Potential Applications of Thyroid Hormone Derivatives in Obesity and Type 2 Diabetes: Focus on 3,5-Diiodothyronine (3,5-T2) in Psammomys obesus (Fat Sand Rat) Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Protocol Design
2.2. Diet Composition
- (i)
- Group I submitted on natural diet (ND): Thirty two P. obesus are strictly maintained ad libitum on ND. This ND group represents the diet-control P. obesus. This ND is composed by halophilic plants of the Chenopodiaceae family (Traganum nudatum, Salsola foetidia, Suaeda fructosa and Artriplex halimus species), freshly harvested in their native biotope [10]. The P. obesus subsists primarily or exclusively of these plants whose sap has a very high salt concentration [11]. The ND is characterized by a low caloric value [12].
- (ii)
- Group submitted on HED before randomization: one hundred twenty five P. obesus are maintained on laboratory synthetic chow pellets (Carfil Quality, Beyntellus, Belgium; https://www.carfil.be, accessed on 1 January 1982). This synthetic diet is intended for all experimental rodents but it is considered to be high-caloric diet (3.25 kcal/g of diet) compared to ND (0.40 kcal/g of diet) and hence, it is used to develop obesity and diabetes [13]. The HED contain: 47.4% carbohydrates, 25% proteins, 7.5% fat, with high energy carbohydrates (33.5% starch and 13.5% total sugar), fatty substances, vitamins, minerals. P. obesus HED-fed group are randomized into three subgroups according experimental protocol described in Figure 1: Group II or HED-controlled group: Thirty P. obesus continue to feed on HED without treatment or supplementation; Group III or 3,5-T2-treated group: Sixty-five P. obesus continue to feed on HED and submitted to a continuous and constant administration 3,5-T2 (3,5-diiodothyronine) pellet implant according subcutaneous technique (25 µg/100 g body weight/day) during 5 weeks. Group IV or Placebo-controlled group: Fifteen P. obesus continue to feed on HED and submitted to a continuous and constant administration of vehicle (isotonic saline solution 0.9% NaCl) according subcutaneous a placebo pellet implant. A placebo pellet was implanted at the same time as the P. obesus treated with 3,5-T2. In fact, 3,5-T2-treated group received the HED for 10 weeks and were subsequently treated for five weeks simultaneously with HED. The pharmacological dose of 25 µg 3,5-T2/100 g body weight (BW) was chosen after reviewing data from previous studies [14] which used 3,5-T2 as treatment and we have adapted for our study in gerbil P. obesus. Indeed, recent studies have shown that chronic treatment during 4 weeks with doses of 50 µg of 3,5-T2/100 g BW to male Rats HED pre-fed did not result in any thyrotoxic effect that might be of clinical relevance.
2.3. 3,5-Diiodothyronine Pellet Implantation Method
2.4. Oxygen Consumption, Energy Expenditure, Basal Metabolic Rate Measurement
2.5. Adipose Tissue Samples and Adiposity Index Estimation
2.6. Plasma and Hepatic Biochemical Analysis
2.7. Plasma Thyroid Hormones Assays
2.8. Hepatocytes Isolation Method
2.9. Intracellular Metabolic Fluxes Analysis
2.10. Assessment of Hepatic Intracellular Intermediary Metabolites
2.11. Determination of Oxygen Consumption Rates
2.12. Hepatic Protein, Glycogen and Lipids Determination
2.13. Statistical Analyses
3. Results
3.1. In Vivo Effects of 3,5-T2 Treatment on Plasma Thyroid Hormone Profile
3.2. In Vivo Effects of 3,5-T2 Treatment on Body Weight, Calorie Intake, Respiratory Quotient and Basal Metabolic Rate
3.3. In Vivo Effects of 3,5-T2 Treatment on Body Adipose Tissue Distribution
3.4. In Vivo Effects of 3,5-T2 Treatment on Plasma and Liver Metabolic Disorders
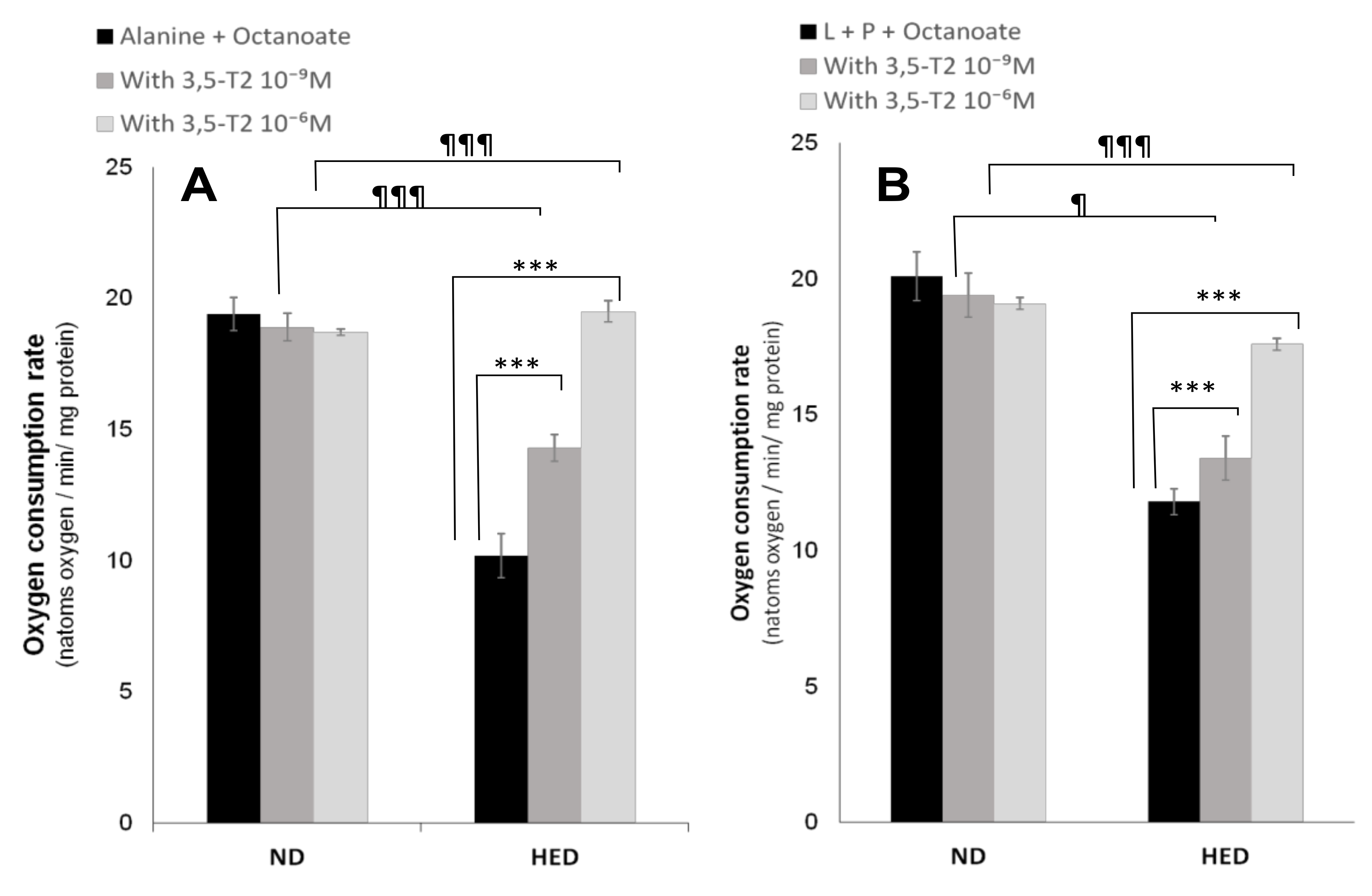
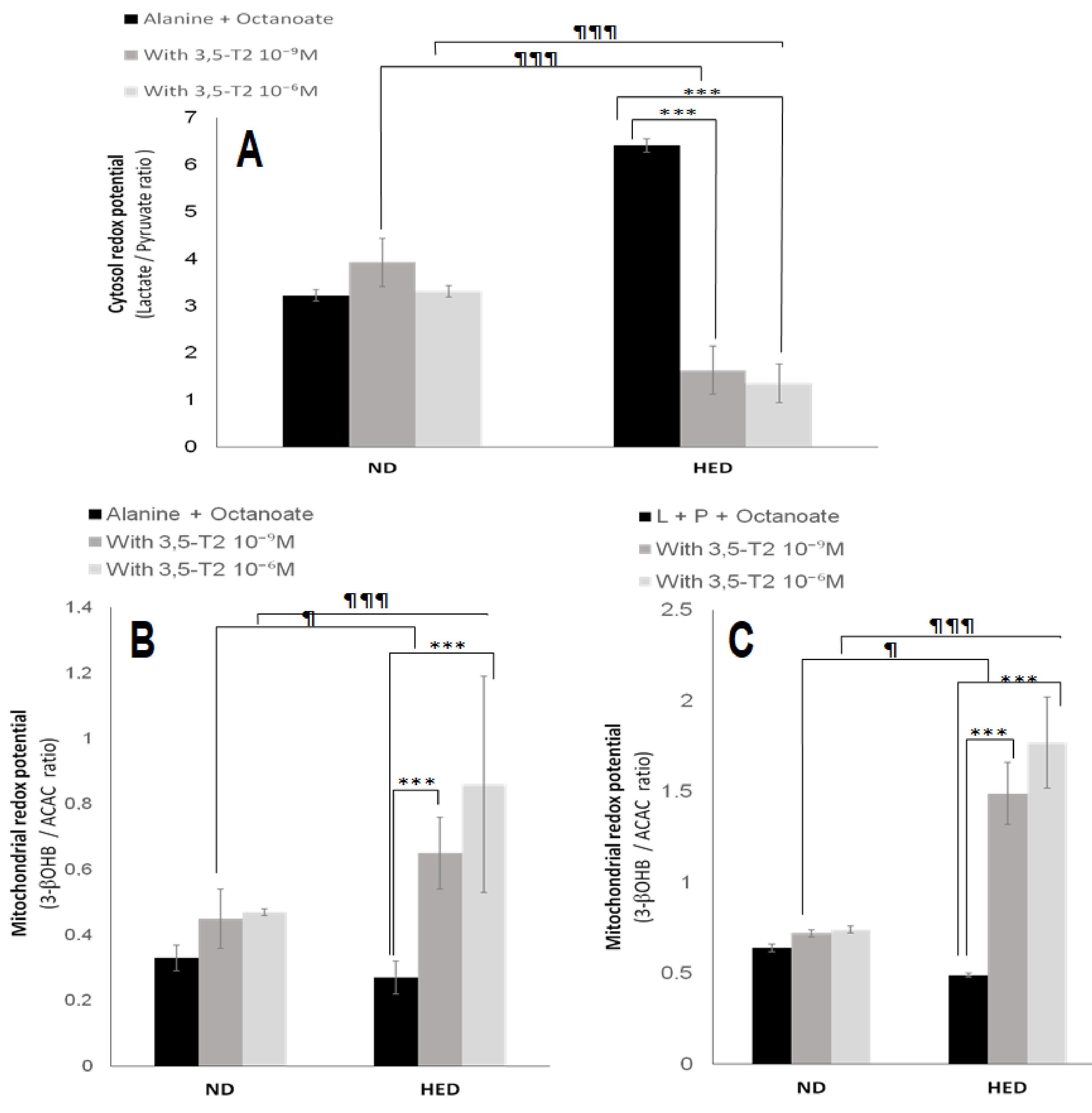
3.5. In Vitro Effects of 3,5-T2 Treatment on Oxygen Consumption, Hepatic Gluconeogenesis, Ketogenesis, Intracellular Intermediary Metabolites, and Cellular Redox-Phosphate Potential
4. Discussion
4.1. The First Point Is Linked to 3,5-T2 Treatment on Plasma Thyroid Hormone Profile
4.2. The Second Point Is Linked to 3,5-T2 Treatment on Body Weight, Calorie Intake, Oxygen Consumption, Respiratory Quotient and Basal Metabolic Rate
4.3. The Third Point Is Linked to 3,5-T2 Treatment on Body Adipose Tissue Distribution
4.4. The Fourth Point Is Linked to 3,5-T2 Treatment on Plasma and Liver Metabolic Disorders
4.5. The Fifth Point Is Linked to 3,5-T2 Treatment on Hepatic Gluconeogenesis, Ketogenesis, Intracellular Intermediary Metabolites, and Cellular Redox-Phosphate Potential
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visser, W.E. Therapeutic applications of thyroid hormone analogues. Ann. Endocrinol. 2021, 82, 170–172. [Google Scholar] [CrossRef]
- Cioffi, F.; Giacco, A.; Goglia, F.; Silvestri, E. Bioenergetic Aspects of Mitochondrial Actions of Thyroid Hormones. Cells 2022, 11, 997. [Google Scholar] [CrossRef]
- Köhrle, J. Thyroid Hormones and Derivatives: Endogenous Thyroid Hormones and Their Targets. Methods Mol. Biol. 2018, 1801, 85–104. [Google Scholar]
- Brenta, G.; Caballero, A.S.; Nunes, M.T. Case finding for hypothyroidism should include type 2 diabetes and metabolic syndrome patients: A Latin American Thyroid Society (LATS) position statement. Endocr. Pract. 2019, 25, 101–105. [Google Scholar] [CrossRef]
- Zucchi, R.; Rutigliano, G.; Saponaro, F. Novel thyroid hormones. Endocrine 2019, 66, 95–104. [Google Scholar] [CrossRef]
- Giammanco, M.; Di Liegro, C.M.; Schiera, G.; Di Liegro, I. Genomic and Non-Genomic Mechanisms of Action of Thyroid Hormones and Their Catabolite 3,5-Diiodo-L-Thyronine in Mammals. Int. J. Mol. Sci. 2020, 21, 4140. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.C.; Salas-Lucia, F.; Bianco, A.C. Deiodinases and the Metabolic Code for Thyroid Hormone Action. Endocrinology 2021, 162, bqab059. [Google Scholar] [CrossRef]
- Chaudhary, R.; Walder, K.R.; Hagemeyer, C.E.; Kanwar, J.R. Psammomys obesus: A Natural Diet-Controlled Model for Diabetes and Cardiovascular Diseases. Curr. Atheroscler. Rep. 2018, 20, 46. [Google Scholar] [CrossRef]
- Koceir, E.A.; Dahmani, Y.; Leverve, X. Low rate of glucose 6-phosphate hydrolysis in liver cells is a physiological feature of non-diabetic wild sand rats (Psammomys obesus). Diabetes Metab. 2003, 29, 363–374. [Google Scholar] [CrossRef]
- Marquié, G.; Duhault, J.; Jacotot, B. Diabetes mellitus in sand rats (Psammomys obesus). Metabolic pattern during development of the diabetic syndrome. Diabetes 1984, 33, 438–443. [Google Scholar] [CrossRef]
- Haines, H.; Hackel, D.B.; Schmidt-Nielsen, K. Experimental diabetes mellitus induced by diet in the sand rat. Am. J. Physiol. 1965, 208, 297–300. [Google Scholar] [CrossRef]
- Daly, M. Early use of solid food by a leaf-eating gerbil (Psammomys obesus). J. Mammal. 1975, 56, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, N.; Cerasi, E.; Leibowitz, G. Diet-induced diabetes in the sand rat (Psammomys obesus). Methods Mol. Biol. 2012, 933, 89–102. [Google Scholar] [PubMed]
- Senese, R.; Cioffi, F.; Petito, G.; Goglia, F.; Lanni, A. Thyroid hormone metabolites and analogues. Endocrine 2019, 66, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Jethwa, P.H.; Warner, A.; Barrett, P.; Nilaweera, K.N.; Brameld, J.M.; Ebling, F.J. Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinology 2012, 153, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNab, B.K. An analysis of the factors that influence the level and scaling of mammalian BMR. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, 5–28. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [Green Version]
- Leopoldo, A.S.; Lima-Leopoldo, A.P.; Nascimento, A.F.; Luvizotto, R.A.; Sugizaki, M.M.; Campos, D.H.; da Silva, D.C.; Padovani, C.R.; Cicogna, A.C. Classification of different degrees of adiposity in sedentary rats. Braz. J. Med. Biol. Res. 2016, 49, e5028. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.; Ryu, S.; Sung, E.; Jang, Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin. Chem. 2007, 53, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Engl, J.; Sturm, W.; Sandhofer, A.; Kaser, S.; Tschoner, A.; Tatarczyk, T.; Weiss, H.; Tilg, H.; Patsch, J.R.; Ebenbichler, C.F. Effect of pronounced weight loss on visceral fat, liver steatosis and adiponectin isoforms. Eur. J. Clin. Invest. 2008, 38, 238–244. [Google Scholar] [CrossRef]
- Antunes, L.C.; Elkfury, J.L.; Jornada, M.N.; Foletto, K.C.; Bertoluci, M.C. Validation of HOMA-IR in a model of insulinresistance induced by a high-fat diet in Wistar rats. Arch. Endocrinol. Metab. 2016, 60, 138–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdge, G.C.; Wright, P.; Jones, A.E.; Wootton, S.A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000, 84, 781–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharrazian, D.; Herbert, M.; Vojdani, A. A Cross-Reactivity between Chemical Antibodies Formed to Serum Proteins and Thyroid Axis Target Sites. Int J Mol Sci. 2020, 21, 7324. [Google Scholar] [CrossRef] [PubMed]
- Jongejan, R.M.S.; Klein, T.; Meima, M.E.; Visser, W.E.; van Heerebeek, R.E.A.; Luider, T.M.; Peeters, R.P.; de Rijke, Y.B. A Mass Spectrometry-Based Panel of Nine Thyroid Hormone Metabolites in Human Serum. Clin. Chem. 2020, 66, 556–566. [Google Scholar] [CrossRef]
- Pangaro, L.; Burman, K.D.; Wartofsky, L.; Cahnmann, H.J.; Smallridge, R.C.; O’Brian, J.T.; Wright, F.D.; Latham, K. Radioimmunoassay for 3,5-diiodothyronine and evidence for dependence on conversion from 3,5,3’-triiodothyronine. J. Clin. Endocrinol. Metab. 1980, 50, 1075–1081. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High yield preparation of isolated rat liver parenchymal cells: A biochemical and fine study. J. Cell Biol. 1969, 43, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Groen, A.K.; Sips, H.J.; Vervoorn, R.C.; Tager, J.M. Intracellular compartmentation and control of alanine metabolism in rat liver parenchymal cells. Eur. J. Biochem. 1982, 122, 87–93. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; Goglia, F. Rapid stimulation in vitro of rat liver cytochrome oxidase activity by 3,5-diiodo-L-thyronine and by 3,3’-diiodo-L-thyronine. Mol. Cell Endocrinol. 1994, 99, 89–94. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods in Enzymatic Analysis; Verlag Chemie Weinheim Academic Press Inc.: New York, NY, USA; San Francisco, CA, USA; London, UK, 1974. [Google Scholar]
- Williamson, D.H.; Lund, P.; Krebs, H.A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm andmitochondria of rat liver. Biochem. J. 1967, 103, 514–526. [Google Scholar] [CrossRef]
- Leverve, X.M.; Fontaine, E.; Putod-Paramelle, F.; Rigoulet, M. Decrease in cytosolic ATP/ADP ratio and activation of pyruvate kinase after in vitro addition of almitrine in hepatocytes isolated from fasted rats. Eur. J. Biochem. 1994, 224, 967–974. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Chan, T.M.; Exton, J.H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal. Biochem. 1976, 71, 96–105. [Google Scholar] [CrossRef]
- Coppola, M.; Cioffi, F.; Moreno, M.; Goglia, F.; Silvestri, E. 3,5-diiodo-L-thyronine: A Possible Pharmacological Agent? Curr. Drug Deliv. 2016, 13, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Gj, P.; Ghosh, S.; Mohanan, A.; Joshi, S.; Mohan, V.; Chowdhury, S.; Dutt, C.; Tandon, N. TRC150094, a Novel Mitochondrial Modulator, Reduces Cardio-Metabolic Risk as an Add-On Treatment: A Phase-2, 24-Week, Multi-Center, Randomized, Double-Blind, Clinical Trial. Diabetes Metab. Syndr. Obes. 2022, 15, 615–631. [Google Scholar] [CrossRef]
- Banta, M.R.; Holcombe, D.W. The effects of thyroxine on metabolism and water balance in a desert-dwelling rodent, Merriam’s kangaroo rat (Dipodomys merriami). J. Comp. Physiol. B. 2002, 172, 17–25. [Google Scholar] [CrossRef]
- Lee, S.; Farwell, A.P. Euthyroid Sick Syndrome. Compr. Physiol. 2016, 6, 1071–1080. [Google Scholar]
- Yousef, M.K.; Johnson, H.D. Thyroid activity in desert rodents: A mechanism for lowered metabolic rate. Am. J. Physiol. 1975, 229, 427–431. [Google Scholar] [CrossRef]
- Ben chaouacha-Chekir, R.; Lachiver, F.; Leloup, J. Thyroid function of Gerbillidae (Meriones (M) libycus, Shawi shawi and Psammomys (P) obesus): Field studies in southern Tunisia. Comp. Biochem. Physiol. 1991, 99A, 27–30. [Google Scholar] [CrossRef]
- Von Dorsche, H.H. Quantitative histological and electron microscopical investigations of endocrine organs of sand rats (Psammomys obesus) during the development of diabetes mellitus. Histochem. J. 1984, 16, 409–411. [Google Scholar] [CrossRef]
- Jonas, W.; Lietzow, J.; Wohlgemuth, F.; Hoefig, C.S.; Wiedmer, P.; Schweizer, U.; Köhrle, J.; Schürmann, A. 3,5-Diiodo-L-Thyronine (3,5-T2) Exerts Thyromimetic Effects on Hypothalamus-Pituitary-Thyroid Axis, Body Composition, and Energy Metabolism in Male Diet-Induced Obese Mice. Endocrinology 2015, 156, 389–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, A.C.; da Conceição, R.R. The Deiodinase Trio and Thyroid Hormone Signaling. Methods Mol. Biol. 2018, 1801, 67–83. [Google Scholar] [PubMed]
- García-G, C.; López-Bojorquez, L.; Nuñez, J.; Valverde-R, C.; Orozco, A. 3,5-Diiodothyronine in vivo maintains euthyroidal expression of type 2 iodothyronine deiodinase, growth hormone, and thyroid hormone receptor beta1in the killifish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R877–R883. [Google Scholar] [CrossRef]
- Mendoza, A.; Navarrete-Ramírez, P.; Hernández-Puga, G.; Villalobos, P.; Holzer, G.; Renaud, J.P.; Laudet, V.; Orozco, A. 3,5-T2 is analternative ligand for the thyroid hormone receptor b1. Endocrinology 2013, 154, 2948–2958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gereben, B.; Zeöld, A.; Dentice, M.; Salvatore, D.; Bianco, A.C. Activation and inactivation of thyroid hormone by deiodinases: Local action with general consequences. Cell Mol. Life Sci. 2008, 65, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Horst, C.; Harneit, A.; Seitz, H.J.; Rokos, H. 3,5-Di-iodo-L-thyronine suppresses TSH in rats in vivo and in rat pituitary fragments in vitro. J. Endocrinol. 1995, 145, 291–297. [Google Scholar] [CrossRef]
- Chen, R.H.; Chen, H.Y.; Man, K.M.; Chen, S.J.; Chen, W.; Liu, P.L.; Chen, Y.H.; Chen, W.C. Thyroid diseases increased the risk of type 2 diabetes mellitus. A nation-wide cohort study. Medicine 2019, 98, e15631. [Google Scholar]
- Karakaya, R.E.; Saka, M.; Ozdemir, D. Determining the relationship between dietary iodine intake, urinary iodine excretion and thyroid functions in people with type 2 diabetes mellitus. Arch. Endocrinol. Metab. 2020, 64, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Shafrir, E. Overnutrition in spiny mice (Acomys cahirinus): B-cell expansion leading to rupture and overt diabetes on fat-rich diet and protective energy-wasting elevation in thyroid hormone on sucrose-rich diet. Diabetes Metab. Res. Rev. 2000, 16, 94–105. [Google Scholar] [CrossRef]
- Faber, J.; Heaf, J.; Kirkegaard, C.; Lumholtz, I.B.; Siersbaek-Nielsen, K.; Kølendorf, K.; Friis, T. Simultaneous turnover studies of thyroxine, 3,5,3’ and 3,3’,5’-triiodothyronine, 3,5-,3,3’-, and 3’,5’-diiodothyronine, and 3’-monoiodothyronine in chronic renal failure. J. Clin. Endocrinol. Metab. 1983, 56, 211–217. [Google Scholar] [CrossRef]
- Riccioni, G.; Menna, V.; Lambo, M.S.; Della Vecchia, R.; Di Ilio, C.; De Lorenzo, A.; D’Orazio, N. Leptin and hypothalamus-hypophysis-thyroid axis. Clin. Ther. 2004, 155, 29–31. [Google Scholar]
- Patel, K.; Joharapurkar, A.; Dhanesha, N.; Patel, V.; Kshirsagar, S.; Raval, P.; Raval, S.; Jain, M.R. Thyroid hormone modulates food intake and glycemia via ghrelin secretion in Zucker fatty rats. Drug Res. 2014, 64, 523–529. [Google Scholar] [CrossRef]
- Varela, L.; Martínez-Sánchez, N.; Gallego, R.; Vázquez, M.J.; Roa, J.; Gándara, M.; Schoenmakers, E.; Nogueiras, R.; Chatterjee, K.; Tena-Sempere, M.; et al. Hypothalamic mTOR pathway mediates thyroid hormone-induced hyperphagia in hyperthyroidism. J. Pathol. 2012, 227, 209–222. [Google Scholar] [CrossRef]
- Sabio, G.; Cavanagh-Kyros, J.; Barrett, T.; Jung, D.Y.; Ko, H.J.; Ong, H.; Morel, C.; Mora, A.; Reilly, J.; Kim, J.K.; et al. Role of the hypothalamic- pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 2010, 24, 256–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goglia, F. The effects of 3,5-diiodothyronine on energy balance. Front. Physiol. 2015, 5, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsibulnikov, S.; Maslov, L.; Voronkov, N.; Oeltgen, P. Thyroid hormones and the mechanisms of adaptation to cold. Hormones 2020, 19, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Degen, A.A. Energy expenditure and efficiency of energy use in rodents: Desert and non-desert species. J. Basic. Clin. Physiol. Pharmacol. 1998, 9, 29–49. [Google Scholar] [CrossRef]
- Cioffi, F.; Zambad, S.P.; Chhipa, L.; Senese, R.; Busiello, R.A.; Tuli, D.; Munshi, S.; Moreno, M.; Lombardi, A.; Gupta, R.C.; et al. TRC150094, a novel functional analog of iodothyronines, reduces adiposity by increasing energy expenditure and fatty acid oxidation in rats receiving a high fat diet. FASEB J. 2010, 24, 3451–3461. [Google Scholar] [CrossRef]
- Louzada, R.A.; Carvalho, D.P. Similarities and Differences in the Peripheral Actions of Thyroid Hormones and Their Metabolites. Front. Endocrinol. 2018, 9, 394. [Google Scholar] [CrossRef]
- Coppola, M.; Glinni, D.; Moreno, M.; Cioffi, F.; Silvestri, E.; Goglia, F. Thyroid hormone analogues and derivatives: Actions in fatty liver. World J. Hepatol. 2014, 6, 114–129. [Google Scholar] [CrossRef]
- Tan, J.T.M.; Nankivell, V.A.; Bilu, C.; Shemesh, T.; Nicholls, S.J.; Zimmet, P.; Kronfeld-Schor, N.; Brown, A.; Bursill, C.A. High-Energy Diet and Shorter Light Exposure Drives Markers of Adipocyte Dysfunction in Visceral and Subcutaneous Adipose Depots of Psammomys obesus. Int. J. Mol. Sci. 2019, 20, 6291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, A.D.; Magnuson, A.M.; Fouts, J.; Wei, Y.; Wang, D.; Pagliassotti, M.J.; Foster, M.T. Subcutaneous adipose tissue accumulation protects systemic glucose tolerance and muscle metabolism. Adipocyte 2018, 7, 261–272. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Lombardi, A.; de Lange, P.; Silvestri, E.; Ragni, M.; Farina, P.; Baccari, G.C.; Fallahi, P.; Antonelli, A.; et al. 3,5-Diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005, 19, 1552–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhrle, J.; Lehmphul, I.; Pietzner, M.; Renko, K.; Rijntjes, E.; Richards, K.; Anselmo, J.; Danielsen, M.; Jonklaas, J. 3,5-T2-A Janus-Faced Thyroid Hormone Metabolite Exerts Both Canonical T3-Mimetic Endocrine and Intracrine Hepatic Action. Front. Endocrinol. 2020, 10, 787. [Google Scholar] [CrossRef]
- Ball, S.G.; Sokolov, J.; Chin, W.W. 3,5-Diiodo-L-thyronine (T2) has selective thyromimetic effects in vivo and in vitro. J. Mol. Endocrinol. 1997, 19, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Lietzow, J.; Golchert, J.; Pietzner, M.; Völker, U.; Poutanen, M.; Ohlsson, C.; Homuth, G.; Köhrle, J. Comparative Analysis of the Effects of Long-Term 3,5-diiodothyronine Treatment on the Murine Hepatic Proteome and Transcriptome Under Conditions of Normal Diet and High-Fat Diet. Thyroid 2021, 31, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Baker, B.; Marsh, H.; Mandarino, L.; Rizza, R.; Bergman, R.; Haymond, M.; Gerich, J. Effects of thyroid hormone excess on action, secretion, and metabolism of insulin in humans. Am. J. Physiol. 1985, 248, E593–E601. [Google Scholar] [CrossRef]
- Da Silva Teixeira, S.; Filgueira, C.; Sieglaff, D.H.; Benod, C.; Villagomez, R.; Minze, L.J.; Zhang, A.; Webb, P.; Nunes, M.T. 3,5-diiodothyronine (3,5-T2) reduces blood glucose independently of insulin sensitization in obese mice. Acta Physiol. 2017, 220, 238–250. [Google Scholar] [CrossRef]
- De Lange, P.; Cioffi, F.; Senese, R.; Moreno, M.; Lombardi, A.; Silvestri, E.; De Matteis, R.; Lionetti, L.; Mollica, M.P.; Goglia, F.; et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-L-thyronine in rats. Diabetes 2011, 60, 2730–2739. [Google Scholar] [CrossRef] [Green Version]
- Taleux, N.; Guigas, B.; Dubouchaud, H.; Moreno, M.; Weitzel, J.M.; Goglia, F.; Favier, R.; Leverve, X.M. High expression of thyroid hormone receptors and mitochondrial glycerol-3-phosphate dehydrogenase in the liver is linked to enhanced fatty acid oxidation in Lou/C, a rat strain resistant to obesity. J. Biol. Chem. 2009, 284, 4308–4316. [Google Scholar] [CrossRef] [Green Version]
- Shafrir, E. Intermediary metabolism during the development of obesity and diabetes in the desert rodent Acomys cahirinus. Int. J. Obes. 1982, 6, 9–20. [Google Scholar]
- Teixeira, S.D.; Panveloski-Costa, A.C.; Carvalho, A.; Monteiro Schiavon, F.P.; Ruiz Marque, A.C.; Campello, R.S.; Bazotte, R.B.; Nunes, M.T. Thyroid hormone treatment decreases hepatic glucose production and renal reabsorption of glucose in alloxan-induced diabetic Wistar rats. Physiol. Rep. 2016, 4, e12961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senese, R.; de Lange, P.; Petito, G.; Moreno, M.; Goglia, F.; Lanni, A. 3,5-Diiodothyronine: A Novel Thyroid Hormone Metabolite and Potent Modulator of Energy Metabolism. Front. Endocrinol. 2018, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, E.; Voci, A.; Canesi, L.; De Matteis, R.; Goglia, F.; Cioffi, F.; Fugassa, E.; Gallo, G.; Vergani, L. Direct effects of iodothyronines on excess fat storage in rat hepatocytes. J. Hepatol. 2011, 54, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Ghelardoni, S.; Chiellini, G.; Frascarelli, S.; Saba, A.; Zucchi, R. Uptake and metabolic effects of 3-iodothyronamine in hepatocytes. J. Endocrinol. 2014, 221, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Gnocchi, D.; Ellis, E.C.S.; Johansson, H.; Eriksson, M.; Bruscalupi, G.; Steffensen, K.R.; Parini, P. Diiodothyronines regulate metabolic homeostasis in primary human hepatocytes by modulating mTORC1 and mTORC2 activity. Mol. Cell. Endocrinol. 2020, 499, 110604. [Google Scholar] [CrossRef] [PubMed]
- Horst, C.; Rokos, H.; Seitz, H.J. Rapid stimulation of hepatic oxygen consumption by 3,5-di-iodo-L-thyronine. Biochem. J. 1989, 261, 945–950. [Google Scholar] [CrossRef] [Green Version]
- Silvestri, E.; Lombardi, A.; Coppola, M.; Gentile, A.; Cioffi, F.; Senese, R.; Goglia, F.; Lanni, A.; Moreno, M.; de Lange, P. Differential Effects of 3,5-Diiodo-L-Thyronine and 3,5,3’-Triiodo-L-Thyronine on Mitochondrial Respiratory Pathways in Liver from Hypothyroid Rats. Cell. Physiol. Biochem. 2018, 47, 2471–2483. [Google Scholar] [CrossRef]
- Cavallo, A.; Taurino, F.; Damiano, F.; Siculella, L.; Sardanelli, A.M.; Gnoni, A. Acute administration of 3,5-diiodo-L-thyronine to hypothyroid rats stimulates bioenergetic parameters in liver mitochondria. J. Bioenerg. Biomembr. 2016, 48, 521–529. [Google Scholar] [CrossRef]
- Nogueira, V.; Walter, L.; Avéret, N.; Fontaine, E.; Rigoulet, M.; Leverve, X.M. Thyroid status is a key regulator of both flux and efficiency of oxidative phosphorylation in rat hepatocytes. J. Bioenerg. Biomembr. 2002, 34, 55–66. [Google Scholar] [CrossRef]
- Harper, M.E.; Brand, M.D. The quantitative contributions of mitochondrial proton leak and ATP turnover reactions to the changed respiration rates of hepatocytes from rats of different thyroid status. J. Biol. Chem. 1993, 268, 14850–14860. [Google Scholar] [CrossRef]

| Parameters/Groups | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| ND-controlled | HED-controlled | HED-3,5-T2-treated | HED-Placebo | |
| Body weight (g) | 81 ± 3 | 144 ± 10 *** | 108 ± 3 ***/¶¶¶ | 141 ± 7 *** |
| Thyroid weight (mg) | 11.5 ± 1.32 | 10.7 ± 2.69 ns | 10.3 ± 1.18 ns/ns | 10.1 ± 1.47 ns |
| Thyroid mass (% body wt) | 14.1 ± 0.44 | 7.43 ± 0.26 *** | 9.53 ± 0.39 ***/¶¶¶ | 7.16 ± 0.21 *** |
| TSH (µIU/mL) | 1.63 ± 0.52 | 1.37 ± 0.17 ns | 1.19 ± 0.49 ns/ns | 1.46 ± 0.81 ns |
| TT4 (ng/mL) | 29.4 ± 2.23 | 24.3 ± 4.11 ns | 23.9 ± 3.22 ns/ns | 23.7 ± 1.34 ns |
| FT4 (pg/mL) | 3.88 ± 0.11 | 3.75 ± 0.57 ns | 3.71 ± 0.66 ns/ns | 3.79 ± 0.45 ns |
| TT3 (ng/mL) | 0.661 ± 0.08 | 0.344 ± 0.07 *** | 0.362 ± 0.03 ***/ns | 0.377 ± 0.09 *** |
| FT3 (pg/mL) | 1.92 ± 0.08 | 1.77 ± 0.07 ** | 1.10 ± 0.09 ***/¶¶¶ | 1.83 ± 0.02 ** |
| 3,5-T2 (pg/mL) | 0.30 ± 0.09 | 0.24 ± 0.07 ns | 0.51 ± 0.05 ***/¶¶¶ | 0.27 ± 0.08 ns |
| FT3/FT4-ratio | 0.49 ± 0.07 | 0.47 ± 0.01 ns | 0.29 ± 0.03 ***/¶¶¶ | 0.48 ± 0.04 ns |
| FT3/3,5-T2-ratio | 6.41 ± 0.88 | 7.37 ± 0.11 ns | 2.15 ± 0.18 ***/¶¶¶ | 6.77 ± 0.25 ns |
| FT4/3,5-T2-ratio | 12.9 ± 1.22 | 15.6 ± 1.42 ** | 7.27 ± 1.32 ***/¶¶¶ | 14.1 ± 0.11 ** |
| Parameters/Groups | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| ND-controlled | HED-controlled | HED-3,5-T2-treated | HED-Placebo | |
| Body weight (g) | 81 ± 3 | 144 ± 10 *** | 108 ± 3 ***/¶¶¶ | 141 ± 7 *** |
| BMI (g/cm2) | 0.38 ± 0.01 | 0.49 ± 0.01 *** | 0.32 ± 0.03 ns/¶¶¶ | 0.47 ± 0.02 *** |
| Caloric intake (Kcal/100g BW) | 43.5 ± 1.9 | 316 ± 11 *** | 534 ± 10 ***/¶¶¶ | 322 ± 17 *** |
| Respiratory quotient | 0.913 ± 0.09 | 0.975 ± 0.03 ns | 0.753 ± 0.05 ***/¶¶¶ | 0.944 ± 0.07 ns |
| BMR (mLO2/h/g BW) | 0.521 ± 0.02 | 0.643 ± 0.09 *** | 0.891 ± 0.05 ***/¶¶¶ | 0.675 ± 0.03 *** |
| Glucose (mmol/L) | 3.21 ± 0.51 | 15.2 ± 1.04 *** | 3.88 ± 0.17 ns/¶¶¶ | 14.9 ± 1.82 *** |
| HbA1c (mmol/mol) | 17.9 ± 0.65 | 68.2 ± 9.44 *** | 20.5 ± 3.85 ns/¶¶¶ | 65.3 ± 7.11 *** |
| Insulin (pmol/L) | 130 ± 21 | 580 ± 47 *** | 110 ± 14 ***/¶¶¶ | 608 ± 85 *** |
| HOMA-IR | 2.57 ± 0.14 | 5.33 ± 0.23 *** | 2.68 ± 0.11 ns/¶¶¶ | 5.58 ± 0.44 *** |
| ALT (IU/L) | 26.3 ± 1.58 | 74.1 ± 2.35 *** | 23.9 ± 1.47 ns/¶¶¶ | 81.3 ± 1.71 *** |
| Triglycerides (mmol/L) | 0.81 ± 0.07 | 4.09 ± 0.61 *** | 1.58 ± 0.01 ***/¶ | 3.07 ± 0.06 *** |
| Total cholesterol (mmol/L) | 1.48 ± 0.70 | 8.06 ± 1.22 *** | 2.81 ± 0.64 ***/¶¶¶ | 7.98 ± 1.15 *** |
| NEFA (μmol/L) | 291 ± 33 | 578 ± 41 *** | 898 ± 66 ***/¶¶¶ | 609 ± 23 *** |
| Ketone bodies (µmol/L) | 250 ± 16 | 303 ± 30 *** | 588 ± 57 ***/¶¶¶ | 278 ± 21 *** |
| Lactate (mM) | 0.65 ± 0.09 | 1.13 ± 0.25 *** | 0.73 ± 0.14 ***/¶¶¶ | 1.05 ± 0.03 *** |
| Parameters/Groups | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| ND-controlled | HED-controlled | HED-3,5-T2-treated | HED-Placebo | |
| Total visceral fat (mg/g BW) | 10.3 ± 3.38 | 25.8 ± 4.32 *** | 10.1 ± 1.85 ***/¶¶¶ | 26.3 ± 4.38 *** |
| Retroperitoneal fat (mg/g BW) | 2.75 ± 0.66 | 3.02 ± 0.57 ns | 2.11 ± 0.44 ns/ns | 2.98 ± 0.75 ns |
| Omental fat (mg/g BW) | 1.27 ± 0.91 | 9.95 ± 1.33 *** | 2.79 ± 0.68***/¶¶¶ | 10.1 ± 2.22 *** |
| Mesenteric fat (mg/g BW) | 6.33 ± 1.81 | 12.9 ± 2.42 *** | 5.18 ± 0.73 ***/¶¶¶ | 13.2 ± 1.41 *** |
| Epididymal fat (mg/g BW) | 3.14 ± 0.72 | 8.69 ± 1.47 *** | 2.25 ± 0.65 *** | 9.02 ± 0.73 *** |
| Subcutaneous fat (mg/g BW) | 1.93 ± 0.17 | 8.33 ± 1.18 *** | 2.09 ± 0.15 ***/¶¶¶ | 7.92 ± 0.22 *** |
| Perirenal fat (mg/g BW) | 2.13 ± 0.51 | 2.82 ± 1.61 ns | 2.03 ± 0.34 ns/ns | 2.57 ± 1.91 ns |
| Suprascapular fat (mg/g BW) | 5.91 ± 2.14 | 12.5 ± 1.08 ** | 4.11 ± 1.33 ***/¶¶¶ | 11.9 ± 2.01 ** |
| Gonadal fat (mg/g BW) | 1.78 ± 0.61 | 1.93 ± 0.22 ns | 1.66 ± 0.55 ns/ns | 1.86 ± 1.24 ns |
| Intramuscular fat (mg/g BW) | 2.51 ± 0.33 | 3.74 ± 0.27 ** | 1.81 ± 0.24 ***/¶¶¶ | 3.67 ± 0.45 ** |
| Adiposity index (% BW) | 2.61 ± 0.35 | 3.87 ± 0.95 *** | 1.07 ± 0.13 **/¶¶¶ | 3.91 ± 0.47 *** |
| Brown adipose tissue (mg/g BW) | 0.46 ± 0.03 | 0.33 ± 0.07 * | 0.55 ± 0.05 */¶ | 0.31 ± 0.02 * |
| Total hepatic lipids (g 100 g wet/wt) | 2.97 ± 0.31 | 6.17 ± 0.94 *** | 3.19 ± 0.11 ***/¶¶¶ | 5.83 ± 0.76 *** |
| Hepatic glycogen (g/100 g wet/wt) | 2.77 ± 0.84 | 2.44 ± 0.93 * | 1.18 ± 0.02 ***/¶¶¶ | 2.65 ± 0.79 |
| Liver mass (% body BW) | 2.51 ± 0.45 | 4.05 ± 0.78 *** | 2.89 ± 0.33 ***/¶¶¶ | 3.64 ± 1.49 *** |
| Metabolic Fluxes (µmol/min/g Dry Cells) | Energy Substrates (mM) | Group I ND-Controlled | Group II HED-Controlled | Group III HED-3,5-T2-Treated | Group IV HED-Placebo | ||
|---|---|---|---|---|---|---|---|
| 10−9 M 3,5-T2 | 10−6 M 3,5-T2 | 10−9 M 3,5-T2 | 10−6 M 3,5-T2 | ||||
| Glucose synthesis | Ala+Octa | 4.33 ± 0.22 | 8.38 ± 0.54 | 2.55 ± 0.31 ***/¶¶¶ | 2.19 ± 0.11 ***/¶¶¶ | 4.22 ± 0.62 | 3.87 ± 0.22 |
| (L+P)+Octa | 5.66 ± 0.31 | 12.1 ± 0.27 | 4.87 ± 0.17 ***/¶ | 2.66 ± 0.33 ***/¶¶¶ | 5.33 ± 0.41 | 5.02 ± 0.77 | |
| Glycolysis | Ala+Octa | 1.83 ± 0.12 | 3.27 ± 0.14 | 1.65 ± 0.41 ***/¶¶¶ | 1.09 ± 0.53 ***/¶¶¶ | 2.02 ± 0.33 | 2.11 ± 0.12 |
| Ketogenesis | Ala+Octa | 4.77 ± 0.33 | 5.65 ± 0.61 | 5.87 ± 0.55 ***/¶¶ | 6.29 ± 0.11 ***/¶¶¶ | 4.08 ± 0.34 | 3.95 ± 0.61 |
| (L+P)+Octa | 3.76 ± 0.51 | 4.61 ± 0.37 | 4.97 ± 0.11 ***/¶ | 5.44 ± 0.13 ***/¶¶¶ | 3.05 ± 0.21 | 3.02 ± 0.17 | |
| G6P (nmol/g dry cells) | 184 ± 62 | 227 ± 51 | 108 ± 31 ***/¶¶¶ | 97 ± 14 ***/¶¶¶ | 172 ± 23 | 181 ± 55 | |
| F6P (nmol/g dry cells) | 299 ± 24 | 338 ± 55 | 317 ± 11 ***/¶¶¶ | 379 ± 19 ***/¶¶¶ | 304 ± 31 | 310 ± 22 | |
| 3PG (nmol/g dry cells) | 951 ± 16 | 1331 ± 77 | 1016 ± 33 ***/¶¶¶ | 1287 ± 46 ***/¶¶¶ | 997 ± 32 | 1008 ± 57 | |
| PEP (μmol/g dry cells) | 575 ± 30 | 805 ± 44 | 895 ± 41 ***/¶¶¶ | 1034 ± 27 ***/¶¶¶ | 609 ± 22 | 645 ± 18 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouazza, A.; Favier, R.; Fontaine, E.; Leverve, X.; Koceir, E.-A. Potential Applications of Thyroid Hormone Derivatives in Obesity and Type 2 Diabetes: Focus on 3,5-Diiodothyronine (3,5-T2) in Psammomys obesus (Fat Sand Rat) Model. Nutrients 2022, 14, 3044. https://doi.org/10.3390/nu14153044
Bouazza A, Favier R, Fontaine E, Leverve X, Koceir E-A. Potential Applications of Thyroid Hormone Derivatives in Obesity and Type 2 Diabetes: Focus on 3,5-Diiodothyronine (3,5-T2) in Psammomys obesus (Fat Sand Rat) Model. Nutrients. 2022; 14(15):3044. https://doi.org/10.3390/nu14153044
Chicago/Turabian StyleBouazza, Asma, Roland Favier, Eric Fontaine, Xavier Leverve, and Elhadj-Ahmed Koceir. 2022. "Potential Applications of Thyroid Hormone Derivatives in Obesity and Type 2 Diabetes: Focus on 3,5-Diiodothyronine (3,5-T2) in Psammomys obesus (Fat Sand Rat) Model" Nutrients 14, no. 15: 3044. https://doi.org/10.3390/nu14153044
APA StyleBouazza, A., Favier, R., Fontaine, E., Leverve, X., & Koceir, E.-A. (2022). Potential Applications of Thyroid Hormone Derivatives in Obesity and Type 2 Diabetes: Focus on 3,5-Diiodothyronine (3,5-T2) in Psammomys obesus (Fat Sand Rat) Model. Nutrients, 14(15), 3044. https://doi.org/10.3390/nu14153044








