Effects of Soy–Whey Protein Nutritional Supplementation on Hematopoiesis and Immune Reconstitution in an Allogeneic Transplanted Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Grouping
2.2. Bone Marrow Transplantation (BMT)
2.3. Protein Supplementation
2.4. Peripheral Blood Cell Count
2.5. Organ Index and Cell Count
2.6. Flow Cytometric Analysis
2.7. Biochemical Parameters
2.8. Quantitative Real-Time Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
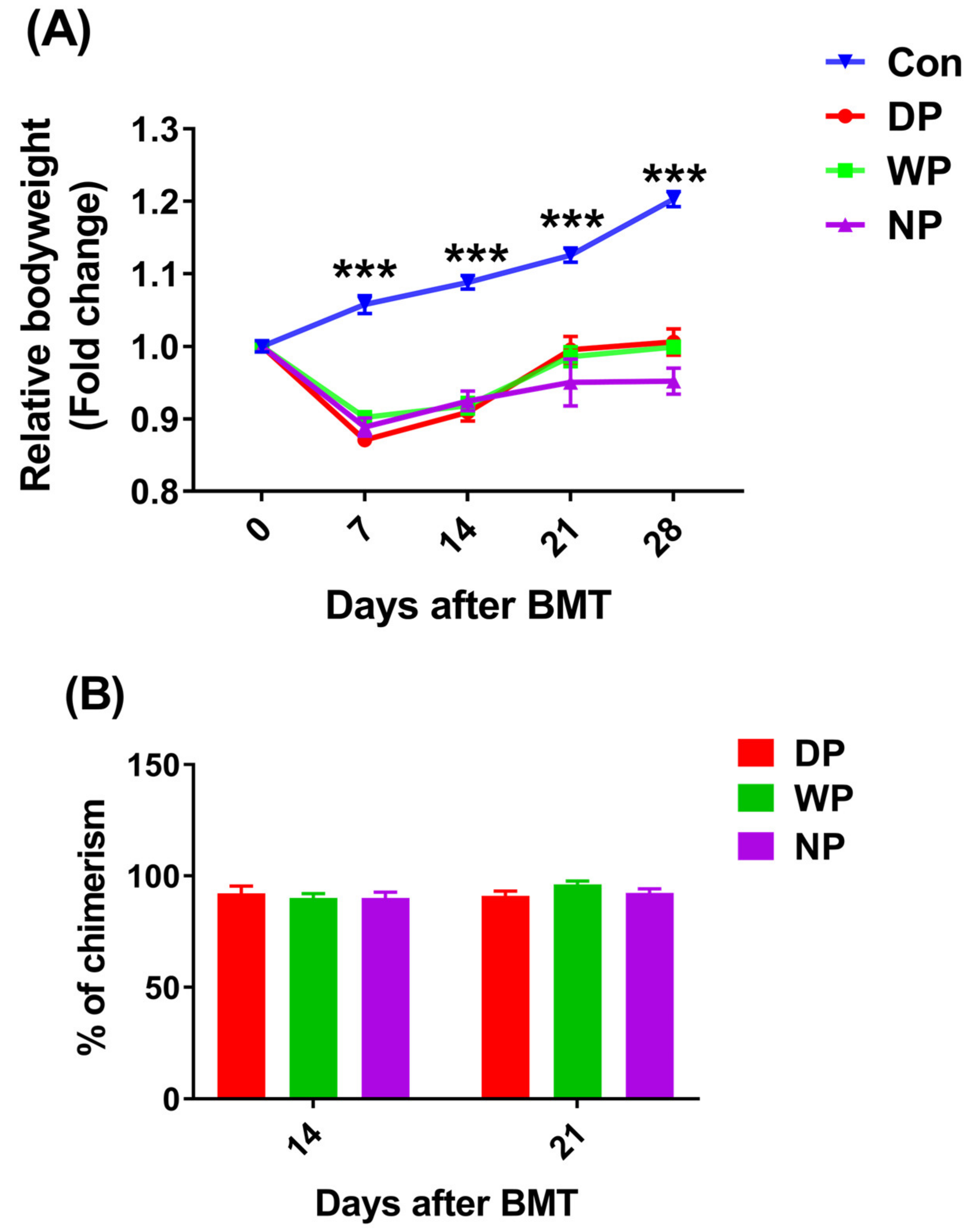
3.1. The Established Allogeneic Transplant Mouse Model, Recipients Successfully Reached Full Bone Marrow Chimerism at 28 Days after BMT
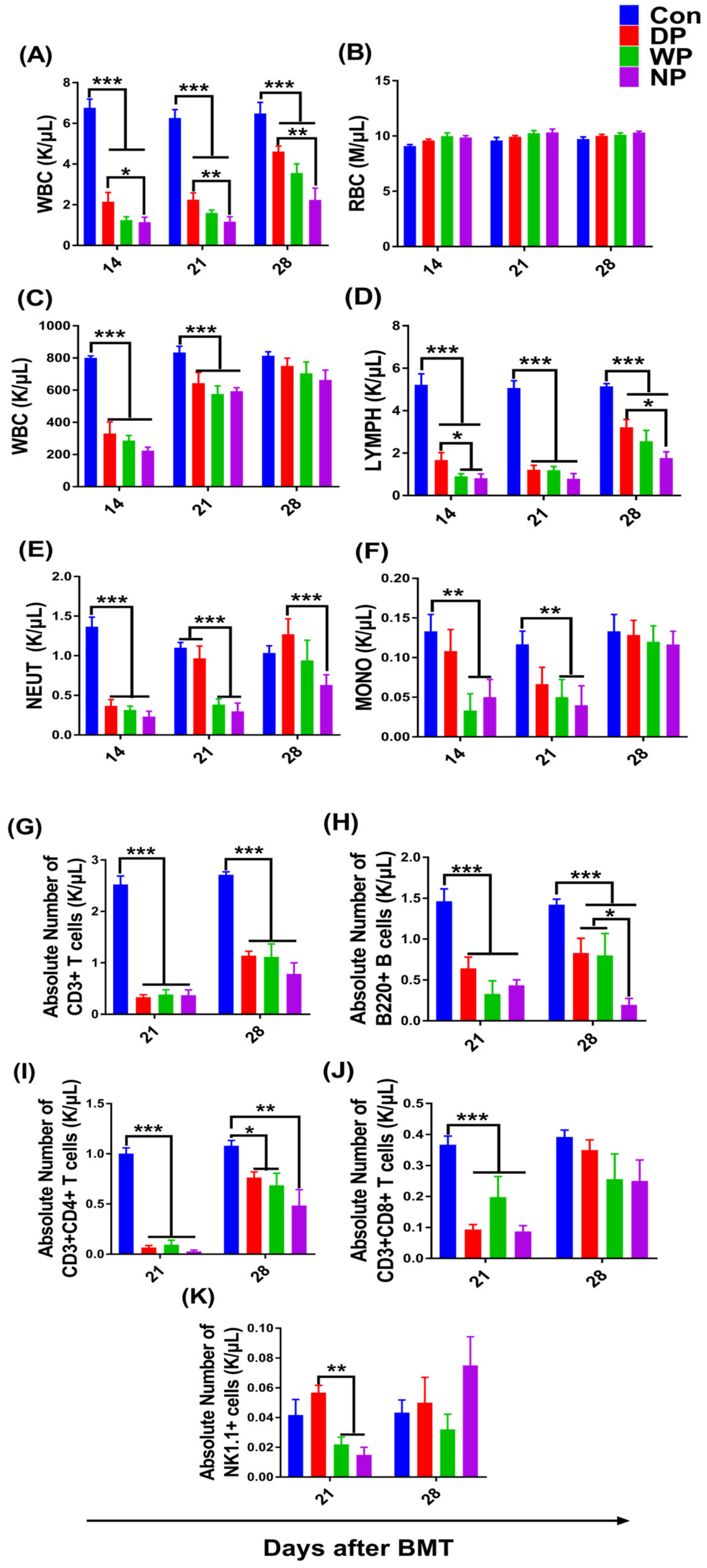
3.2. Hematopoiesis and Lymphocyte Subsets Recovery in Peripheral Blood
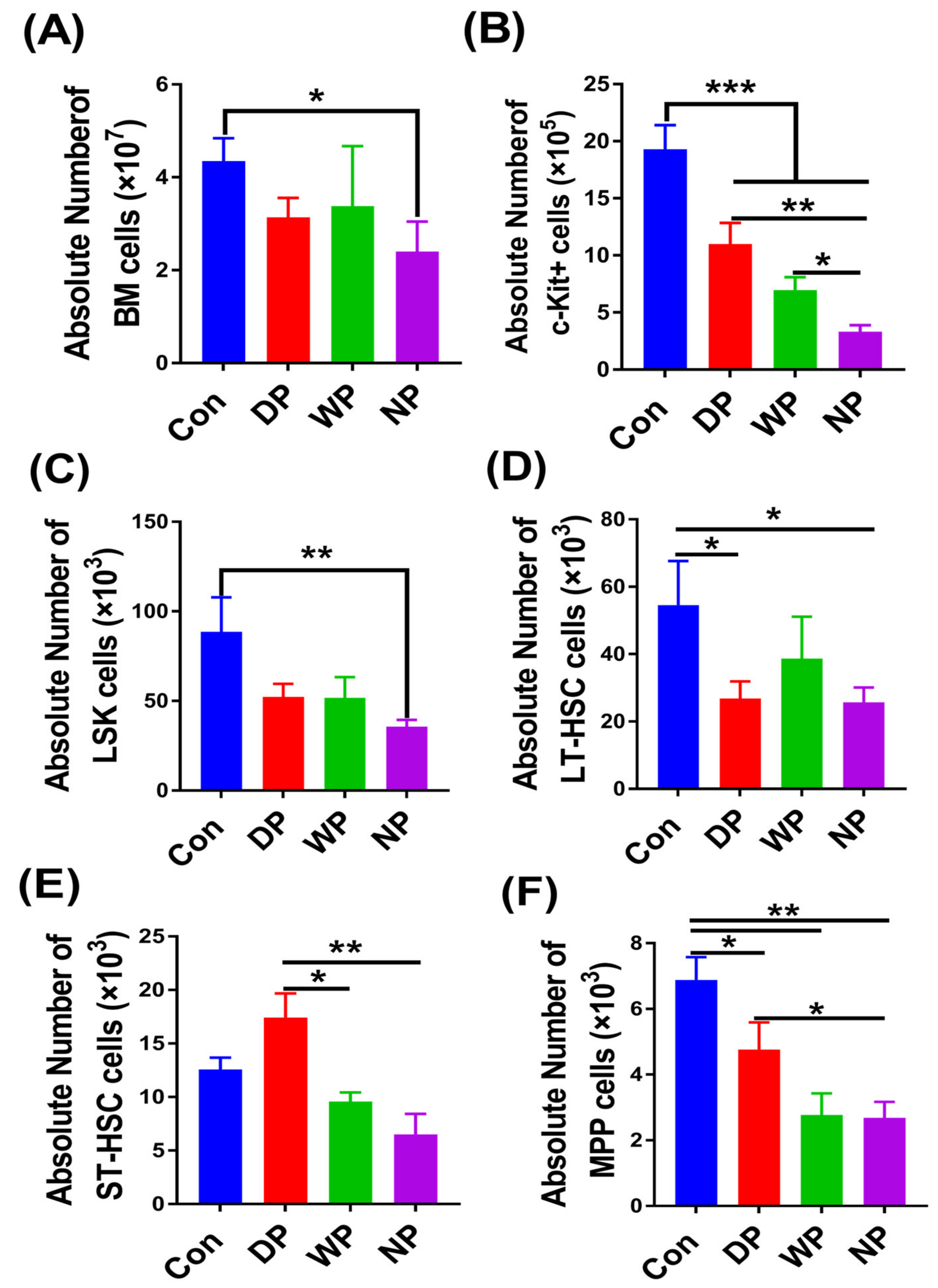
3.3. The Recovery of Hematopoietic Stem Cells and Progenitor Cell Pools
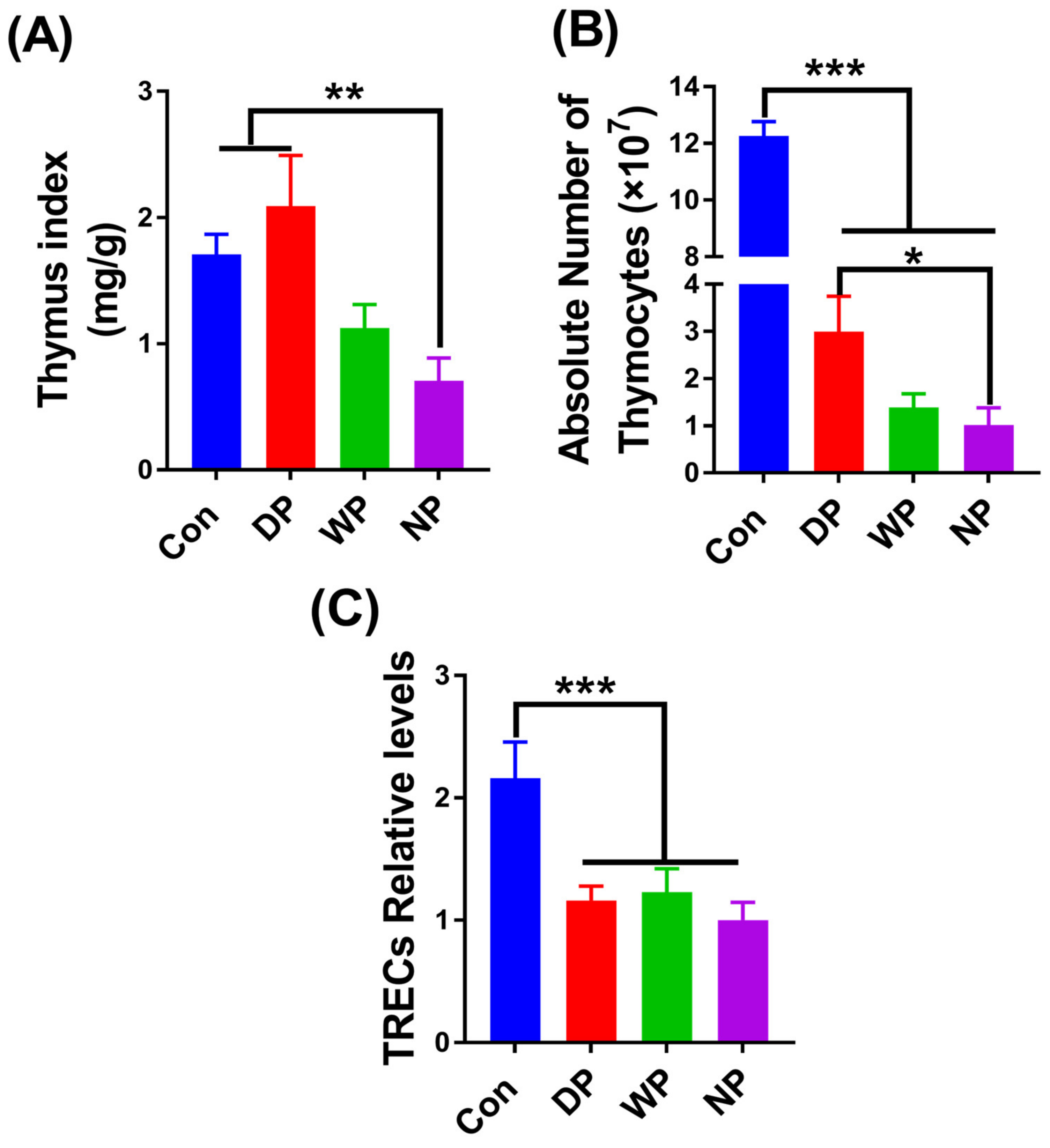
3.4. Thymus Re-Establishment Post-BMT
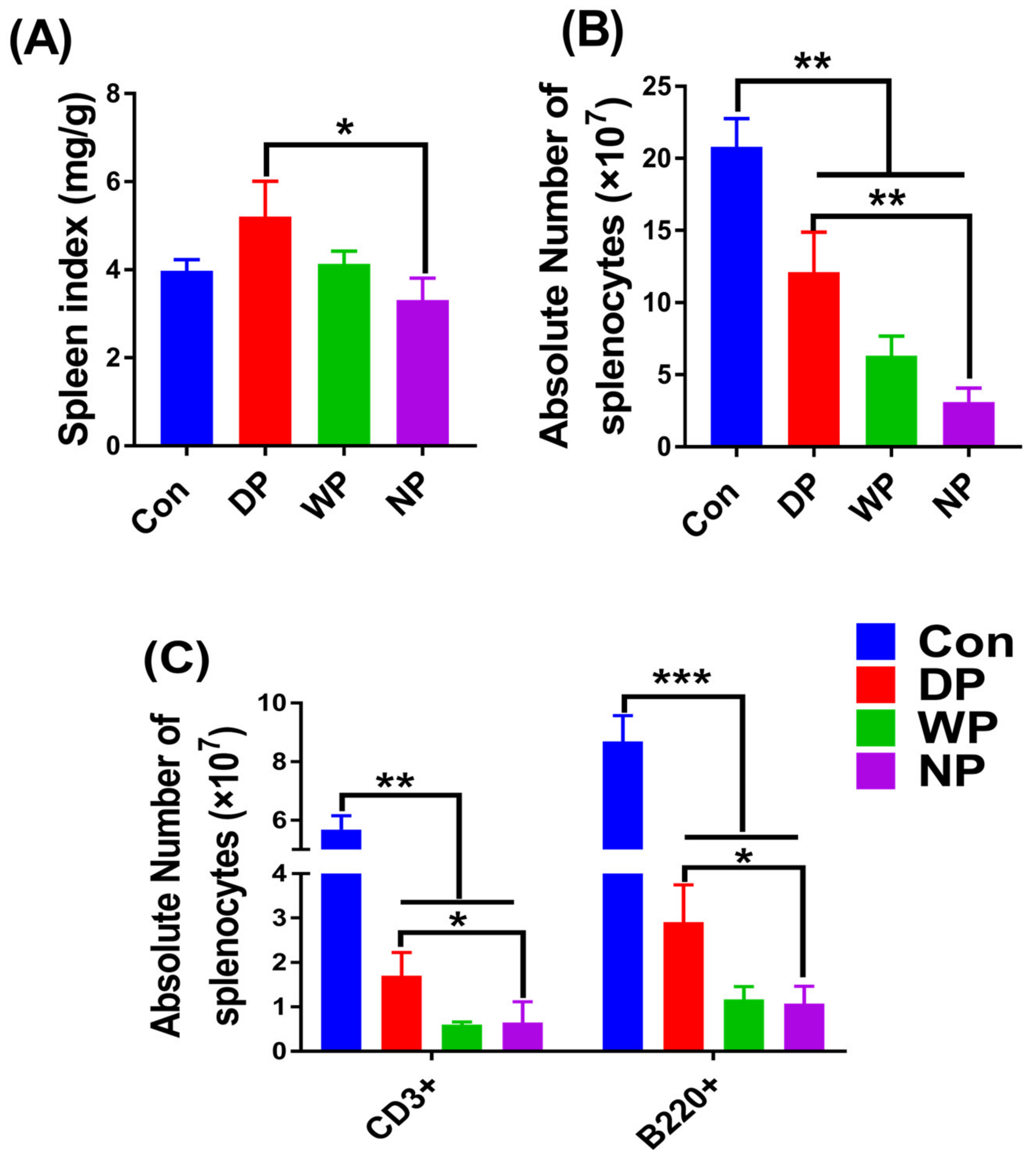
3.5. Splenic Subset Recovery Post-BMT
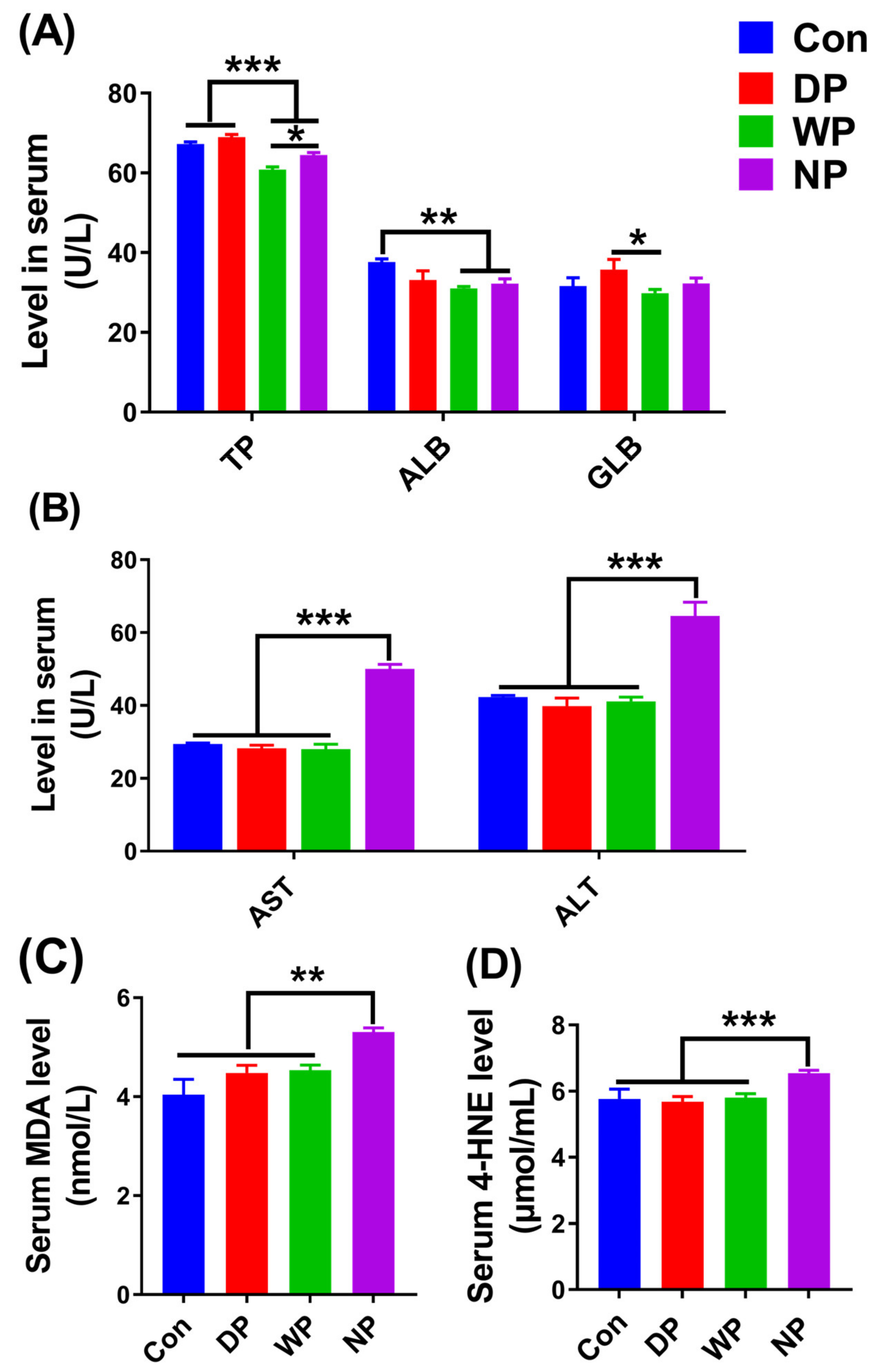
3.6. Biochemical Indicators in Serum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMT | Bone marrow transplantation |
| WP | whey protein |
| WBC | white blood cells |
| GVHD | graft-versus-host disease |
| LSK | Lin-Sca1+c-kit+ |
| ST-HSCs | short-term HSC cells |
| LMPs | lymphoid myeloid progenitors |
| EAAs | essential amino acids |
| DP | soy–whey blended double protein |
| TBI | total body irradiation |
| RBC | red blood cell |
| HS/PCs | hematopoietic stem/progenitor cells |
| LT-HSCs | long-term HSC cells |
| MPPs | primitive multipotent progenitors |
| TRECs | T cell receptor excision circles |
| BCAAs | branched-chain amino acids |
References
- Thomas, E.D.; Storb, R.; Clift, R.A.; Fefer, A.; Johnson, L.; Neiman, P.E.; Lerner, K.G.; Glucksberg, H.; Buckner, C.D. Bone marrow transplantation. N. Engl. J. Med. 1975, 292, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Maury, S.; Mary, J.Y.; Rabian, C.; Schwarzinger, M.; Toubert, A.; Scieux, C.; Carmagnat, M.; Esperou, H.; Ribaud, P.; Devergie, A.; et al. Prolonged immune deficiency following allogeneic stem cell transplantation: Risk factor and complications in adult patients. Br. J. Haematol. 2001, 115, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Storek, J.; Gooley, T.; Witherspoon, R.P.; Sullivan, K.M.; Storb, R. Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am. J. Hematol. 1997, 54, 131–138. [Google Scholar] [CrossRef]
- Chang, Y.J.; Zhao, X.Y.; Huang, X.J. Effects of the NK cell recovery on transplantation for patients with outcomes of unmanipulated haploidentical blood and marrow hematologic malignancies. Biol. Blood Marrow Transpl. 2008, 14, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.G.; Park, C.J.; Jang, S.; Chi, H.S.; Kim, D.Y.; Lee, J.H.; Lee, J.H.; Lee, K.H. Reconstitution of lymphocyte subpopulations after hematopoietic stem cell transplantation: Comparison of hematologic malignancies and donor types in event-free patients. Leuk. Res. 2015, 39, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Ogonek, J.; Kralj Juric, M.; Ghimire, S.; Varanasi, P.R.; Holler, E.; Greinix, H.; Weissinger, E. Immune reconstitution after allogeneic hematopoietic stem cell transplantation. Front. Immunol. 2016, 7, 507. [Google Scholar] [CrossRef] [Green Version]
- Porrata, L.F.; Markovic, S.N. Timely reconstitution of immune competence affects clinical outcome following autologous stem cell transplantation. Clin. Exp. Med. 2004, 4, 78–85. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Zhao, X.Y.; Huo, M.R.; Xu, L.P.; Liu, D.H.; Liu, K.Y.; Huang, X.J. Influence of lymphocyte recovery on outcome of haploidentical transplantation for hematologic malignancies. Medicine 2009, 88, 322–330. [Google Scholar] [CrossRef]
- Broers, A.E.; Posthumus-Van, S.S.; Spits, H.; van der Holt, B.; Löwenberg, B.; Braakman, E.; Cornelissen, J.J. Interleukin-7 improves T-cell recovery after experimental T-cell-depleted bone marrow transplantation in T-cell-deficient mice by strong expansion of recent thymic emigrants. Blood 2003, 102, 1534–1540. [Google Scholar] [CrossRef] [Green Version]
- Tormo, A.; Khodayarian, F.; Cui, Y.; Al-Chami, E.; Kanjarawi, R.; Noé, B.; Wang, H.; Rafei, M. Interleukin-21 promotes thymopoiesis recovery following hematopoietic stem cell transplantation. J. Hematol. Oncol. 2017, 10, 120. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, J.; Tian, X.; Sun, Q.; Xu, J.; Huang, Y.; Wu, B. Ghrelin protects the thymic epithelium from conditioning-regimen-induced damage and promotes the restoration of CD4+ T cells in mice after bone marrow transplantation. Transplantation 2017, 101, e293–e300. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, C.; Song, G.; Yan, Z.; Xu, S.; Jia, L.; Ding, S.; Cao, J.; Chen, W.; Cheng, H.; et al. Infusion of endothelial progenitor cells accelerates hematopoietic and immune reconstitution, and ameliorates the Graft-Versus-Host Disease after hematopoietic stem cell transplantation. Cell Biochem. Biophys. 2012, 64, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.L.; King, C.G.; Nejat, R.A.; Suh, D.Y.; Smith, O.M.; Bretz, J.C.; Samstein, R.M.; Dudakov, J.A.; Chidgey, A.P.; Chen-Kiang, S.; et al. Luteinizing Hormone-Releasing Hormone enhances T cell recovery following allogeneic bone marrow transplantation. J. Immunol. 2009, 182, 5846–5854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Koning, C.; Nierkens, S.; Boelens, J.J. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood 2016, 128, 2607–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rzepecki, P.; Barzal, J.; Sarosiek, T.; Szczylik, C. Biochemical indices for the assessment of nutritional status during hematopoietic stem cell transplantation: Are they worth using? A single center experience. Bone Marrow Transpl. 2007, 40, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Scolapio, J.S.; Smith, D.J. Nutrition support in gastrointestinal disease. World Rev. Nutr. Diet. 2015, 111, 141–145. [Google Scholar]
- Murray, S.M.; Pindoria, S. Nutrition support for bone marrow transplant patients. Cochrane Database Syst. Rev. 2009, CD002920. [Google Scholar] [CrossRef]
- Ordóñez, G.F.; Jiménez, J.F.; Delgado, P.J. Parenteral nutrition in hematologic patients treated with hematopoietic stem cell transplantation. Nutr. Hosp. 2000, 15 (Suppl. 1), 114–120. [Google Scholar]
- Keller, U.; Kraenzlin, M.E.; Gratwohl, A.; Thélin, A.; Straumann, E.; Arnaud, M.J.; Speck, B.; Stauffacherm, W. Protein metabolism assessed by 1-13C leucine infusions in patients undergoing bone marrow transplantation. JPEN J. Parenter. Enteral Nutr. 1990, 14, 480–484. [Google Scholar] [CrossRef]
- Forchielli, M.L.; Azzi, N.; Cadranel, S.; Paolucci, G. Total parenteral nutrition in bone marrow transplant: What is the appropriate energy level? Oncology 2003, 64, 7–13. [Google Scholar] [CrossRef]
- Martin-Salces, M.; de Paz, R.; Canales, M.A.; Mesejo, A.; Hernandez-Navarro, F. Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition 2008, 24, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Thompson, J. Graft-vs-host disease: Nutrition therapy in a challenging condition. Nutr. Clin. Pract. 2005, 20, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Sheean, P.M. Nutrition support of blood or marrow transplant recipients: How much do we really know? Pract. Gastroenterol. 2005, 26, 84–97. [Google Scholar]
- Hughes, G.J.; Ryan, D.J.; Mukherjea, R.; Schasteen, C.S. Protein digestibility-corrected amino acid scores (PDCAAS) for soy protein isolates and concentrate: Criteria for evaluation. J. Agric. Food Chem. 2011, 59, 12707–12712. [Google Scholar] [CrossRef]
- Tang, J.E.; Phillips, S.M. Maximizing muscle protein anabolism: The role of protein quality. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, C.; Metges, C.C.; Gaudichon, C.; Petzke, K.J.; Pueyo, M.E.; Morens, C.; Everwand, J.; Benamouzig, R.; Tomé, D. Postprandial kinetics of dietary amino acids are the main determinant of their metabolism after soy or milk protein ingestion in humans. J. Nutr. 2003, 133, 1308–1315. [Google Scholar] [CrossRef]
- Farnfield, M.M.; Trenerry, C.; Carey, K.A.; Cameron-Smith, D. Plasma amino acid response after ingestion of different whey protein fractions. Int. J. Food Sci. Nutr. 2009, 60, 476–486. [Google Scholar] [CrossRef]
- Aoki, H.; Otaka, Y.; Igarashi, K.; Takenaka, A. Soy protein reduces paraquat-induced oxidative stress in rats. J. Nutr. 2002, 132, 2258–2262. [Google Scholar] [CrossRef]
- Tosukhowong, P.; Boonla, C.; Dissayabutra, T.; Kaewwilai, L.; Muensri, S.; Chotipanich, C.; Joutsa, J.; Rinne, J.; Bhidayasiri, R. Biochemical and clinical effects of Whey protein supplementation in Parkinson’s disease: A pilot study. J. Neurol. Sci. 2016, 367, 162–170. [Google Scholar] [CrossRef]
- Wong, C.W.; Watson, D.L. Immunomodulatory effects of dietary whey proteins in mice. J. Dairy Res. 1995, 62, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Low, P.P.; Rutherfurd, K.J.; Gill, H.S.; Cross, M.L. Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice. Int. Immunopharmacol. 2003, 3, 393–401. [Google Scholar] [CrossRef]
- Ford, J.T.; Wong, C.W.; Colditz, I.G. Effects of dietary protein types on immune responses and levels of infection with Eimeria vermiformis in mice. Immunol. Cell Biol. 2001, 79, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, J.; Li, M.; Yi, S.; Xie, J.; Zhang, H.; Wang, J. Protein blend ingestion before allogeneic stem cell transplantation improves protein-energy malnutrition in patients with leukemia. Nutr. Res. 2017, 46, 68–77. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Geng, J.; Zhang, H.; Guo, L. Apolipoprotein E deletion has no effect on copper-induced oxidative stress in the mice brain. Biosci. Rep. 2018, 38, 20180719. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Chen, X.; Qian, L.; Shen, J.; Cai, J. Administration of Hydrogen-Rich Saline in Mice with Allogeneic Hematopoietic Stem-Cell Transplantation. Med. Sci. Monit. 2015, 21, 749–754. [Google Scholar]
- Ryan, M.R.; Shepherd, R.; Leavey, J.K.; Gao, Y.; Grassi, F.; Schnell, F.J.; Qian, W.P.; Kersh, G.J.; Weitzmann, M.N.; Pacifici, R. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc. Natl. Acad. Sci. USA 2005, 102, 16735–16740. [Google Scholar] [CrossRef] [Green Version]
- Politikos, I.; Boussiotis, V.A. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood 2014, 124, 3201–3211. [Google Scholar] [CrossRef] [Green Version]
- Douek, D.C.; Vescio, R.A.; Betts, M.R.; Brenchley, J.M.; Hill, B.J.; Zhang, L.; Berenson, J.R.; Collins, R.H.; Koup, R.A. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 2000, 355, 1875–1881. [Google Scholar] [CrossRef]
- Le Na, N.T.; Duc Loc, S.; Minh Tri, N.L.; Bich Loan, N.T.; Anh Son, H.; Linh Toan, N.; Phuong Thu, H.; My Nhung, H.T.; Lai Thanh, N.; Van Anh, N.T.; et al. Nanomelanin potentially protects the spleen from radiotherapy-associated damage and enhances immunoactivity in tumor-bearing mice. Materials 2019, 12, 1725. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, R.; Cortés, L.; Cortés, E.; Medina, H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin. Exp. Immunol. 2009, 155, 96–106. [Google Scholar] [CrossRef]
- Rzepecki, P.; Barzal, J.; Oborska, S. Blood and marrow transplantation and nutritional support. Support. Care Cancer 2010, 18 (Suppl. 2), S57–S65. [Google Scholar] [CrossRef]
- Zhang, H.; Zhai, Z.; Wang, Y.; Zhang, J.; Wu, H.; Wang, Y.; Li, C.; Li, D.; Lu, L.; Wang, X.; et al. Resveratrol ameliorates ionizing irradiation-induced long-term hematopoietic stem cell injury in mice. Free Radic. Biol. Med. 2013, 54, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kim, S.; Park, Y.; Shin, A.R.; Yeom, H. Nutritional intervention for a patient with acute lymphoblastic leukemia on allogeneic peripheral blood stem cell transplantation. Clin. Nutr. Res. 2018, 7, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lum, L.G. The kinetics of immune reconstitution after human marrow transplantation. Blood 1987, 69, 369–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkman, R.; Cohen, G.; Carter, S.L.; Weinberg, K.I.; Masinsin, B.; Guinan, E.; Kurtzberg, J.; Wagner, J.E.; Kernan, N.A. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol. Blood Marrow Transpl. 2006, 12, 919–927. [Google Scholar]
- Liu, P.; Wang, B.; Yan, X.; Cai, J.; Wang, Y. Comprehensive evaluation of nutritional status before and after hematopoietic stem cell transplantation in 170 patients with hematological diseases. Chin. J. Cancer Res. 2016, 28, 626–633. [Google Scholar] [CrossRef] [Green Version]
- Planas, M.; Puiggrós, C.; Redecillas, S. Contribution of nutritional support to fight cancer cachexia. Nutr. Hosp. 2006, 21 (Suppl. 3), 27–36. [Google Scholar]
- Herrmann, V.M.; Petruska, P.J. Nutrition support in bone marrow transplant recipients. Nutr. Clin. Pract. 1993, 8, 19–27. [Google Scholar] [CrossRef]
- Weisdorf, S.A.; Salati, L.M.; Longsdorf, J.A.; Ramsay, N.K.; Sharp, H.L. Graft-versus-host disease of the intestine: A protein losing enterophathy characterized by fecal alpha-1-antitrypsin. Gastroenterology 1983, 85, 1076–1081. [Google Scholar] [CrossRef]
- Ito, K.; Bonora, M.; Ito, K. Metabolism as master of hematopoietic stem cell fate. Int. J. Hematol. 2019, 109, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanescu, S.; Belanger-Quintana, A.; Fernandez-Felix, B.M.; Arrieta, F.; Quintero, V.; Maldonado, M.S.; Alcaide, P.; Martínez-Pardo, M. Severe anemia in patients with Propionic acidemia is associated with branched-chain amino acid imbalance. Orphanet J. Rare Dis. 2021, 16, 226. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Morita, M.; Nakauchi, H.; Yamazaki, S. Branched-chain amino acid depletion conditions bone marrow for hematopoietic stem cell transplantation avoiding amino acid imbalance-associated toxicity. Exp. Hematol. 2018, 63, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.S.; Zhao, H.L.; Gao, Y.; Wang, Q.; Xiang, H.; Xu, X.P.; Huang, S.; Yan, D.-L.; Qin, X.-M. Branched-Chain Amino Acids Catabolism Pathway Regulation Plays a Critical Role in the Improvement of Leukopenia Induced by Cyclophosphamide in 4T1 Tumor-Bearing Mice Treated with Lvjiaobuxue Granule. Front. Pharmacol. 2021, 12, 657047. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Lee, M.C.; Hsu, Y.J.; Huang, W.C.; Huang, C.C.; Huang, S.W. Isolated soy protein supplementation and exercise improve fatigue-related biomarker levels and bone strength in ovariectomized mice. Nutrients 2018, 10, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sah, B.N.; Mcainch, A.J.; Vasiljevic, T. Modulation of bovine whey protein digestion in gastrointestinal tract: A comprehensive review. Int. Dairy J. 2016, 62, 10–18. [Google Scholar] [CrossRef]
- Wilkinson, A.C.; Yamazaki, S. The hematopoietic stem cell diet. Int. J. Hematol. 2018, 107, 634–641. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, A.C.; Igarashi, K.J.; Nakauchi, H. Haematopoietic stem cell self-renewal in vivo and ex vivo. Nat. Rev. Genet. 2020, 21, 541–554. [Google Scholar] [CrossRef]
- Santos, E.W.; Oliveira, D.C.; Silva, G.B.; Tsujita, M.; Beltran, J.O.; Hastreiter, A.; Fock, R.A.; Borelli, P. Hematological alterations in protein malnutrition. Nutr. Rev. 2017, 75, 909–919. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamura, C.; Bouladoux, N.; Belkaid, Y.; Sher, A.; Jankovic, D. Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood 2017, 129, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josefsdottir, K.S.; Baldridge, M.T.; Kadmon, C.S.; King, K.Y. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood 2017, 129, 729–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staffas, A.; Burgos da Silva, M.; Slingerland, A.E.; Lazrak, A.; Bare, C.J.; Holman, C.D.; Docampo, M.D.; Shono, Y.; Durham, B.; Pickard, A.J.; et al. Nutritional support from the intestinal microbiota improves hematopoietic reconstitution after bone marrow transplantation in mice. Cell Host Microbe 2018, 23, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.I.; Yatsunenko, T.; Manary, M.J.; Trehan, I.; Mkakosya, R.; Cheng, J.; Docampo, M.D.; Shono, Y.; Durham, B.; Pickard, A.; et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013, 339, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Shono, Y.; van den Brink, M.R.M. Gut Microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat. Rev. Cancer 2018, 18, 283–295. [Google Scholar] [CrossRef]
- Cronin, O.; Barton, W.; Skuse, P.; Penney, N.C.; Garcia-Perez, I.; Murphy, E.F.; Woods, T.; Nugent, H.; Fanning, A.; Melgar, S.; et al. A prospective metagenomic and metabolomic analysis of the impact of exercise and/or whey protein supplementation on the gut microbiome of sedentary adults. mSystems 2018, 3, e00044-18. [Google Scholar] [CrossRef] [Green Version]
- Butteiger, D.N.; Hibberd, A.A.; McGraw, N.J.; Napawan, N.; Hall-Porter, J.M.; Krul, E.S. Soy protein compared with milk protein in a western diet increases gut microbial diversity and reduces serum lipids in golden Syrian hamsters. J. Nutr. 2016, 146, 697–705. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.A. The influence of the diet on the maturation of the immune system. Allergy 1998, 53 (Suppl. 46), 26–28. [Google Scholar] [CrossRef]
- Luo, H.; Li, J.; Chen, X. Potential role of N-Succinyl-Chitosan in immune reconstitution after umbilical cord blood transplantation in mice. Biomed. Pharm. 2011, 65, 578–584. [Google Scholar] [CrossRef]
- Gonçalves, T.L.; Benvegnú, D.M.; Bonfanti, G.; Frediani, A.V.; Pereira, D.V.; Rocha, J.B. Oxidative stress and δ-ALA-D activity in different conditioning regimens in allogeneic bone marrow transplantation patients. Clin. Biochem. 2009, 42, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Dürken, M.; Agbenu, J.; Finckh, B.; Hübner, C.; Pichlmeier, U.; Zeller, W.; Winkler, K.; Zander, A.; Kohlschütter, A. Deteriorating free radical-trapping capacity and antioxidant status in plasma during bone marrow transplantation. Bone Marrow Transpl. 1995, 15, 757–762. [Google Scholar]
- Smeyne, M.; Smeyne, R.J. Glutathione metabolism and Parkinson’s disease. Free Radic. Biol. Med. 2013, 62, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Marshall, K. Therapeutic applications of Whey protein. Altern. Med. Rev. 2004, 9, 136–156. [Google Scholar] [PubMed]
- Gariballa, S.E. Malnutrition in hospitalized elderly patients: When does it matter? Clin. Nutr. 2001, 20, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.F.; Wochos, D.N. The utility of serum albumin values in the nutritional assessment of hospitalized patients. Mayo Clin. Proc. 1982, 57, 181–184. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, J.; Li, M.; Tang, Z.; Yang, Z.; Cheng, G.; Wang, J. Gut microbiota composition influences outcomes of skeletal muscle nutritional intervention via blended protein supplementation in posttransplant patients with hematological malignancies. Clin. Nutr. 2021, 40, 94–102. [Google Scholar] [CrossRef]
- Van Erp, A.C.; Hoeksma, D.; Rebolledo, R.A.; Ottens, P.J.; Jochmans, I.; Monbaliu, D.; Pirenne, J.; Leuvenink, H.G.D.; Decuypere, J.P. The Crosstalk between ROS and Autophagy in the Field of Transplantation Medicine. Oxid. Med. Cell Longev. 2017, 2017, 7120962. [Google Scholar] [CrossRef] [Green Version]
- Ludin, A.; Gur-Cohen, S.; Golan, K.; Kaufmann, K.B.; Itkin, T.; Medaglia, C.; Lu, X.-J.; Ledergor, G.; Kollet, O.; Lapidot, T. Reactive Oxygen Species Regulate Hematopoietic Stem Cell Self-Renewal, Migration and Development, as well as Their Bone Marrow Microenvironment. Antioxid. Redox Signal. 2014, 21, 1605–1619. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Hou, Q.; Zhao, Z.; Wang, J.; Guo, Y.; Lu, L.; Han, J. Effects of Soy–Whey Protein Nutritional Supplementation on Hematopoiesis and Immune Reconstitution in an Allogeneic Transplanted Mice. Nutrients 2022, 14, 3014. https://doi.org/10.3390/nu14153014
Wu X, Hou Q, Zhao Z, Wang J, Guo Y, Lu L, Han J. Effects of Soy–Whey Protein Nutritional Supplementation on Hematopoiesis and Immune Reconstitution in an Allogeneic Transplanted Mice. Nutrients. 2022; 14(15):3014. https://doi.org/10.3390/nu14153014
Chicago/Turabian StyleWu, Xiaoliang, Qinghua Hou, Zhenyu Zhao, Jing Wang, Yanzhi Guo, Lingang Lu, and Juan Han. 2022. "Effects of Soy–Whey Protein Nutritional Supplementation on Hematopoiesis and Immune Reconstitution in an Allogeneic Transplanted Mice" Nutrients 14, no. 15: 3014. https://doi.org/10.3390/nu14153014
APA StyleWu, X., Hou, Q., Zhao, Z., Wang, J., Guo, Y., Lu, L., & Han, J. (2022). Effects of Soy–Whey Protein Nutritional Supplementation on Hematopoiesis and Immune Reconstitution in an Allogeneic Transplanted Mice. Nutrients, 14(15), 3014. https://doi.org/10.3390/nu14153014






