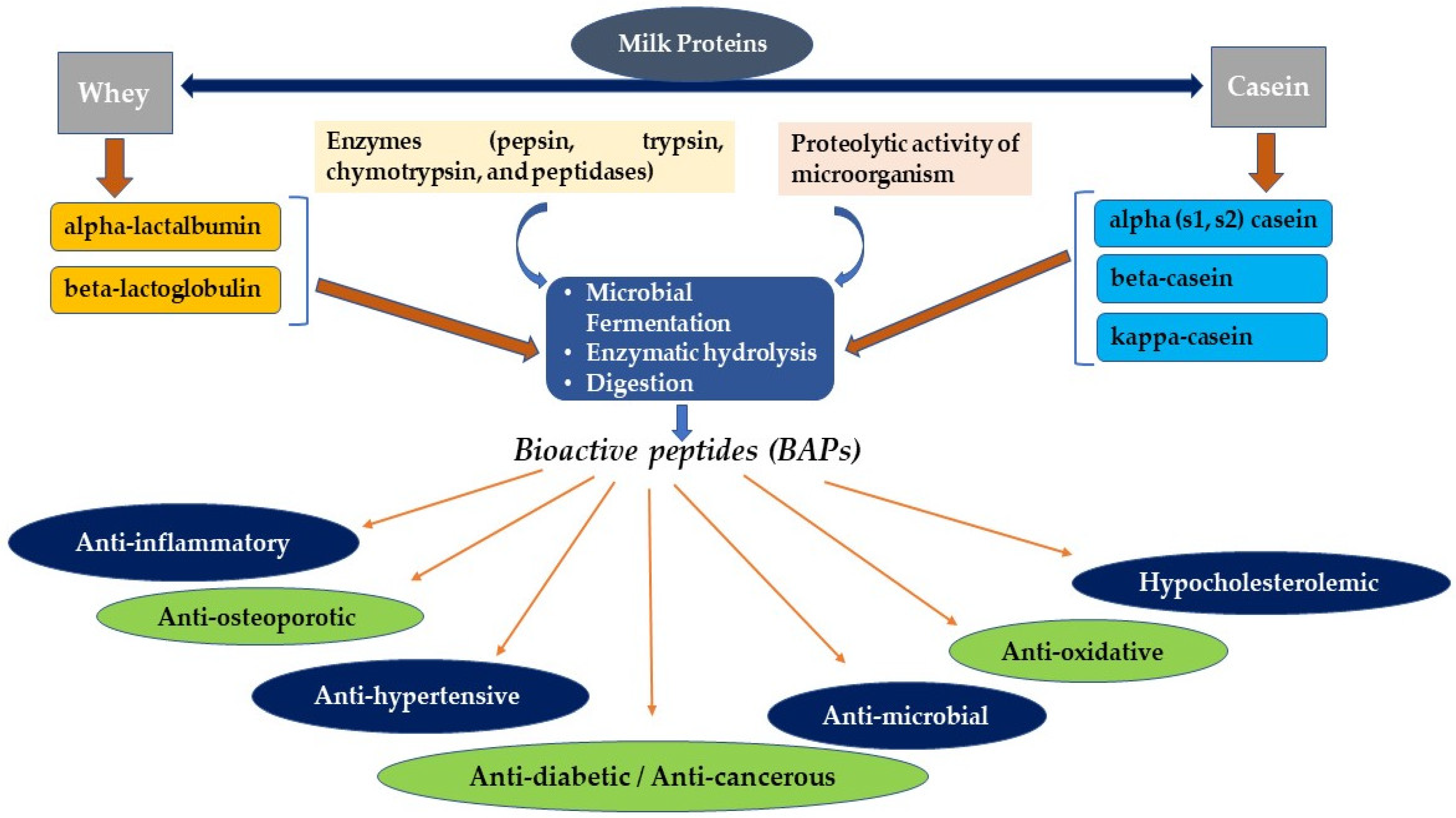
2. Brief Comparison of Milk and Milk Proteins Composition
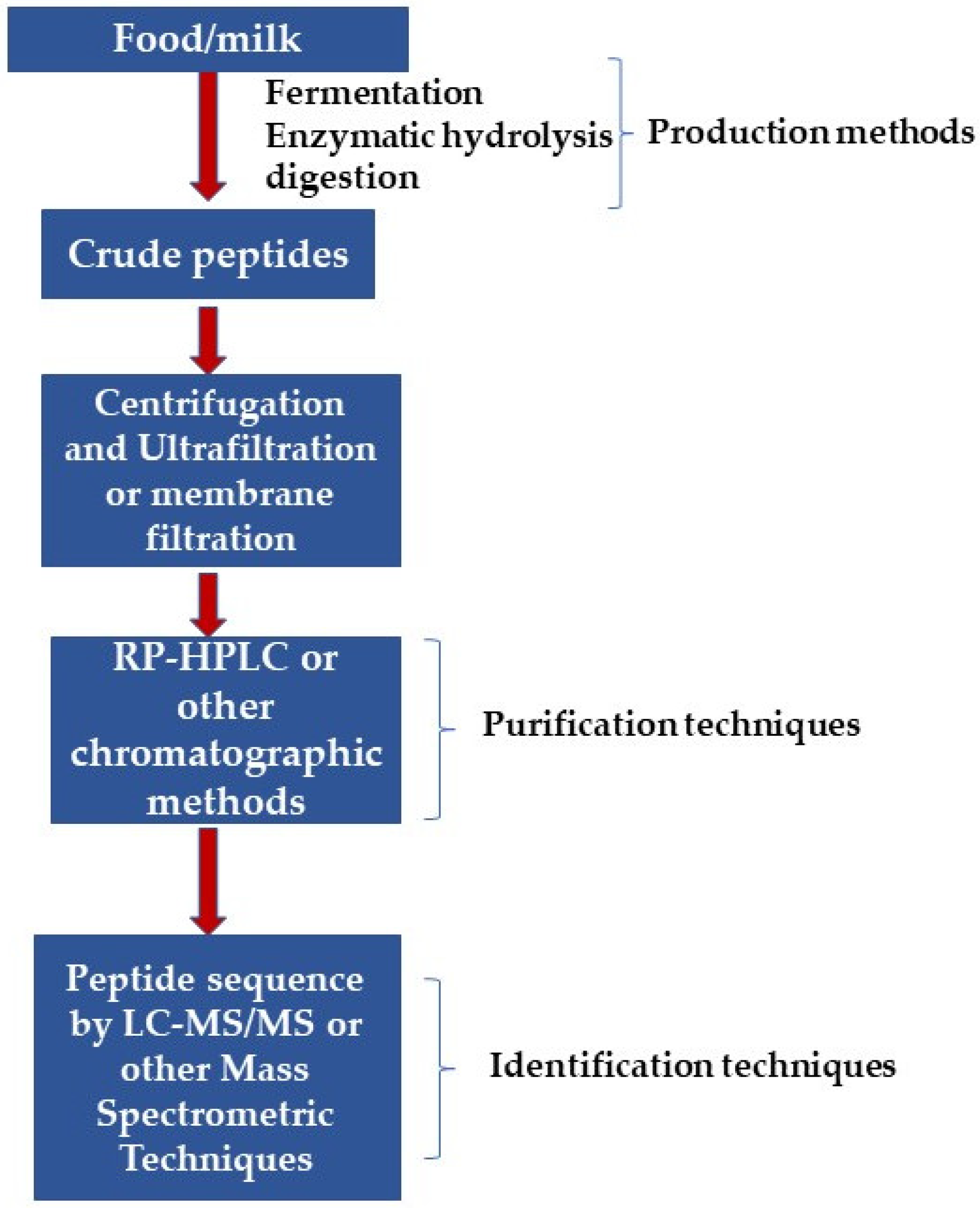
3. Production of Milk-Derived BAPs
3.1. Fermentation and Enzymatic Hydrolysis Are the Two Most Common Methods Widely Used to Produce BAPs
3.1.1. Fermentation
3.1.2. Enzymatic Hydrolysis
4. BAPs’ Purification and Identification
Advantage of AAs Identification
5. Therapeutic Potentials of Milk-Derived BAPs
5.1. Anti-Osteoporotic Effect
5.2. Anti-Hypertensive
5.3. Anti-Hypercholesterolemia
5.4. Anti-Oxidative
5.5. Anti-Microbial
5.6. Immunomodulatory/Anti-Inflammatory
5.7. Anti-Cancer
5.8. Anti-Diabetic
6. Products from Dairy Peptides
7. Safety of BAPs
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Milk and Dairy Products in Human Nutrition. 2013. Available online: https://www.fao.org/3/i3396e/i3396e.pdf (accessed on 3 January 2022).
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Prod. Process. Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2020–2029; FAO: Rome, Italy; OECD Publishing: Paris, France, 2020. [Google Scholar] [CrossRef]
- Martínez-Sánchez, S.M.; Gabaldón-Hernández, J.A.; Montoro-García, S. Unravelling the molecular mechanisms associated with the role of food-derived bioactive peptides in promoting cardiovascular health. J. Funct. Foods 2020, 64, 103645. [Google Scholar] [CrossRef]
- Park, Y.W.; Nam, M.S. Bioactive peptides in milk and dairy products: A review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, C.B.; Je, J.Y. Bone health-promoting bioactive peptides. J. Food Biochem. 2019, 43, e12529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samtiya, M.; Acharya, S.; Pandey, K.K.; Aluko, R.E.; Udenigwe, C.C.; Dhewa, T. Production, Purification, and Potential Health Applications of Edible Seeds’ Bioactive Peptides: A Concise Review. Foods 2021, 10, 2696. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S. Growth and bioactive peptides production potential of Lactobacillus plantarum strain C2 in soy milk: A LC-MS/MS based revelation for peptides biofunctionality. LWT 2017, 86, 293–301. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Kumari, A.; Bhawal, S.; Kapila, S.; Yadav, H.; Kapila, R. Health-promoting role of dietary bioactive compounds through epigenetic modulations: A novel prophylactic and therapeutic approach. Crit. Rev. Food Sci. Nutr. 2020, 62, 619–639. [Google Scholar] [CrossRef]
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive peptides in the management of lifestyle-related diseases: Current trends and future perspectives. Crit. Rev. Food Sci. Nutr. 2021, 62, 4593–4606. [Google Scholar] [CrossRef]
- Wu, S.; Bekhit, A.E.D.A.; Wu, Q.; Chen, M.; Liao, X.; Wang, J.; Ding, Y. Bioactive peptides and gut microbiota: Candidates for a novel strategy for reduction and control of neurodegenerative diseases. Trends Food Sci. Technol. 2021, 108, 164–176. [Google Scholar] [CrossRef]
- Sowmya, K.; Bhat, M.I.; Bajaj, R.K.; Kapila, S.; Kapila, R. Buffalo milk casein derived decapeptide (YQEPVLGPVR) having bifunctional anti-inflammatory and antioxidative features under cellular milieu. Int. J. Pept. Res. Ther. 2019, 25, 623–633. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Białas, W.; Grajek, W. Integrated approach for obtaining bioactive peptides from whey proteins hydrolysed using a new proteolytic lactic acid bacteria. Food Chem. 2020, 312, 126035. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Vij, S. Effect of bioactive peptides derived from fermented whey based drink against food borne pathogens. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 936–941. [Google Scholar]
- Shivanna, S.K.; Nataraj, B.H. Revisiting therapeutic and toxicological fingerprints of milk-derived bioactive peptides: An overview. Food Biosci. 2020, 38, 100771. [Google Scholar] [CrossRef]
- Vincenzetti, S.; Pucciarelli, S.; Polzonetti, V.; Polidori, P. Role of proteins and of some bioactive peptides on the nutritional quality of donkey milk and their impact on human health. Beverages 2017, 3, 34. [Google Scholar] [CrossRef]
- Shori, A.B. Comparative study of chemical composition, isolation and identification of micro-flora in traditional fermented camel milk products: Gariss, Suusac, and Shubat. J. Saudi Soc. Agric. Sci. 2012, 11, 79–88. [Google Scholar] [CrossRef]
- Ellouze, S.; Kamoun, M. Evolution of the composition of camel milk according to the stage of lactation. In Milk in the Mediterranean Region; Tisserand, J.-L., Ed.; Mediterranean Options: Series A. Mediterranean Seminars; CIHEAM: Paris, France, 1989; pp. 307–311. Available online: http://om.ciheam.org/om/pdf/a06/CI000495.pdf (accessed on 3 January 2022).
- Derdak, R.; Sakoui, S.; Pop, O.L.; Muresan, C.I.; Vodnar, D.C.; Addoum, B.; Vulturar, R.; Chis, A.; Suharoschi, R.; Soukri, A.; et al. Insights on Health and Food Applications of Equus asinus (Donkey) Milk Bioactive Proteins and Peptides—An Overview. Foods 2020, 9, 1302. [Google Scholar] [CrossRef]
- Mohanty, D.P.; Mohapatra, S.; Misra, S.; Sahu, P.S. Milk derived bioactive peptides and their impact on human health–A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Sharma, P.; Jaimni, S.; Kehinde, B.A.; Kaur, S. Recent developments in purification techniques and industrial applications for whey valorization: A review. Chem. Eng. Commun. 2020, 207, 123–138. [Google Scholar] [CrossRef]
- Mati, A.; Senoussi-Ghezali, C.; Zennia, S.S.A.; Almi-Sebbane, D.; El-Hatmi, H.; Girardet, J.M. Dromedary camel milk proteins, a source of peptides having biological activities—A review. Int. Dairy J. 2017, 73, 25–37. [Google Scholar] [CrossRef]
- Hazebrouck, S. Goat, donkey and camel milk: An alternative in case of cow’s milk allergy? Agron. Innov. 2016, 52, 73–84. [Google Scholar]
- Alichanidis, E.; Moatsou, G.; Polychroniadou, A. Composition and properties of non-cow milk and products. In Non-Bovine Milk and Milk Products; Academic Press: Cambridge, MA, USA, 2016; pp. 81–116. [Google Scholar]
- Medhammar, E.; Wijesinha-Bettoni, R.; Stadlmayr, B.; Nilsson, E.; Charrondiere, U.R.; Burlingame, B. Composition of milk from minor dairy animals and buffalo breeds: A biodiversity perspective. J. Sci. Food Agric. 2012, 92, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Potočnik, K.; Gantner, V.; Kuterovac, K.; Cividini, A. Mare’s milk: Composition and protein fraction in comparison with different milk species. Mljek. Čas. Unapr. Proizv. Prerade Mlijeka 2011, 61, 107–113. [Google Scholar]
- Mohanty, D.; Jena, R.; Choudhury, P.K.; Pattnaik, R.; Mohapatra, S.; Saini, M.R. Milk derived antimicrobial bioactive peptides: A review. Int. J. Food Prop. 2016, 19, 837–846. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Mada, S.B.; Ugwu, C.P.; Abarshi, M.M. Health promoting effects of food-derived bioactive peptides: A review. Int. J. Pept. Res. Ther. 2019, 26, 831–848. [Google Scholar] [CrossRef]
- De Brito, R.C.; Cardoso, J.M.D.O.; Reis, L.E.; Vieira, J.F.; Mathias, F.A.; Roatt, B.M.; Aguiar-Soares, R.D.; Ruiz, J.C.; Resende, D.D.M.; Reis, A.B. Peptide vaccines for leishmaniasis. Front. Immunol. 2018, 9, 1043. [Google Scholar] [CrossRef] [Green Version]
- Schrimpf, A.; Hempel, F.; Li, A.; Linne, U.; Maier, U.G.; Reetz, M.T.; Geyer, A. Hinge-type dimerization of proteins by a tetracysteine peptide of high pairing specificity. Biochemistry 2018, 57, 3658–3664. [Google Scholar] [CrossRef]
- Danquah, M.K.; Agyei, D. Pharmaceutical applications of bioactive peptides. OA Biotechnol. 2012, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; de Oliveira, D.E.; Fleuri, L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Sawh, F.; Green-Johnson, J.M.; Taggart, H.J.; Strap, J.L. Characterization of casein-derived peptide bioactivity: Differential effects on angiotensin-converting enzyme inhibition and cytokine and nitric oxide production. J. Dairy Sci. 2020, 103, 5805–5815. [Google Scholar] [CrossRef] [PubMed]
- Gaspar-Pintiliescu, A.; Oancea, A.; Cotarlet, M.; Vasile, A.M.; Bahrim, G.E.; Shaposhnikov, S.; Craciunescu, O.; Oprita, E.I. Angiotensin-converting enzyme inhibition, antioxidant activity and cytotoxicity of bioactive peptides from fermented bovine colostrum. Int. J. Dairy Technol. 2020, 73, 108–116. [Google Scholar] [CrossRef]
- Pihlanto, A. Lactic fermentation and bioactive peptides. In Lactic Acid Bacteria–R & D for Food, Health and Livestock Purposes; IntechOpen: London, UK, 2013; pp. 310–331. [Google Scholar]
- Wu, N.; Xu, W.; Liu, K.; Xia, Y. Angiotensin-converting enzyme inhibitory peptides from Lactobacillus delbrueckii QS306 fermented milk. J. Dairy Sci. 2019, 102, 5913–5921. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of new peptides from fermented milk showing antioxidant properties: Mechanism of action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Guo, T.; Li, W.; Chen, J.; Li, F.; Wang, C.; Shi, Y.; Li, D.X.A.; Zhang, S. Isolation and identification of novel casein-derived bioactive peptides and potential functions in fermented casein with Lactobacillus helveticus. Food Sci. Hum. Wellness 2019, 8, 156–176. [Google Scholar] [CrossRef]
- Rubak, Y.T.; Nuraida, L.; Iswantini, D.; Prangdimurti, E. Angiotensin-I-converting enzyme inhibitory peptides in milk fermented by indigenous lactic acid bacteria. Vet. World 2020, 13, 345. [Google Scholar] [CrossRef]
- Kinariwala, D.; Panchal, G.; Sakure, A.; Hati, S. Exploring the potentiality of Lactobacillus cultures on the production of milk-derived bioactive peptides with antidiabetic activity. Int. J. Pept. Res. Ther. 2019, 26, 1613–1627. [Google Scholar] [CrossRef]
- Soleymanzadeh, N.; Mirdamadi, S.; Mirzaei, M.; Kianirad, M. Novel β-casein derived antioxidant and ACE-inhibitory active peptide from camel milk fermented by Leuconostoc lactis PTCC1899: Identification and molecular docking. Int. Dairy J. 2019, 97, 201–208. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Lee, B.H.; Park, B.J.; Kim, S.H.; Oh, D.H. Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Sci. Biotechnol. 2018, 27, 1781–1789. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Akalın, A.S. Dairy-derived antimicrobial peptides: Action mechanisms, pharmaceutical uses and production proposals. Trends Food Sci. Technol. 2014, 36, 79–95. [Google Scholar] [CrossRef]
- Meisel, H.; FitzGerald, R.J. Biofunctional peptides from milk proteins: Mineral binding and cytomodulatory effects. Curr. Pharm. Des. 2003, 9, 1289–1296. [Google Scholar]
- Yamamoto, N.; Ejiri, M.; Mizuno, S. Biogenic peptides and their potential use. Curr. Pharm. Des. 2003, 9, 1345–1355. [Google Scholar] [CrossRef]
- Shazly, A.B.; Mu, H.; Liu, Z.; Abd El-Aziz, M.; Zeng, M.; Qin, F.; Zhang, S.; He, Z.; Chen, J. Release of antioxidant peptides from buffalo and bovine caseins: Influence of proteases on antioxidant capacities. Food Chem. 2019, 274, 261–267. [Google Scholar] [CrossRef]
- Fajardo-Espinoza, F.S.; Romero-Rojas, A.; Hernández-Sánchez, H. Production of bioactive peptides from bovine colostrum whey using enzymatic hydrolysis. Rev. Mex. Ing. Quím. 2020, 19, 1. [Google Scholar] [CrossRef] [Green Version]
- Shazly, A.B.; He, Z.; Abd El-Aziz, M.; Zeng, M.; Zhang, S.; Qin, F.; Chen, J. Fractionation and identification of novel antioxidant peptides from buffalo and bovine casein hydrolysates. Food Chem. 2017, 232, 753–762. [Google Scholar] [CrossRef]
- Shanmugam, V.P.; Kapila, S.; Sonfack, T.K.; Kapila, R. Antioxidative peptide derived from enzymatic digestion of buffalo casein. Int. Dairy J. 2015, 42, 1–5. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Bechaux, J.; Gatellier, P.; Le Page, J.F.; Drillet, Y.; Sante-Lhoutellier, V. A comprehensive review of bioactive peptides obtained from animal byproducts and their applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Perez Espitia, P.J.; de Fátima Ferreira Soares, N.; dos Reis Coimbra, J.S.; de Andrade, N.J.; Souza Cruz, R.; Alves Medeiros, E.A. Bioactive peptides: Synthesis, properties, and applications in the packaging and preservation of food. Compr. Rev. Food Sci. Food Saf. 2012, 11, 187–204. [Google Scholar] [CrossRef]
- Mada, S.B.; Abaya, P.C.; James, D.B.; Abarshi, M.M.; Tanko, M.S. Milk-derived bioactive peptides with antiosteoporotic effect: A mini review. Fudma J. Sci. 2020, 4, 351–357. [Google Scholar] [CrossRef]
- Firdaous, L.; Dhulster, P.; Amiot, J.; Gaudreau, A.; Lecouturier, D.; Kapel, R.; Lutin, F.; Vézina, L.P.; Bazinet, L. Concentration and selective separation of bioactive peptides from an alfalfa white protein hydrolysate by electrodialysis with ultrafiltration membranes. J. Membr. Sci. 2009, 329, 60–67. [Google Scholar] [CrossRef]
- Doyen, A.; Beaulieu, L.; Saucier, L.; Pouliot, Y.; Bazinet, L. Impact of ultrafiltration membrane material on peptide separation from a snow crab byproduct hydrolysate by electrodialysis with ultrafiltration membranes. J. Agric. Food Chem. 2011, 59, 1784–1792. [Google Scholar] [CrossRef]
- Doyen, A.; Beaulieu, L.; Saucier, L.; Pouliot, Y.; Bazinet, L. Demonstration of in vitro anticancer properties of peptide fractions from a snow crab by-products hydrolysate after separation by electrodialysis with ultrafiltration membranes. Sep. Purif. Technol. 2011, 78, 321–329. [Google Scholar] [CrossRef]
- Bamdad, F.; Shin, S.H.; Suh, J.W.; Nimalaratne, C.; Sunwoo, H. Anti-inflammatory and antioxidant properties of casein hydrolysate produced using high hydrostatic pressure combined with proteolytic enzymes. Molecules 2017, 22, 609. [Google Scholar] [CrossRef]
- Franca-Oliveira, G.; Fornari, T.; Hernández-Ledesma, B. A review on the extraction and processing of natural source-derived proteins through eco-innovative approaches. Processes 2021, 9, 1626. [Google Scholar] [CrossRef]
- Marson, G.V.; Belleville, M.P.; Lacour, S.; Hubinger, M.D. Membrane fractionation of protein hydrolysates from by-products: Recovery of valuable compounds from spent yeasts. Membranes 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Yuguchi, Y.; Bui, L.M.; Takebe, S.; Suzuki, S.; Nakajima, N.; Kitamura, S.; Thanh, T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016, 147, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Vitha, M.F. Chromatography: Principles and Instrumentation; John Wiley & Sons: New York, NY, USA, 2016; Volume 185. [Google Scholar]
- Ho, C.S.; Lam, C.W.K.; Chan, M.H.M.; Cheung, R.C.K.; Law, L.K.; Lit, L.C.W.; Ng, K.F.; Suen, M.W.M.; Tai, H. Electrospray ionisation mass spectrometry: Principles and clinical applications. Clin. Biochem. Rev. 2003, 24, 3. [Google Scholar]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure−activity relationship study of di-and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Zhao, M.; Zhao, K.; Tian, Y.; Yang, Y. Purification of peptide fraction with antioxidant activity from Moringa oleifera leaf hydrolysate and protective effect of its in vitro gastrointestinal digest on oxidatively damaged erythrocytes. Int. J. Food Sci. Technol. 2019, 54, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Bu, T.; Zheng, J.; Liu, L.; He, G.; Wu, J. Preparation, bioavailability, and mechanism of emerging activities of ile-pro-pro and val-pro-pro. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1097–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Reddi, S.; Shanmugam, V.P.; Kapila, S.; Kapila, R. Identification of buffalo casein-derived bioactive peptides with osteoblast proliferation activity. Eur. Food Res. Technol. 2016, 242, 2139–2146. [Google Scholar] [CrossRef]
- Reddi, S.; Kumar, N.; Vij, R.; Mada, S.B.; Kapila, S.; Kapila, R. Akt drives buffalo casein-derived novel peptide-mediated osteoblast differentiation. J. Nutr. Biochem. 2016, 38, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Kapila, R.; Kapila, S. Osteoanabolic activity of whey-derived anti-oxidative (MHIRL and YVEEL) and angiotensin-converting enzyme inhibitory (YLLF, ALPMHIR, IPA and WLAHK) bioactive peptides. Peptides 2018, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Reddi, S.; Shanmugam, V.P.; Tanedjeu, K.S.; Kapila, S.; Kapila, R. Effect of buffalo casein-derived novel bioactive peptides on osteoblast differentiation. Eur. J. Nutr. 2018, 57, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, D.; Kaur, T.; Kapila, R.; Kapila, S. Repertoire of Structure–Activity-Based Novel Modified Peptides Elicits Enhanced Osteogenic Potential. J. Agric. Food Chem. 2020, 68, 8308–8320. [Google Scholar] [CrossRef]
- Pandey, M.; Kapila, S.; Kapila, R.; Trivedi, R.; Karvande, A. Evaluation of the osteoprotective potential of whey derived-antioxidative (YVEEL) and angiotensin-converting enzyme inhibitory (YLLF) bioactive peptides in ovariectomised rats. Food Funct. 2018, 9, 4791–4801. [Google Scholar] [CrossRef] [PubMed]
- Dzudie, A.; Twagirumukiza, M.; Cornick, R.; Abdou Ba, S.; Damasceno, A.; Rayner, B.; Kane, A.; Sliwa, K.; Anzouan Kacou, J.B.; Mocumbi, A.O.; et al. Roadmap to achieve 25% hypertension control in Africa by 2025. Cardiovasc. J. Afr. 2017, 28, 262–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarajan, N.; Jalal, D. Resistant hypertension: Diagnosis and management. Adv. Chronic Kidney Dis. 2019, 26, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Technological options for the production of health-promoting proteins and peptides derived from milk and colostrum. Curr. Pharm. Des. 2007, 13, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shangguan, W.; Bao, C.; Shu, G.; Chen, H. Collaborative optimization and molecular docking exploration of novel ACE-inhibitory peptides from bovine milk by complex proteases hydrolysis. Artif. Cells Nanomed. Biotechnol. 2020, 48, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, V.P.; Kapila, S.; Kemgang, T.S.; Reddi, S.; Kapila, R.; Muthukumar, S.; Rajesh, D. Isolation and Characterization of Angiotensin Converting Enzyme Inhibitory Peptide from Buffalo Casein. Int. J. Pept. Res. Ther. 2021, 27, 1481–1491. [Google Scholar] [CrossRef]
- Chanson-Rolle, A.; Aubin, F.; Braesco, V.; Hamasaki, T.; Kitakaze, M. Influence of the lactotripeptides isoleucine–proline–proline and valine–proline–proline on systolic blood pressure in Japanese subjects: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2015, 10, e0142235. [Google Scholar] [CrossRef]
- Espejo-Carpio, F.J.; De Gobba, C.; Guadix, A.; Guadix, E.M.; Otte, J. Angiotensin I-converting enzyme inhibitory activity of enzymatic hydrolysates of goat milk protein fractions. Int. Dairy J. 2013, 32, 175–183. [Google Scholar] [CrossRef]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Groop, P.H.; Korpela, R. Long-term intervention with Lactobacillus helveticus fermented milk reduces augmentation index in hypertensive subjects. Eur. J. Clin. Nutr. 2010, 64, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Siow, H.L.; Choi, S.B.; Gan, C.Y. Structure–activity studies of protease activating, lipase inhibiting, bile acid binding and cholesterol-lowering effects of pre-screened cumin seed bioactive peptides. J. Funct. Foods 2016, 27, 600–611. [Google Scholar] [CrossRef]
- Lapphanichayakool, P.; Sutheerawattananonda, M.; Limpeanchob, N. Hypocholesterolemic effect of sericin-derived oligopeptides in high-cholesterol fed rats. J. Nat. Med. 2017, 71, 208–215. [Google Scholar] [CrossRef]
- Kalyan, S.; Meena, S.; Kapila, S.; Sowmya, K.; Kumar, R. Evaluation of goat milk fat and goat milk casein fraction for anti-hypercholesterolaemic and antioxidative properties in hypercholesterolaemic rats. Int. Dairy J. 2018, 84, 23–27. [Google Scholar] [CrossRef]
- Morikawa, K.; Kondo, I.; Kanamaru, Y.; Nagaoka, S. A novel regulatory pathway for cholesterol degradation via lactostatin. Biochem. Biophys. Res. Commun. 2007, 352, 697–702. [Google Scholar] [CrossRef]
- Nagaoka, S.; Futamura, Y.; Miwa, K.; Awano, T.; Yamauchi, K.; Kanamaru, Y.; Tadashi, K.; Kuwata, T. Identification of novel hypocholesterolemic peptides derived from bovine milk β-lactoglobulin. Biochem. Biophys. Res. Commun. 2001, 281, 11–17. [Google Scholar] [CrossRef]
- Boachie, R.; Yao, S.; Udenigwe, C.C. Molecular mechanisms of cholesterol-lowering peptides derived from food proteins. Curr. Opin. Food Sci. 2018, 20, 58–63. [Google Scholar] [CrossRef]
- Jiang, X.; Pan, D.; Zhang, T.; Liu, C.; Zhang, J.; Su, M.; Wu, Z.; Zeng, X.; Sun, Y.; Guo, Y. Novel milk casein–derived peptides decrease cholesterol micellar solubility and cholesterol intestinal absorption in Caco-2 cells. J. Dairy Sci. 2020, 103, 3924–3936. [Google Scholar] [CrossRef]
- Waili, Y.; Gahafu, Y.; Aobulitalifu, A.; Chang, Z.; Xie, X.; Kawuli, G. Isolation, purification, and characterization of antioxidant peptides from fresh mare’s milk. Food Sci. Nutr. 2021, 9, 4018–4027. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Muramoto, K.; Yamauchi, F. Structural analysis of antioxidative peptides from Soybean. beta.-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Carrasco-Castilla, J.; Hernández-Álvarez, A.J.; Jiménez-Martínez, C.; Jacinto-Hernández, C.; Alaiz, M.; Girón-Calle, J.; Vioque, J.; Dávila-Ortiz, G. Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates. Food Chem. 2012, 131, 1157–1164. [Google Scholar] [CrossRef]
- Huang, S.M.; Chen, K.N.; Chen, Y.P.; Hong, W.S.; Chen, M.J. Immunomodulatory properties of the milk whey products obtained by enzymatic and microbial hydrolysis. Int. J. Food Sci. Technol. 2010, 45, 1061–1067. [Google Scholar] [CrossRef]
- Mada, S.B.; Reddi, S.; Kumar, N.; Kapila, S.; Kapila, R. Protective effects of casein-derived peptide VLPVPQK against hydrogen peroxide–induced dysfunction and cellular oxidative damage in rat osteoblastic cells. Hum. Exp. Toxicol. 2017, 36, 967–980. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, B. Characterization of structure–antioxidant activity relationship of peptides in free radical systems using QSAR models: Key sequence positions and their amino acid properties. J. Theor. Biol. 2013, 318, 29–43. [Google Scholar] [CrossRef]
- Kumar, N.; Raghavendra, M.; Tokas, J.; Singal, H.R. Milk Proteins: Precursors of antioxidative peptides and their health benefits. In Dairy in Human Health and Disease Across the Lifespan; Academic Press: New York, NY, USA, 2017; pp. 313–323. [Google Scholar]
- Sowmya, K.; Bhat, M.I.; Bajaj, R.; Kapila, S.; Kapila, R. Antioxidative and anti-inflammatory potential with trans-epithelial transport of a buffalo casein-derived hexapeptide (YFYPQL). Food Biosci. 2019, 28, 151–163. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Ferro, S.; Folda, A.; Scalcon, V.; Sandre, M.; Fiorese, F.; Marin, O.; Bindoli, A.; Rigobello, M.P. Insight into antioxidant properties of milk-derived bioactive peptides in vitro and in a cellular model. J. Pept. Sci. 2019, 25, e3162. [Google Scholar] [CrossRef]
- Khan, M.U.; Pirzadeh, M.; Förster, C.Y.; Shityakov, S.; Shariati, M.A. Role of milk-derived antibacterial peptides in modern food biotechnology: Their synthesis, applications and future perspectives. Biomolecules 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.; Hsiao, F.S.H.; Ho, Y.H.; Chen, C.S. The proteome targets of intracellular targeting antimicrobial peptides. Proteomics 2016, 16, 1225–1237. [Google Scholar] [CrossRef]
- Taniguchi, M.; Ochiai, A.; Kondo, H.; Fukuda, S.; Ishiyama, Y.; Saitoh, E.; Kato, T.; Tanaka, T. Pyrrhocoricin, a proline-rich antimicrobial peptide derived from insect, inhibits the translation process in the cell-free Escherichia coli protein synthesis system. J. Biosci. Bioeng. 2016, 121, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Kulyar, M.F.E.A.; Yao, W.; Ding, Y.; Li, K.; Zhang, L.; Li, A.; Waqas, M.; Huachun, P.; Quan, M.; Zeng, Z.; et al. Bioactive potential of yak’s milk and its products; pathophysiological and molecular role as an immune booster in antibiotic resistance. Food Biosci. 2020, 39, 100838. [Google Scholar] [CrossRef]
- Abu-qatouseh, L.; Mallah, E.; Issa, H.; Sabri, I.; Shihab, P. Antimicrobial and anti-inflammatory activity of camel milk derived immune proteins and peptides against Propionibacterium acnes. Int. J. Biol. Pharm. Allied Sci. 2019, 8, 163–173. [Google Scholar]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Saporito, P.; Osman, A.; Mateiu, R.V.; Mojsoska, B.; Jenssen, H. Camel milk whey hydrolysate inhibits growth and biofilm formation of Pseudomonas aeruginosa PAO1 and methicillin-resistant Staphylococcus aureus. Food Control. 2020, 111, 107056. [Google Scholar] [CrossRef]
- Cai, J.; Li, X.; Du, H.; Jiang, C.; Xu, S.; Cao, Y. Immunomodulatory significance of natural peptides in mammalians: Promising agents for medical application. Immunobiology 2020, 225, 151936. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, L.; Zhang, L.; Qiao, Q.; Farooq, M.Z.; Xu, Q. The potential of food protein-derived bioactive peptides against chronic intestinal inflammation. Mediat. Inflamm. 2020, 2020, 6817156. [Google Scholar] [CrossRef]
- Minshawi, F.; Lanvermann, S.; McKenzie, E.; Jeffery, R.; Couper, K.; Papoutsopoulou, S.; Roers, A.; Muller, W. The generation of an engineered interleukin-10 protein with improved stability and biological function. Front. Immunol. 2020, 11, 1794. [Google Scholar] [CrossRef]
- Pavlou, S.; Wang, L.; Xu, H.; Chen, M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J. Inflamm. 2017, 14, 4. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Yin and yang interplay of IFN-γ in inflammation and autoimmune disease. J. Clin. Investig. 2007, 117, 871–873. [Google Scholar] [CrossRef]
- Marcone, S.; Haughton, K.; Simpson, P.J.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides inhibit human endothelial-monocyte interactions via PPAR-γ dependent regulation of NF-κB. J. Inflamm. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived bioactive peptides and their health promoting effects: A potential role in atherosclerosis. Br. J. Clin. Pharmacol. 2017, 83, 152–162. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Kaur, H.; Kehinde, B.A.; Chhikara, N.; Sharma, D.; Panghal, A. Food-derived anticancer peptides: A review. Int. J. Pept. Res. Ther. 2021, 27, 55–70. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Li, Z.; Lan, X.; Leung, P.H.M.; Li, J.; Yang, M.; Ko, F.; Qin, L. Mechanism of anticancer effects of antimicrobial peptides. J. Fiber. Bioeng. Inf. 2015, 8, 25–36. [Google Scholar] [CrossRef]
- Wu, M.L.; Li, H.; Yu, L.J.; Chen, X.Y.; Kong, Q.Y.; Song, X.; Shu, X.H.; Liu, J. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS ONE 2014, 9, e89806. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Arias, M.; Hilchie, A.L.; Haney, E.F.; Bolscher, J.G.; Hyndman, M.E.; Hancock, R.E.; Vogel, H.J. Anticancer activities of bovine and human lactoferricin-derived peptides. Biochem. Cell Biol. 2017, 95, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Zhao, M.; Tang, Y.; Wang, J.; Wei, C.; Gu, F.; Lei, T.; Chen, Z.; Qin, Y. The milk-derived fusion peptide, ACFP, suppresses the growth of primary human ovarian cancer cells by regulating apoptotic gene expression and signaling pathways. BMC Cancer 2016, 16, 246. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, S.; Huma, N.; Rakariyatham, K.; Hussain, I.; Gulzar, N.; Hayat, I. Anti-inflammatory and anticancer activities of water-soluble peptide extracts of buffalo and cow milk Cheddar cheeses. Int. J. Dairy Technol. 2018, 71, 432–438. [Google Scholar] [CrossRef]
- Parodi P W A role for milk proteins and their peptides in cancer prevention. Curr. Pharm. Des. 2007, 13, 813–828. [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquah, C.; Dzuvor, C.K.; Tosh, S.; Agyei, D. Anti-diabetic effects of bioactive peptides: Recent advances and clinical implications. Crit. Rev. Food Sci. Nutr. 2020, 62, 2158–2171. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Bourque, S.L.; Wu, J. A comprehensive review on the glucoregulatory properties of food-derived bioactive peptides. Food Chem. 2022, X, 100222. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Hou, T. A comprehensive review of corn protein-derived bioactive peptides: Production, characterization, bioactivities, and transport pathways. Compr. Rev. Food Sci. Food Saf. 2019, 18, 329–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, R.; Ma, H.; Chen, S. Isolation and identification of dipeptidyl peptidase IV-inhibitory peptides from trypsin/chymotrypsin-treated goat milk casein hydrolysates by 2D-TLC and LC–MS/MS. J. Agric. Food Chem. 2015, 63, 8819–8828. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Cadamuro, C.; Le Gouic, A.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of a camel whey protein enriched hydrolysate preparation. Food Chem. 2019, 279, 70–79. [Google Scholar] [CrossRef]
- Jia, C.L.; Hussain, N.; Ujiroghene, O.J.; Pang, X.Y.; Zhang, S.W.; Lu, J.; Liu, L.; Lv, J.P. Generation and characterization of dipeptidyl peptidase-IV inhibitory peptides from trypsin-hydrolyzed α-lactalbumin-rich whey proteins. Food Chem. 2020, 318, 126333. [Google Scholar] [CrossRef]
- El-Sayed, M.I.; Awad, S.; Wahba, A.; El Attar, A.; Yousef, M.I.; Zedan, M. In Vivo anti-diabetic and biological activities of milk protein and milk protein hydrolyaste. Adv. Dairy Res. 2016, 4, 2. [Google Scholar]
- Yan, J.; Zhao, J.; Yang, R.; Zhao, W. Bioactive peptides with antidiabetic properties: A review. Int. J. Food Sci. Technol. 2019, 54, 1909–1919. [Google Scholar] [CrossRef] [Green Version]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Huma, N.; Gulzar, N.; Murtaza, M.A.; Hussain, I. Effect of cheddar cheese peptide extracts on growth inhibition, cell cycle arrest and apoptosis induction in human lung cancer (H-1299) cell line. Int. J. Dairy Technol. 2018, 71, 975–980. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Liao, W.; Davidge, S.T.; Wu, J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) differentially modulate angiotensin II effects on vascular smooth muscle cells. J. Funct. Foods 2017, 30, 151–158. [Google Scholar] [CrossRef]
- Hirota, T.; Ohki, K.; Kawagishi, R.; Kajimoto, Y.; Mizuno, S.; Nakamura, Y.; Kitakaze, M. Casein hydrolysate containing the Antihypertensive Tripeptides Val-Pro-Pro and Ile-Pro-Pro improves vascular endothelial function independent of Blood Pressure–Lowering Effects: Contribution of the inhibitory action of Angiotensin-Converting enzyme. Hypertens. Res. 2007, 30, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, N.; Kawaguchi, K.; Yamamoto, N. Study of the mechanism of antihypertensive peptides VPP and IPP in spontaneously hypertensive rats by DNA microarray analysis. Eur. J. Pharmacol. 2009, 620, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Lu, H.; Zeng, X. A newly isolated Ca binding peptide from whey protein. Int. J. Food Prop. 2013, 16, 1127–1134. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J.; Wang, Y.; Luo, Q.W.; Guo, K.R.; Ren, F.Z.; Mao, X.Y. Identification of novel peptides from goat milk casein that ameliorate high-glucose-induced insulin resistance in HepG2 cells. J. Dairy Sci. 2020, 103, 4907–4918. [Google Scholar] [CrossRef]
- Ishida, Y.; Shibata, Y.; Fukuhara, I.; Yano, Y.; Takehara, I.; Kaneko, K. Effect of an excess intake of casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro in subjects with normal blood pressure, high-normal blood pressure, or mild hypertension. Biosci. Biotechnol. Biochem. 2011, 75, 427–433. [Google Scholar] [CrossRef]
- Kajimoto, O.; Kurosaki, T.; Mizutani, J.; Ikeda, N.; Kaneko, K.; Aihara, K.; Yabune, M.; Nakamura, Y. Antihypertensive effects of liquid yogurts containing “lactotripeptides (VPP, IPP)” in mild hypertensive subjects. J. Nutr. Food 2002, 5, 55–66. [Google Scholar]
- Nakamura, T.; Mizutani, J.; Ohki, K.; Yamada, K.; Yamamoto, N.; Takeshi, M.; Takazawa, K. Casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro improves central blood pressure and arterial stiffness in hypertensive subjects: A randomized, double-blind, placebo-controlled trial. Atherosclerosis 2011, 219, 298–303. [Google Scholar] [CrossRef]
- Jauhiainen, T.; Rönnback, M.; Vapaatalo, H.; Wuolle, K.; Kautiainen, H.; Korpela, R. Lactobacillus helveticus fermented milk reduces arterial stiffness in hypertensive subjects. Int. Dairy J. 2007, 17, 1209–1211. [Google Scholar] [CrossRef]
- Kaido, T.; Ogura, Y.; Ogawa, K.; Hata, K.; Yoshizawa, A.; Yagi, S.; Uemoto, S. Effects of post-transplant enteral nutrition with an immunomodulating diet containing hydrolyzed whey peptide after liver transplantation. World J. Surg. 2012, 36, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernández-Ledesma, B.; Fernández-Tomé, S.; Weinborn, V.; Barile, D.; de Moura Bell, J.M.L.N. Milk proteins, peptides, and oligosaccharides: Effects against the 21st century disorders. BioMed Res. Int. 2015, 2015, 146840. [Google Scholar] [CrossRef]
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, E.; Chand, R.; Kapila, S. Biofunctional properties of bioactive peptides of milk origin. Food Rev. Int. 2008, 25, 28–43. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides-opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [Green Version]
- Ricci, I.; Artacho, R.; Olalla, M. Milk protein peptides with angiotensin I-converting enzyme inhibitory (ACEI) activity. Crit. Rev. Food Sci. Nutr. 2010, 50, 390–402. [Google Scholar] [CrossRef]
- FitzGerald, R.J.; Murray, B.A.; Walsh, D.J. Hypotensive peptides from milk proteins. J. Nutr. 2004, 134, 980S–988S. [Google Scholar] [CrossRef] [Green Version]
- Ricci-Cabello, I.; Olalla Herrera, M.; Artacho, R. Possible role of milk-derived bioactive peptides in the treatment and prevention of metabolic syndrome. Nutr. Rev. 2012, 70, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto-Leppäla, A.; Rantamäki, P.; Tupasela, T. Impact of processing on bioactive proteins and peptides. Trends Food Sci. Technol. 1998, 9, 307–319. [Google Scholar] [CrossRef]
- Chaudhary, K.; Kumar, R.; Singh, S.; Tuknait, A.; Gautam, A.; Mathur, D.; Anand, P.; Varshney, G.C.; Raghava, G.P. A web server and mobile app for computing hemolytic potency of peptides. Sci. Rep. 2016, 6, 22843. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Raghava, G.P. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef] [Green Version]
- Verhoeckx, K.C.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Golkar, A.; Milani, J.M.; Vasiljevic, T. Altering allergenicity of cow’s milk by food processing for applications in infant formula. Crit. Rev. Food Sci. Nutr. 2019, 59, 159–172. [Google Scholar] [CrossRef]
- Hill, D.J.; Cameron, D.J.S.; Francis, D.E.M.; Gonzalez-Andaya, A.M.; Hosking, C.S. Challenge confirmation of late-onset reactions to extensively hydrolyzed formulas in infants with multiple food protein intolerance. J. Allergy Clin. Immunol. 1995, 96, 386–394. [Google Scholar] [CrossRef]
- Saylor, J.D.; Bahna, S.L. Anaphylaxis to casein hydrolysate formula. J. Pediatr. 1991, 118, 71–74. [Google Scholar] [CrossRef]
- Dziuba, M.; Minkiewicz, P.; Dabek, M. Peptides, specific proteolysis products, as molecular markers of allergenic proteins-in silico studies. Acta Sci. Pol. Technol. Aliment. 2013, 12, 101–112. [Google Scholar]
- Picariello, G.; Ferranti, P.; Fierro, O.; Mamone, G.; Caira, S.; Di Luccia, A.; Monica, S.; Addeo, F. Peptides surviving the simulated gastrointestinal digestion of milk proteins: Biological and toxicological implications. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Abioye, R.O.; Okagu, I.U.; Obeme-Nmom, J.I. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Kapila, R.; Kapila, S.; Vij, R. Efficacy of milk-derived bioactive peptides on health by cellular and animal models. In Nutrients in Dairy and Their Implications on Health and Disease; Academic Press: New York, NY, USA, 2017; pp. 303–311. [Google Scholar]
| Species | Energy (KJ/Kg) | Ash | Fat | Proteins | Lactose | Dry Matter | Water | References |
|---|---|---|---|---|---|---|---|---|
| Percent | ||||||||
| Camel | 2745.80 | 0.85 | 1.80 | 1.80 | 2.91 | 11.30 | 90.60 | [17,18,19,20] |
| Cow | 2983.00 | 0.78 | 3.46 | 3.43 | 4.71 | 12.38 | 87.62 | |
| Donkey | 1939.40 | 0.43 | 1.21 | 1.74 | 6.23 | 9.61 | 90.39 | |
| Goat | 3399.50 | 0.73 | 4.62 | 3.41 | 4.47 | 13.23 | 86.77 | |
| Human | 2855.60 | 0.22 | 3.38 | 1.64 | 6.69 | 12.43 | 87.57 | |
| Species | Protein | Total Casein | αS1-Casein | αS2 Casein | κ-Casein | β-Casein | Total Whey | α-Lactalbumin | β-Lactoglobulin | References |
|---|---|---|---|---|---|---|---|---|---|---|
| (g/L) | ||||||||||
| Camel | 25–45 | 26.4 | 5 | 2.2 | 0.8 | 12.8 | 6.6 | 3.5 | - | [17,20,23,24] |
| Cow | 31–38 | 27.2 | 10–15 | 3–4 | 3–4 | 9–11 | 4.5 | 1–1.5 | 3.3–4 | |
| Donkey | 13–28 | 27.2 | 0.2–1 | 0.2 | - | 3.9 | 7.5 | 1.8–3 | 3.2–3.7 | |
| Goat | 25–39 | 25 | 0–7 | 4.2 | 4–4.6 | 11–18 | 6 | 1.2 | 2.1 | |
| Human | 9–17 | 5.6 | 0.3–0.8 | - | 0.6–1 | 1.8–4 | 8 | 1.9–2.6 | - | |
| Source | Sequence/Peptide/Fragment | Fermenting Microorganisms | References |
|---|---|---|---|
| Colostrum powder (bovine) | Peptides lower than 10 kDa MW (P1 and P2 fractions) | Candida lipolytica | [39] |
| Milk | LPYPY peptide | Lactobacillus delbrueckii | [41] |
| Casein protein | DELQDKIHPF peptide | Lactobacillus helveticus | [43] |
| Milk (bovine) | MKLFVPALLSLGALGLCLAA peptide | Lactobacillus fermentum | [45] |
| Milk (camel) | MVPYPQR peptide | Leuconostoc lactis | [46] |
| Whey protein | Peptides lower than (<7 kDa) | Pediococcus acidilactici | [47] |
| Source | Sequence/Peptide/Fragment | Enzymes Used | References |
|---|---|---|---|
| Whey protein (bovine colostrum) | Three fractions obtained having >30, 10 to 30 and <10 kDa MW | Pepsin and pancreatin | [53] |
| Buffalo casein (CB) | Highest degree of hydrolysis obtained in molecular weights <3.5 kDa using alcalase | Alcalase, trypsin, pepsin, or papain. | [52] |
| Buffalo casein hydrolysates (BCH) | RELEE, MEDNKQ, and TVA, EQL peptides | Trypsin and alcalase | [54] |
| Milk casein (buffalo) | VLPVPQK peptide | Pepsin, trypsin, chymotrypsin | [55] |
| Skimmed milk (buffalo) | PGPIPK, IPPK, IVPN, and QPPQ peptides | Papain, pepsin or trypsin | [56] |
| Source | Peptide Sequence/Fragment | Model/Method Used | Potential Attributes | References |
|---|---|---|---|---|
| Casein-derived | VPP and IPP | THP-1 human monocytic cell line | Immunomodulatory effect | [38] |
| Milk | VLPVPQK/PepC | Rat osteoblast cultures | Anti-osteoporotic effect | [79] |
| Whey-derived | YVEEL and YLLF | Ovariectomized (OVX) osteoporotic rat model | Anti-osteoporotic effect | [80] |
| Bovine milk | VLPVPQ and VAPFPE | Molecular docking | Anti-hypertensive effect | [84] |
| Buffalo milk casein | VLPVPQK | In vitro methods | Anti-hypertensive effect | [85] |
| Goat milk protein | WY | In vitro methods | Anti-hypertensive effect | [87] |
| Goat milk | Casein fraction | Hypercholesterolaemic rats | Hypocholesterolemic effect | [92] |
| Bovine milk β-lactoglobulin | IIAEK | Male rats (Wistar strain) | Hypocholesterolemic effect | [94] |
| Bovine milk | Lactostatin or IIAEK | HepG2, a human liver cell line. | Hypocholesterolemic effect | [93] |
| Casein-derived | VLPVPQK | Rat osteoblastic cells | Anti-oxidative effect | [101] |
| Buffalo casein-derived | YFYPQL | In vitro Caco-2 cell model | Anti-oxidative effect | [104] |
| Buffalo casein-derived | YFYPQL | Mice splenocytes culture | Anti-inflammatory effects | [104] |
| Milk-derived | RHPHPHLSFM, VPYPQR, HPHPHLSFM, YVPR | In vitro Caco-2 cell model | Anti-oxidative effect | [105] |
| Camel milk | Peptidoglycan recognition proteins PGRPs (PGRP), lactoferrin | Micro broth dilution assay (in vitro) | Anti-microbial effect | [110] |
| Camel milk | Whey hydrolysate | Biofilm inhibition, disc diffusion assay, biofilm reduction assay | Anti-microbial effect | [112] |
| Milk | Milk-derived hydrolysate | Endothelial cells | Immunomodulatory effect | [118] |
| Bovine milk protein | Anti-cancer fusion peptide (ACFP) | Ovarian cancer cells | Anti-cancerous effect | [125] |
| Buffalo and cow milk cheddar cheeses | Water-soluble peptide (WSP) extracts | Colon cancer model (HT-29) cells | Anti-cancerous effect | [126] |
| Goat milk casein | INNQFLPYPY | In vitro assay (DPP-IV-inhibitory activity) | Anti-diabetic effect | [133] |
| Camel milk proteins | VPV, YPI, and VPF | In vitro assay (DPP-IV-inhibitory activity) | Anti-diabetic effect | [134] |
| Milk | Milk protein hydrolysate | Diabetic rat | Anti-diabetic effect | [136] |
| Camel milk protein | KDLWDDFKGL, MPSKPPLL | In vitro assay (DPP-IV-inhibitory activity, porcine pancreatic α-amylase) | Anti-diabetic effect | [138] |
| Cheddar cheeses (cow and buffalo milk) | Water-soluble peptide (WSP) extracts | Lung cancer (H-1299) cell line | Anti-cancerous effect | [139] |
| Milk | IPP and VPP | Vascular smooth muscle cells | Anti-hypertensive effect | [140] |
| Milk (casein hydrolysate) | IPP and VPP | 25 male subjects (low hypertension) | Anti-hypertensive effect | [141] |
| Milk | IPP and VPP | Spontaneously hypertensive rats (SHRs) | Anti-hypertensive effect | [142] |
| Milk (α-lactalbumin) | STEYG | Mice | Improve bone health | [143] |
| Goat milk casein | QEPVLGPVRGPFP, SLSSSEESITH, NPWDQVKR, and SDIPNPIGSE | Insulin-resistant HepG2 cells | Anti-diabetic effect | [144] |
| Casein hydrolysate | VPP and IPP | 48 subjects | Anti-hypertensive effect | [145] |
| Milk | Yogurts containing IPP and VPP | 64subjects (men and women) | Anti-hypertensive effect | [146] |
| Milk | Casein hydrolysate (VPP and IPP) | 70 subjects (men and women) | Anti-hypertensive effect | [147] |
| L. helveticus fermented milk | VPP and IPP | 94 subjects (men and women) hypertensive | Anti-hypertensive effect | [148] |
| Milk | Hydrolyzed whey peptide | 76 consecutive adult patients (underwent living-donor liver transplantation) | Reduce post-transplant hyperglycemia | [149] |
| Product Name | Protein Source | Processing Method | Peptide | Company | References |
|---|---|---|---|---|---|
| Evolus® | Casein | Fermentation | IPP, VPP | Valio, Helsinki, Finland | [150,156,157,158] |
| BioZate® | Whey proteins | Hydrolysis with trypsin | Whey peptides | Davisco, Minnesota, USA | |
| Calpis® | Casein | Fermentation | IPP, VPP | Calpis Co., Tokyo, Japan | |
| Danaten® | Fermentation | ND | Danone, Paris, France | ||
| Ameal S® | Casein | Fermentation | IPP, VPP | Calpis Co., Tokyo, Japan | |
| C12 peptide® | Casein | Hydrolysis with trypsin | FFVAPFPEVFGK | DMV International, Holland, Netherlands |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides. Nutrients 2022, 14, 3001. https://doi.org/10.3390/nu14153001
Samtiya M, Samtiya S, Badgujar PC, Puniya AK, Dhewa T, Aluko RE. Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides. Nutrients. 2022; 14(15):3001. https://doi.org/10.3390/nu14153001
Chicago/Turabian StyleSamtiya, Mrinal, Sweta Samtiya, Prarabdh C. Badgujar, Anil Kumar Puniya, Tejpal Dhewa, and Rotimi E. Aluko. 2022. "Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides" Nutrients 14, no. 15: 3001. https://doi.org/10.3390/nu14153001






