Quantification of Phytochemicals, Cellular Antioxidant Activities and Antiproliferative Activities of Raw and Roasted American Pistachios (Pistacia vera L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Cancer Cells and Cell Culture
2.3. Extraction of Soluble Free and Bound Phytochemical Compounds
2.4. Total Phenolic and Flavonoid Content Measurement
2.5. Phytochemical Profiles Analysis by High-Performance Liquid Chromatography (HPLC)
2.6. Extraction of Vitamin E and Carotenoids
2.7. Vitamin E and Carotenoid Profile Analysis by HPLC
2.8. Antioxidant Activity Assay
2.8.1. Determination of Oxygen-Radical-Scavenging Capacity
2.8.2. Determination of Peroxyl-Radical-Scavenging Capacity (PSC) Assay
2.8.3. Cellular Antioxidant Activity (CAA) Assay
2.9. Cytotoxicity and Antiproliferative Activity Assays
2.10. Statistical Analysis
3. Results
3.1. Total Phenolics and Total Flavonoids
3.2. Phenolic Profiles
3.3. Profiles of Vitamin E and Carotenoids
3.4. Total Antioxidant Activity
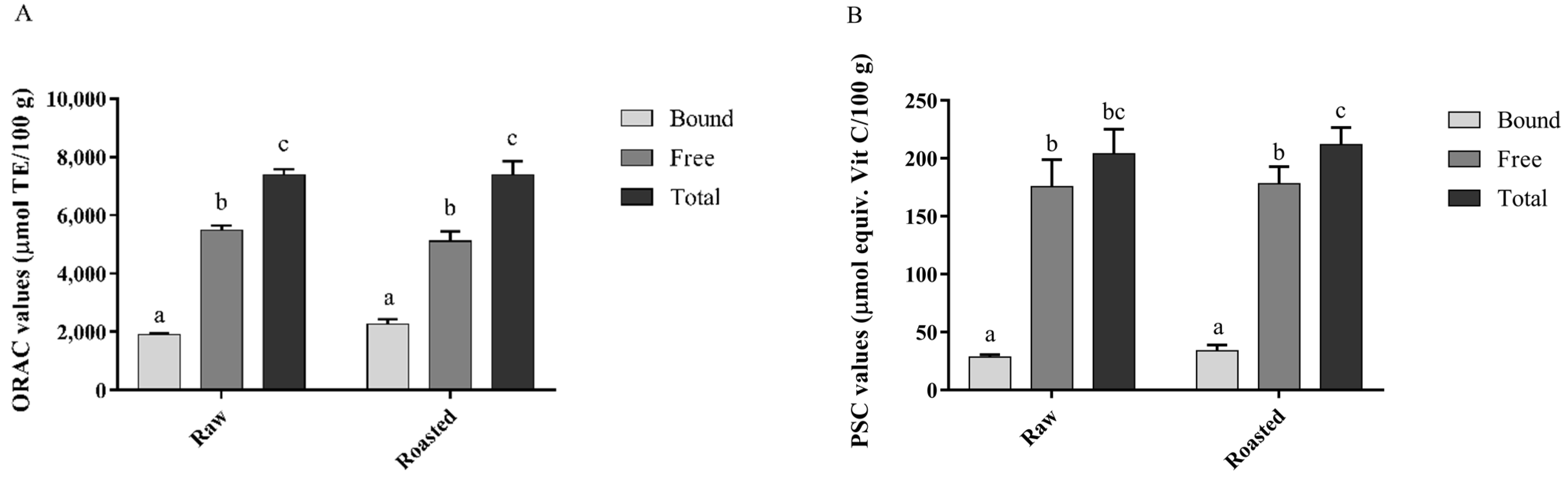
3.4.1. ORAC and PSC Assays
3.4.2. CAA Assay
3.5. Antiproliferative Activity in Cancer Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis of observational studies. Cancer Med. 2015, 4, 1933–1947. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Does a Mediterranean-Type Diet Reduce Cancer Risk? Curr. Nutr. Rep. 2016, 5, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndlovu, T.; Jaarsveld, F.V.; Caleb, O. French and Mediterranean-style diets: Contradictions, misconceptions and scientific facts-A review. Food Res. Int. 2019, 116, 840–858. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. Chemical composition, antioxidant and anti-inflammatory properties of pistachio hull extracts. Food Chem. 2016, 210, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Monnier, G. Neanderthal Behavior. Nat. Educ. Knowl. 2012, 3, 11. [Google Scholar]
- Shahbandeh, M. Production Share of Pistachios Worldwide in 2021/2022, by Country. Available online: https://www.statista.com/statistics/933042/global-pistachio-production-by-country/ (accessed on 10 May 2022).
- Dreher, M.L. Pistachio nuts: Composition and potential health benefits. Nutr. Rev. 2012, 70, 234–240. [Google Scholar] [CrossRef]
- Bulló, M.; Juanola-Falgarona, M.; Hernández-Alonso, P.; Salas-Salvadó, J. Nutrition attributes and health effects of pistachio nuts. Br. J. Nutr. 2015, 113, S79–S93. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Blumberg, J.B.; Chen, C.-Y.O. Quantification and bioaccessibility of California pistachio bioactives (259.1). J. Agric. Food Chem. 2014, 28, 259.1. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [Green Version]
- Grace, M.H.; Esposito, D.; Timmers, M.A.; Xiong, J.; Yousef, G.; Komarnytsky, S.; Lila, M.A. In vitro lipolytic, antioxidant and anti-inflammatory activities of roasted pistachio kernel and skin constituents. Food Funct. 2016, 7, 4285–4298. [Google Scholar] [CrossRef]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Saro, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Santos, J.L.; de Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [Green Version]
- West, S.G.; Gebauer, S.K.; Kay, C.D.; Bagshaw, D.M.; Savastano, D.M.; Diefenbach, C.; Kris-Etherton, P.M. Diets containing pistachios reduce systolic blood pressure and peripheral vascular responses to stress in adults with dyslipidemia. Hypertension 2012, 60, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Baldrich-Mora, M.; Juanola-Falgarona, M.; Bulló, M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: A randomized clinical trial. Diabetes Care 2014, 37, 3098–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, P.D.M.; Silva, A.; Almeida, A.P.; Hermsdorff, H.H.; Alfenas, R.C. Effect of chronic consumption of pistachios (Pistacia vera L.) on glucose metabolism in pre-diabetics and type 2 diabetics: A systematic review. Crit. Rev. Food Sci. Nutr. 2017, 59, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Bin Mohamad, J.; Muhamad, N.A. In Vitro and In Vivo Anticancer Activity of the Most Cytotoxic Fraction of Pistachio Hull Extract in Breast Cancer. Molecules 2020, 25, 1776. [Google Scholar] [CrossRef] [Green Version]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef]
- Canudas, S.; Hernandez-Alonso, P.; Galie, S.; Muralidharan, J.; Morell-Azanza, L.; Zalba, G.; Garcia-Gavilan, J.; Marti, A.; Salas-Salvado, J.; Bullo, M. Pistachio consumption modulates DNA oxidation and genes related to telomere maintenance: A crossover randomized clinical trial. Am. J. Clin. Nutr. 2019, 109, 1738–1745. [Google Scholar] [CrossRef] [Green Version]
- Kocyigit, A.; Keles, H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr. Metab. Cardiovasc. 2006, 16, 202–209. [Google Scholar] [CrossRef]
- Fabani, M.P.; Luna, L.; Baroni, M.V.; Monferran, M.V.; Ighani, M.; Tapia, A.; Wunderlin, D.A.; Egly Feresin, G. Pistachio (Pistacia vera var Kerman) from Argentinean cultivars. A natural product with potential to improve human health. J. Funct. Foods 2013, 5, 1347–1356. [Google Scholar] [CrossRef]
- Fischer, S.; Glei, M. Health-Potential of Nuts. Ernahr. Umsch. 2013, 60, 206–215. [Google Scholar]
- Glei, M.; Ludwig, D.; Lamberty, J.; Fischer, S.; Lorkowski, S.; Schlormann, W. Chemopreventive Potential of Raw and Roasted Pistachios Regarding Colon Carcinogenesis. Nutrients 2017, 9, 1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreca, D.; Lagana, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Seifaddinipour, M.; Farghadani, R.; Namvar, F.; Mohamad, J.; Abdul Kadir, H. Cytotoxic Effects and Anti-Angiogenesis Potential of Pistachio (Pistacia vera L.) Hulls against MCF-7 Human Breast Cancer Cells. Molecules 2018, 23, 110. [Google Scholar] [CrossRef] [Green Version]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef]
- Balbay, A. Effects of environmental temperature and relative humidity on the rehydration of dried pistachios. Dry Technol. 2018, 37, 1239–1250. [Google Scholar] [CrossRef]
- Liu, R.H.; Sun, J. Antiproliferative Activity of Apples Is Not Due to Phenolic-Induced Hydrogen Peroxide Formation. J. Agric. Food Chem. 2003, 51, 1718–1723. [Google Scholar] [CrossRef]
- He, X.; Liu, D.; Liu, R.H. Sodium Borohydride/Chloranil-Based Assay for Quantifying Total Flavonoids. J. Agric. Food Chem. 2008, 56, 9337–9344. [Google Scholar] [CrossRef]
- Xiong, L.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Goji berry (Lycium spp.) extracts exhibit antiproliferative activity via modulating cell cycle arrest, cell apoptosis, and the p53 signaling pathway. Food Funct. 2021, 12, 6513–6525. [Google Scholar] [CrossRef]
- Xie, L.; Yu, Y.; Mao, J.; Liu, H.; Hu, J.G.; Li, T.; Guo, X.; Liu, R.H. Evaluation of Biosynthesis, Accumulation and Antioxidant Activityof Vitamin E in Sweet Corn (Zea mays L.) during Kernel Development. Int. J. Mol. Sci. 2017, 18, 2780. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Xiang, N.; Hu, J.G.; Shijuan, Y.; Xie, L.; Brennan, C.S.; Huang, W.; Guo, X. The manipulation of gene expression and the biosynthesis of Vitamin C, E and folate in light-and dark-germination of sweet corn seeds. Sci. Rep. 2017, 7, 5657–5671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Cao, G.; Prior, R.L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45, 304–309. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Guo, X.; Brennan, C.S.; Li, T.; Fu, X.; Chen, G.; Liu, R.H. Effect of germination on lignan biosynthesis, and antioxidant and antiproliferative activities in flaxseed (Linum usitatissimum L.). Food Chem. 2016, 205, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Liu, R.H. Rapid Peroxyl Radical Scavenging Capacity (PSC) Assay for Assessing both Hydrophilic and Lipophilic Antioxidants. J. Agric. Food Chem. 2005, 53, 6572–6580. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Felice, D.L.; Sun, J.; Liu, R.H. A modified methylene blue assay for accurate cell counting. J. Funct. Foods 2009, 1, 109–118. [Google Scholar] [CrossRef]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Sci. Technol. 2009, 42, 7449–7454. [Google Scholar] [CrossRef]
- Stuetz, W.; Schlörmann, W.; Glei, M. B-vitamins, carotenoids and α-/γ-tocopherol in raw and roasted nuts. Food Chem. 2017, 221, 222–227. [Google Scholar] [CrossRef]
- Rodriguez-Bencomo, J.J.; Kelebek, H.; Sonmezdag, A.S.; Rodriguez-Alcala, L.M.; Fontecha, J.; Selli, S. Characterization of the Aroma-Active, Phenolic, and Lipid Profiles of the Pistachio (Pistacia vera L.) Nut as Affected by the Single and Double Roasting Process. J. Agric. Food Chem. 2015, 63, 7830–7839. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekara, N.; Shahidi, F. Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J. Agric. Food Chem. 2011, 59, 5006–5014. [Google Scholar] [CrossRef] [PubMed]
- Garrido, I.; Monagas, M.; Gomez-Cordoves, C.; Bartolome, B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. J. Food Sci. 2008, 73, C106–C115. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Gong, E.; Lin, Y.; Li, T.; Lian, F.; Zheng, B.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and antiproliferative activities in Chinese bayberry (Myrica rubra Sieb. et Zucc.) fruits. J. Food Sci. 2021, 86, 4691–4703. [Google Scholar] [CrossRef]
- Xia, W.; Lin, Y.; Gong, E.; Li, T.; Lian, F.; Zheng, B.; Liu, R. Wild pink bayberry fruit: The effect of in vitro gastrointestinal digestion on phytochemical profiles, and antioxidant and antiproliferative activities. Food Funct. 2021, 12, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Estrada, R.A.; Gámez-Meza, N.; Medina-Juárez, L.A.; Castillón-Campaña, L.G.; Molina-Domínguez, C.C.; Rascón-Valenzuela, L.A.; García-Galaz, A. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of Wastes from Pecan Nut [Carya illinoinensis (Wagenh) K. Koch]. Waste Biomass Valorization 2019, 11, 3419–3432. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Pereira, R.S.; Pereira, A.B.; Ferreira, A.; Mecha, E.; Silva, A.B.; Serra, A.T.; Bronze, M.R. Identification of functional compounds in baru (Dipteryx alata Vog.) nuts: Nutritional value, volatile and phenolic composition, antioxidant activity and antiproliferative effect. Food Res. Int 2020, 131, 109026. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J.; Giampieri, F.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Manna, P.P.; et al. Effect of pistachio kernel extracts in MCF-7 breast cancer cells: Inhibition of cell proliferation, induction of ROS production, modulation of glycolysis and of mitochondrial respiration. J. Funct. Foods 2018, 45, 155–164. [Google Scholar] [CrossRef]
- Lux, S.; Scharlau, D.; Schloermann, W.; Birringer, M.; Glei, M. In vitro fermented nuts exhibit chemopreventive effects in HT29 colon cancer cells. Br. J. Nutr. 2012, 108, 1177–1186. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-L.; Chen, Y.-G. Natural compounds regulate glycolysis in hypoxic tumor microenvironment. Biomed. Res. Int. 2015, 2015, 354143. [Google Scholar] [CrossRef]


| Pistachios | Total Phenolics (mg GAE/100 g) | Total Flavonoids (mg CE/100 g) | ||||
|---|---|---|---|---|---|---|
| Bound (Percentage of Total) | Free (Percentage of Total) | Total | Bound (Percentage of Total) | Free (Percentage of Total) | Total | |
| Raw | 85.12 ± 3.00 a (18) | 394.8 ± 13.12 c (82) | 479.9 ± 10.2 e | 62.56 ± 2.78 a (35) | 115.8 ± 13.3 c (65) | 178.4 ± 10.6 e |
| Roasted | 73.11 ± 2.11 a (16) | 374.8 ± 8.47 b (84) | 447.9 ± 9.4 d | 42.76 ± 2.73 b (30) | 101.4 ± 4.64 d (70) | 144.1 ± 7.4 f |
| Compounds | Raw (mg/100 g) | Roasted (mg/100 g) | ||||
|---|---|---|---|---|---|---|
| Bound | Free | Total | Bound | Free | Total | |
| Phenolic acids | ||||||
| Gallic acid | 3.45 ± 0.30 a | 9.88 ± 0.11 b | 13.33 ± 0.22 c | 2.05 ± 0.09 d | 16.05 ± 1.04 e | 18.11 ± 1.07 f |
| Protocatechuic acid | 0.23 ± 0.01 a | 2.13 ± 0.14 bc | 2.36 ± 0.14 c | 0.89 ± 0.04 ab | 2.37 ± 1.22 c | 3.25 ± 1.25 c |
| Gentisic acid | 3.09 ± 0.30 a | 117.67 ± 70.74 b | 120.76 ± 70.81 b | 9.25 ± 0.66 a | 202.90 ± 11.88 c | 212.15 ± 12.34 c |
| Flavonoids | ||||||
| Catechin | 0.17 ± 0.01 a | 20.93± 1.48 a | 21.10 ± 1.49 d | 0.35 ± 0.03 b | 33.44 ± 3.00 c | 33.80 ± 3.03 c |
| Epigallocatechin gallate | 3.48 ± 0.19 a | 14.31 ± 1.49 b | 17.78 ± 1.66 c | N/A | 6.26 ± 0.79 d | 6.26 ± 0.79 d |
| Other polyphenols | ||||||
| Catechol | 0.27 ± 0.02 a | N/A | 0.27 ± 0.02 a | 0.57 ± 0.09 b | 7.30 ± 0.34 c | 7.88 ± 0.31 d |
| Compounds | Vit. E (μg per 100 g) | Compounds | Carotenoids (μg per 100 g) | ||
|---|---|---|---|---|---|
| Raw | Roasted | Raw | Roasted | ||
| α-Tocotrienol | 52.58 ± 4.20 a | 85.64 ± 4.74 b | lutein | 1366.7 ± 76 a | 951.2 ± 51 b |
| β-Tocopherol | 1252.7 ± 16 a | 1346.7 ± 7.9 b | zeaxanthin | 42.05± 9.46 a | 43.49 ± 3.24 a |
| γ-Tocopherol | 918.3 ± 85.3 a | 1028.6 ± 2.3 a | β-carotene | 94.96 ± 6.22 a | 70.39 ± 1.33 b |
| γ-Tocotrienol | 246.6 ± 15.2 a | 260.0 ± 1.8 a | Total carotenoids | 1503.64 ± 69 a | 1065.04 ± 55 b |
| Total Vit. E | 2470.2 ± 89 a | 2720.9 ± 1.0 b | |||
| Free | CAA Values (μmol QE per 100 g) | |
|---|---|---|
| Wash | No Wash | |
| Raw | 77.39 ± 4.25 a | 253.71 ± 19.18 b |
| Roasted | 115.62 ± 3.02 c | 216.76 ± 6.60 d |
| Pistachios | Raw (mg/mL) | Roasted (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cells | Bound | SI | Free | SI | Bound | SI | Free | SI | |
| HepG2 | EC50 | 47.84 ± 5.44 b | 2.41 | 100.72 ± 4.72 c | 2.01 | 117.48 ± 6.11 d | 3.17 | 34.73 ± 1.64 a | 4.20 |
| CC50 | 115.49 ± 2.20 a | 202.73 ± 6.46 c | 372.60 ± 0.76 d | 145.96 ± 7.14 b | |||||
| Caco-2 | EC50 | 51.49 ± 4.4 a | 2.20 | 117.13 ± 2.9 b | 2.24 | 151.67 ± 14.8 c | 3.81 | 36.66 ± 3.3 a | 5.43 |
| CC50 | 113.44 ± 8.0 a | 262.87 ± 24.0 b | 578.53 ± 44.5 d | 198.92 ± 20.4 c | |||||
| MDA-MB-231 | EC50 | 51.04 ± 0.69 c | 1.85 | 28.12 ± 1.43 b | 1.50 | 57.61 ± 2.18 d | 2.42 | 7.41 ± 0.82 a | 8.01 |
| CC50 | 94.20 ± 3.91 c | 42.15 ± 6.71 a | 139.22 ± 1.31 d | 59.39 ± 2.96 b | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Zheng, B.; Li, T.; Liu, R.H. Quantification of Phytochemicals, Cellular Antioxidant Activities and Antiproliferative Activities of Raw and Roasted American Pistachios (Pistacia vera L.). Nutrients 2022, 14, 3002. https://doi.org/10.3390/nu14153002
Yuan W, Zheng B, Li T, Liu RH. Quantification of Phytochemicals, Cellular Antioxidant Activities and Antiproliferative Activities of Raw and Roasted American Pistachios (Pistacia vera L.). Nutrients. 2022; 14(15):3002. https://doi.org/10.3390/nu14153002
Chicago/Turabian StyleYuan, Wang, Bisheng Zheng, Tong Li, and Rui Hai Liu. 2022. "Quantification of Phytochemicals, Cellular Antioxidant Activities and Antiproliferative Activities of Raw and Roasted American Pistachios (Pistacia vera L.)" Nutrients 14, no. 15: 3002. https://doi.org/10.3390/nu14153002
APA StyleYuan, W., Zheng, B., Li, T., & Liu, R. H. (2022). Quantification of Phytochemicals, Cellular Antioxidant Activities and Antiproliferative Activities of Raw and Roasted American Pistachios (Pistacia vera L.). Nutrients, 14(15), 3002. https://doi.org/10.3390/nu14153002






