The Effect of Laminaria japonica on Metabolic Syndrome: A Systematic Review of Its Efficacy and Mechanism of Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Registration
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment and Risk of Bias
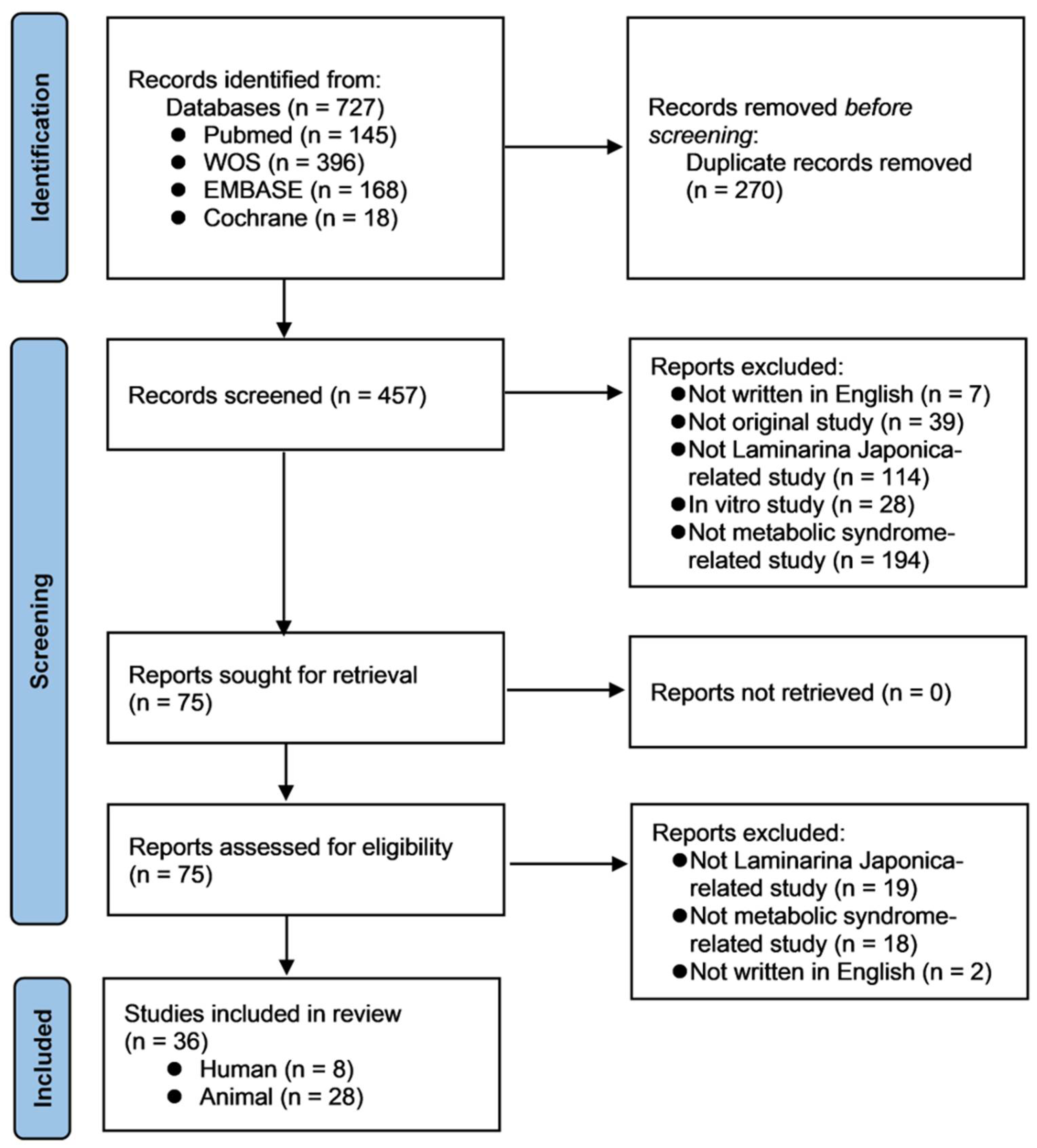
2.5. Literature Search
2.6. Studies Characteristics
3. Results
3.1. Animal Studies
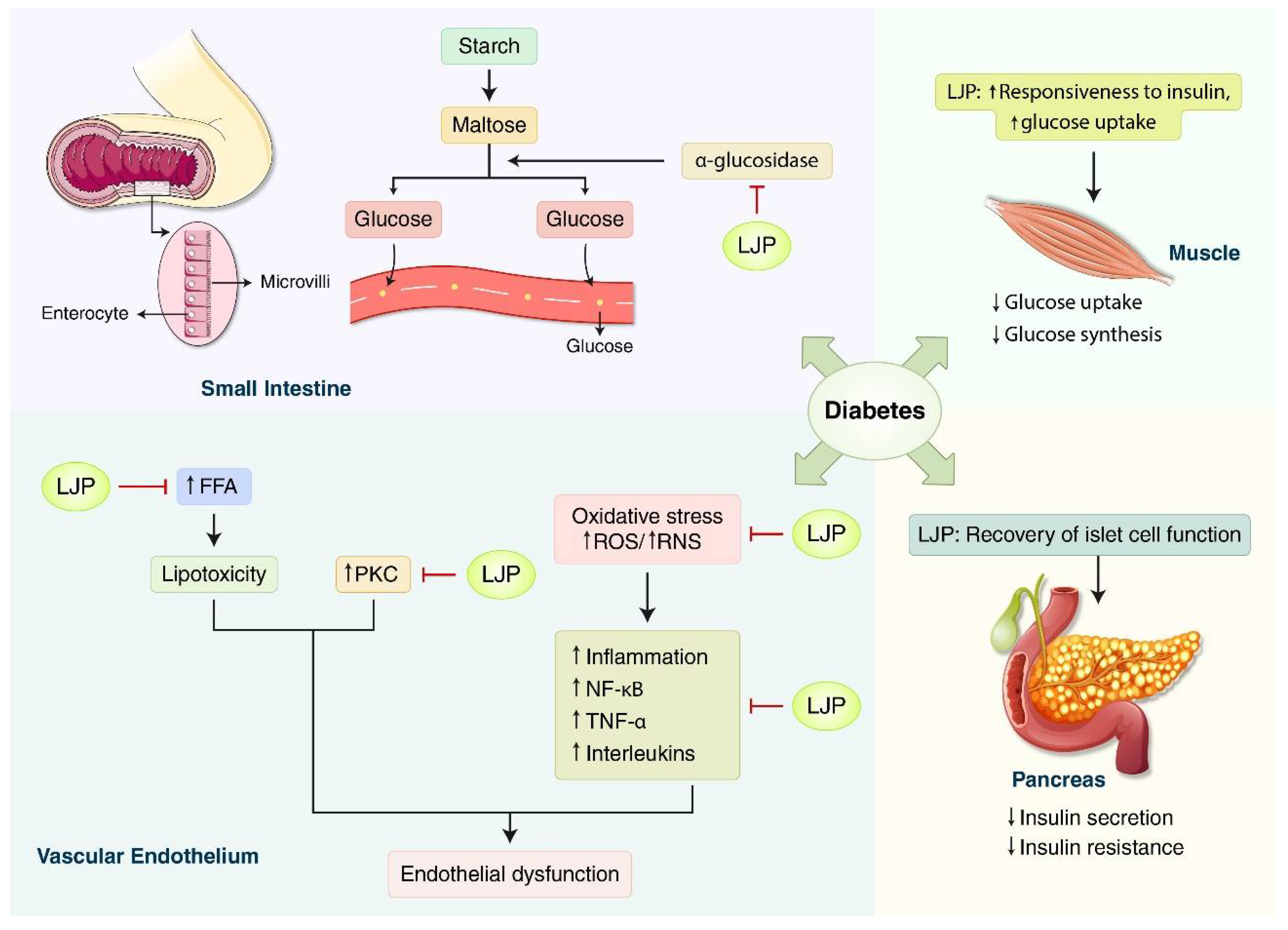
3.1.1. Diabetes
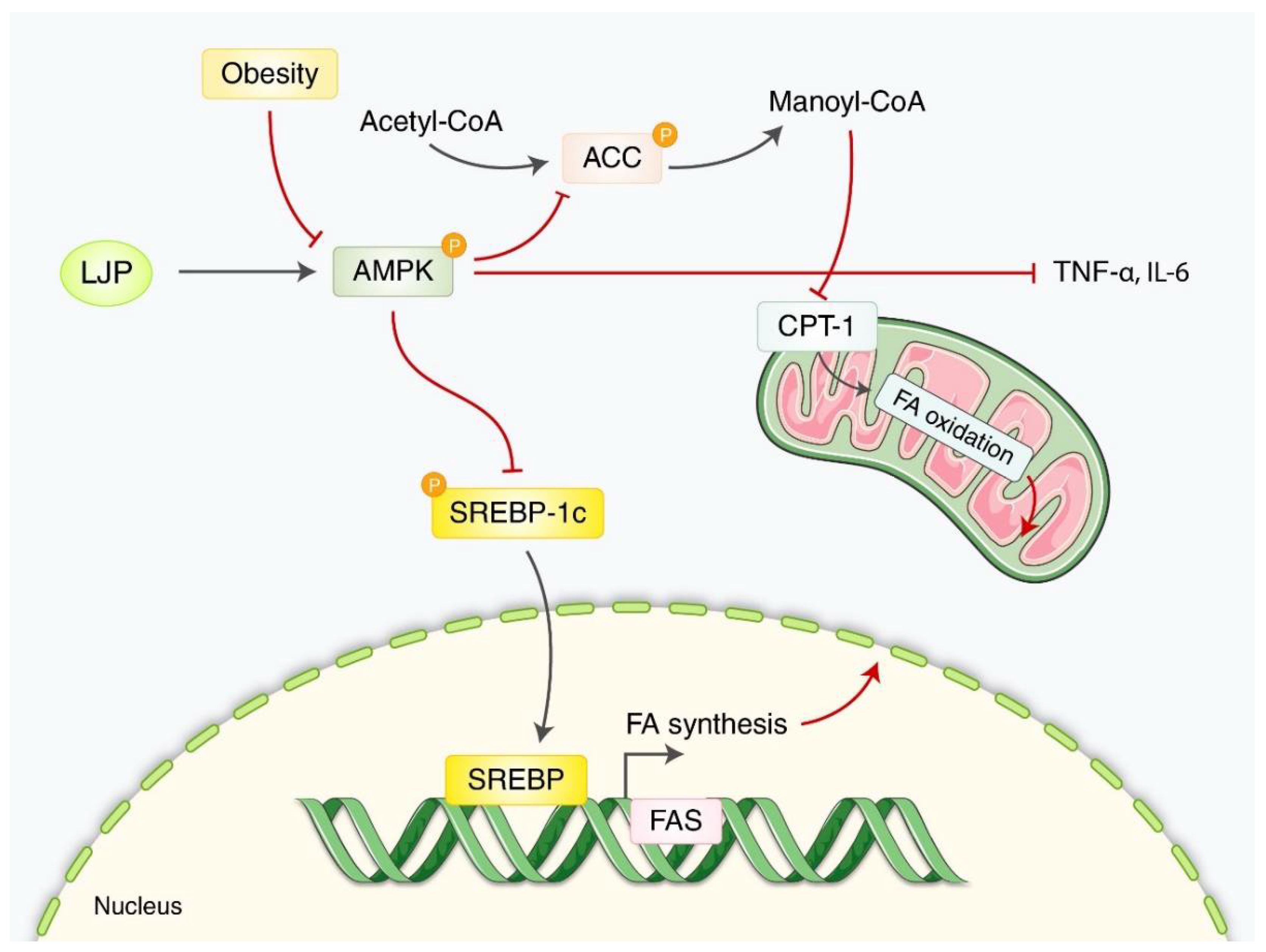
3.1.2. Obesity
3.1.3. Atherosclerosis
3.1.4. Hyperlipidemia/Fatty Liver
3.2. Clinical Studies
3.3. Quality and Risk of Bias
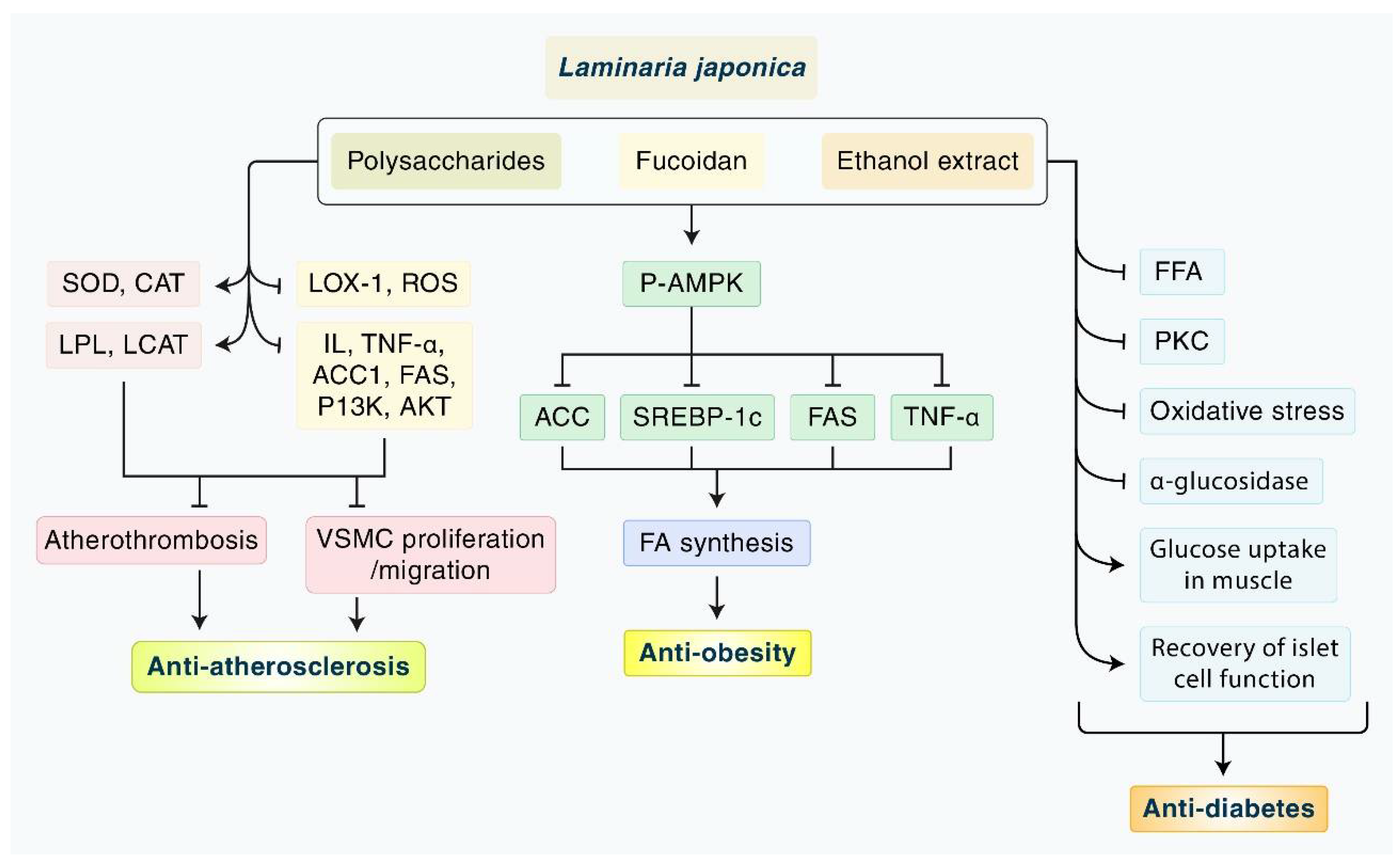
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, B.; Lee, I.S.; Ko, S.J. The efficacy and safety of laminaria japonica for metabolic syndrome: A protocol for systematic review. Medicine 2022, 101, e28892. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Wei, C.; Wang, H.; Gao, H. Deep eutectic solvents as active pharmaceutical ingredient delivery systems in the treatment of metabolic related diseases. Front. Pharmacol. 2021, 12, 794939. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Yang, J.; Wang, Z.; Liu, R.; Xie, R. Polysaccharides from laminaria japonica show hypoglycemic and hypolipidemic activities in mice with experimentally induced diabetes. Exp. Biol. Med. 2014, 239, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Naghipour, M.; Joukar, F.; Nikbakht, H.-A.; Hassanipour, S.; Asgharnezhad, M.; Arab-Zozani, M.; Mansour-Ghanaei, F. High prevalence of metabolic syndrome and its related demographic factors in north of iran: Results from the persian guilan cohort study. Int. J. Endocrinol. 2021, 2021, 8862456. [Google Scholar] [CrossRef]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in antidiabetic drug discovery: Fda approved drugs, new drugs in clinical trials and global sales. Front. Pharmacol. 2021, 12, 807548. [Google Scholar] [CrossRef]
- Ritter, K.; Buning, C.; Halland, N.; Poverlein, C.; Schwink, L. G protein-coupled receptor 119 (gpr119) agonists for the treatment of diabetes: Recent progress and prevailing challenges. J. Med. Chem. 2016, 59, 3579–3592. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, E.; Kang, I.; Lee, M.; Lee, Y. Anti-diabetic effects and anti-inflammatory effects of laminaria japonica and hizikia fusiforme in skeletal muscle: In vitro and in vivo model. Nutrients 2018, 10, 491. [Google Scholar] [CrossRef]
- Shirosaki, M.; Koyama, T. Laminaria japonica as a food for the prevention of obesity and diabetes. Adv. Food Nutr. Res. 2011, 64, 199–212. [Google Scholar]
- Wang, X.; Zhang, L.; Qin, L.; Wang, Y.; Chen, F.; Qu, C.; Miao, J. Physicochemical properties of the soluble dietary fiber from laminaria japonica and its role in the regulation of type 2 diabetes mice. Nutrients 2022, 14, 329. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Li, L.; Kwong, J.S.; Jia, P.; Zhao, P.; Wang, W.; Zhou, X.; Zhang, M.; Sun, X. Alpha-glucosidase inhibitors and hepatotoxicity in type 2 diabetes: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 32649. [Google Scholar] [CrossRef]
- Luan, F.; Zou, J.; Rao, Z.; Ji, Y.; Lei, Z.; Peng, L.; Yang, Y.; He, X.; Zeng, N. Polysaccharides from laminaria japonica: An insight into the current research on structural features and biological properties. Food Funct. 2021, 12, 4254–4283. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-H.; Kim, J.; Lee, Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr. Res. Pract. 2016, 10, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xiang, H.; You, L.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Hypolipidaemic and antioxidant capacities of polysaccharides obtained from laminaria japonica by different extraction media in diet-induced mouse model. Int. J. Food Sci. Technol. 2017, 52, 2274–2281. [Google Scholar] [CrossRef]
- Xie, L.; Chen, M.-H.; Li, J.; Yang, X.-M.; Huang, Q.-J. Antithrombotic effect of a polysaccharide fraction from laminaria japonica from the south china sea. Phytother. Res. 2011, 25, 1362–1366. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Long, S.; Guo, Y.; Duan, D. Hypoglycemic effect of laminaria japonica polysaccharide in a type 2 diabetes mellitus mouse model. ISRN Endocrinol. 2012, 2012, 507462. [Google Scholar] [CrossRef]
- Bu, T.; Liu, M.; Zheng, L.; Guo, Y.; Lin, X. A-glucosidase inhibition and the in vivo hypoglycemic effect of butyl-isobutyl-phthalate derived from the laminaria japonica rhizoid. Phytother. Res. 2010, 24, 1588–1591. [Google Scholar] [CrossRef]
- Jin, D.Q.; Li, G.; Kim, J.S.; Yong, C.S.; Kim, J.A.; Huh, K. Preventive effects of laminaria japonica aqueous extract on the oxidative stress and xanthine oxidase activity in streptozotocin-induced diabetic rat liver. Biol. Pharm. Bull. 2004, 27, 1037–1040. [Google Scholar] [CrossRef][Green Version]
- Liang, Z.; Zheng, Y.; Wang, J.; Zhang, Q.; Ren, S.; Liu, T.; Wang, Z.; Luo, D. Low molecular weight fucoidan ameliorates streptozotocin-induced hyper-responsiveness of aortic smooth muscles in type 1 diabetes rats. J. Ethnopharmacol. 2016, 191, 341–349. [Google Scholar] [CrossRef]
- Park, M.Y.; Kim, E.; Kim, M.S.; Kim, K.H.; Kim, H.A. Dietary supplementation of sea tangle (Laminaria japonica) improves blood glucose and lipid metabolism in the streptozotocin-induced diabetic rats. Food Sci. Biotechnol. 2009, 18, 712–716. [Google Scholar]
- Xu, J.; Wang, Y.; Wang, Z.; Guo, L.; Li, X. Fucoidan mitigated diabetic nephropathy through the downregulation of pkc and modulation of nf-κb signaling pathway: In vitro and in vivo investigations. Phytother. Res. 2021, 35, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Long, S.H.; Yu, Z.Q.; Shuai, L.; Guo, Y.L.; Duan, D.L.; Xu, X.Y.; Li, X.D. The hypoglycemic effect of the kelp on diabetes mellitus model induced by alloxan in rats. Int. J. Mol. Sci. 2012, 13, 3354–3365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, T.; Chen, X.; You, H.; Zhang, Q.; Xue, J.; Zheng, Y.; Luo, D. Low molecular weight fucoidan ameliorates hindlimb ischemic injury in type 2 diabetic rats. J. Ethnopharmacol. 2018, 210, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Song, G.; Zhang, M.; Shi, J.; Xu, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan improves metabolic syndrome in association with increased akkermansia population in the gut microbiota of high-fat diet-fed mice. J. Funct. Foods 2017, 28, 138–146. [Google Scholar] [CrossRef]
- Jang, W.S.; Choung, S.Y. Antiobesity effects of the ethanol extract of laminaria japonica areshoung in high-fat-diet-induced obese rat. Evid.-Based Complement. Altern. Med. 2013, 2013, 492807. [Google Scholar]
- Zhang, Y.; Zhao, N.; Yang, L.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J.; Cui, C.-B. Insoluble dietary fiber derived from brown seaweed laminaria japonica ameliorate obesity-related features via modulating gut microbiota dysbiosis in high-fat diet–fed mice. Food Funct. 2021, 12, 587–601. [Google Scholar] [CrossRef]
- Li, N.; Fu, X.; Xiao, M.; Wei, X.; Yang, M.; Liu, Z.; Mou, H. Enzymatic preparation of a low-molecular-weight polysaccharide rich in uronic acid from the seaweed laminaria japonica and evaluation of its hypolipidemic effect in mice. Food Funct. 2020, 11, 2395–2405. [Google Scholar] [CrossRef]
- Duan, M.; Sun, X.; Ma, N.; Liu, Y.; Luo, T.; Song, S.; Ai, C. Polysaccharides from laminaria japonica alleviated metabolic syndrome in balb/c mice by normalizing the gut microbiota. Int. J. Biol. Macromol. 2019, 121, 996–1004. [Google Scholar] [CrossRef]
- Han, A.R.; Kim, J.H.; Kim, E.; Cui, J.; Chai, I.S.; Zhang, G.; Lee, Y. Hypotriglyceridemic effects of brown seaweed consumption via regulation of bile acid excretion and hepatic lipogenesis in high fat diet-induced obese mice. Nutr. Res. Pract. 2020, 14, 580–592. [Google Scholar] [CrossRef]
- Li, Q.M.; Zha, X.Q.; Zhang, W.N.; Liu, J.; Pan, L.H.; Luo, J.P. Laminaria japonica polysaccharide prevents high-fat-diet-induced insulin resistance in mice via regulating gut microbiota. Food Funct. 2021, 12, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.H.; Zha, X.Q.; Cui, S.H.; Asghar, M.N.; Pan, L.H.; Wang, J.H.; Luo, J.P. Purification, structure features and anti-atherosclerosis activity of a laminaria japonica polysaccharide. Int. J. Biol. Macromol. 2015, 81, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Duan, M.; Jia, J.; Song, S.; Ai, C. Low-molecular alginate improved diet-induced obesity and metabolic syndrome through modulating the gut microbiota in balb/c mice. Int. J. Biol. Macromol. 2021, 187, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.-Q.; Xiao, J.-J.; Zhang, H.-N.; Wang, J.-H.; Pan, L.-H.; Yang, X.-F.; Luo, J.-P.J.F.C. Polysaccharides in laminaria japonica (lp): Extraction, physicochemical properties and their hypolipidemic activities in diet-induced mouse model of atherosclerosis. Food Chem. 2012, 134, 244–252. [Google Scholar] [CrossRef]
- Wang, X.; Pei, L.; Liu, H.; Qv, K.; Xian, W.; Liu, J.; Zhang, G. Fucoidan attenuates atherosclerosis in ldlr-/- mice through inhibition of inflammation and oxidative stress. Int. J. Clin. Exp. Pathol. 2016, 9, 6896–6904. [Google Scholar]
- Zha, X.Q.; Zhang, W.N.; Peng, F.H.; Xue, L.; Liu, J.; Luo, J.P. Alleviating vldl overproduction is an important mechanism for laminaria japonica polysaccharide to inhibit atherosclerosis in ldlr−/− mice with diet-induced insulin resistance. Mol. Nutr. Food Res. 2017, 61, 1600456. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, X.Y.; Guo, W.L.; Cao, Y.J.; Lin, Y.C.; Cheng, W.J.; Chen, L.J.; Rao, P.F.; Ni, L.; Lv, X.C. The protective mechanisms of macroalgae laminaria japonica consumption against lipid metabolism disorders in high-fat diet-induced hyperlipidemic rats. Food Funct. 2020, 11, 3256–3270. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, X.-Y.; Cao, Y.-J.; Zheng, T.-T.; Cheng, W.-J.; Chen, L.-J.; Lv, X.-C.; Ni, L.; Rao, P.-F.; Liang, P. The beneficial effects of lactobacillus brevis fzu0713-fermented laminaria japonica on lipid metabolism and intestinal microbiota in hyperlipidemic rats fed with a high-fat diet. Food Funct. 2021, 12, 7145–7160. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Liu, G.-M.; Chen, Q.; Lu, Z. Ameliorative effect of dieckol-enriched extraction from laminaria japonica on hepatic steatosis induced by a high-fat diet via β-oxidation pathway in icr mice. J. Funct. Foods 2019, 58, 44–55. [Google Scholar] [CrossRef]
- You, J.S.; Sung, M.J.; Chang, K.J. Evaluation of 8-week body weight control program including sea tangle (Laminaria japonica) supplementation in korean female college students. Nutr. Res. Pract. 2009, 3, 307–314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishimura, M.; Sugawara, M.; Kudo, M.; Kinoshita, Y.; Yoshino, H.; Nishihira, J. Effects of daily intake of harudori-kombu: A randomized, double-blind, placebo-controlled, parallel-group study. Funct. Foods Health Dis. 2019, 9, 205–223. [Google Scholar] [CrossRef]
- Nishiumi, S.; Izumi, Y.; Kobayashi, T.; Yoshida, M.J.F.S.; Research, T. A pilot study: Effects of kombu intake on lifestyle-related diseases-possibility that kombu intake is effective in individuals with abnormally high serum triglyceride levels. Food Sci. Technol. Res. 2019, 25, 827–834. [Google Scholar] [CrossRef]
- Nishiumi, S.; Izumi, Y.; Kobayashi, T.; Yoshida, M. Possible involvement of lipids in the effectiveness of kombu in individuals with abnormally high serum triglyceride levels. J. Nutr. Sci. Vitaminol. 2020, 66, 185–190. [Google Scholar] [CrossRef]
- Aoe, S.; Yamanaka, C.; Ohtoshi, H.; Nakamura, F.; Fujiwara, S. Effects of daily kelp (Laminaria japonica) intake on body composition, serum lipid levels, and thyroid hormone levels in healthy japanese adults: A randomized, double-blind study. Mar. Drugs 2021, 19, 352. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (laminaria japonica) by lactobacillus brevis bj20 in individuals with high level of gamma-gt: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012, 50, 1166–1169. [Google Scholar] [CrossRef]
- Ko, S.J.; Kim, J.; Han, G.; Kim, S.K.; Kim, H.G.; Yeo, I.; Ryu, B.; Park, J.W. Laminaria japonica combined with probiotics improves intestinal microbiota: A randomized clinical trial. J. Med. Food 2014, 17, 76–82. [Google Scholar] [CrossRef]
- Saklayen, M.G.J.C.h.r. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Choi, W.-c.; Reid, S.N.; Ryu, J.-k.; Kim, Y.; Jo, Y.-H.; Jeon, B.H.J.A. Effects of γ-aminobutyric acid-enriched fermented sea tangle (laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae 2016, 31, 175–187. [Google Scholar] [CrossRef]
- D’Orazio, N.; Gemello, E.; Gammone, M.A.; de Girolamo, M.; Ficoneri, C.; Riccioni, G. Fucoxantin: A treasure from the sea. Mar. Drugs 2012, 10, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; De Martin, S. Brown seaweeds for the management of metabolic syndrome and associated diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.B.; Katanić Stanković, J.S.; Mihailović, V.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Algae as a source of bioactive compounds to prevent the development of type 2 diabetes mellitus. Curr. Med. Chem. 2021, 28, 4592–4615. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J.L. Antisense to lox-1 inhibits oxidized ldl-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 2000, 101, 2889–2895. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Corrigall, V.; Taylor, P.R.; Poston, R.N. The role of the chemokines mcp-1, gro-alpha, il-8 and their receptors in the adhesion of monocytic cells to human atherosclerotic plaques. Cytokine 2008, 43, 181–186. [Google Scholar] [CrossRef]
- Zhao, Y.; Qian, Y.; Sun, Z.; Shen, X.; Cai, Y.; Li, L.; Wang, Z. Role of pi3k in the progression and regression of atherosclerosis. Front. Pharmacol. 2021, 12, 632378. [Google Scholar] [CrossRef]
- Bonetti, J.; Corti, A.; Lerouge, L.; Pompella, A.; Gaucher, C. Phenotypic modulation of macrophages and vascular smooth muscle cells in atherosclerosis-nitro-redox interconnections. Antioxidants 2021, 10, 516. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jorgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular smooth muscle cells in atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. A-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef]
- Boden, G.; Shulman, G.I. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur. J. Clin. Investig. 2002, 32 (Suppl. S3), 14–23. [Google Scholar] [CrossRef]
- Unger, R.H.; Zhou, Y.T. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001, 50 (Suppl. S1), S118–S121. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta 2014, 1840, 2709–2729. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, Q.; Yuan, H.; Zhou, L.; Li, F.F.; Wang, S.M.; Shi, G.; Wang, M. Nitric oxide mediates inflammation in type ii diabetes mellitus through the pparγ/enos signaling pathway. PPAR Res. 2020, 2020, 8889612. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef]
- Yamagishi, S.; Matsui, T. Nitric oxide, a janus-faced therapeutic target for diabetic microangiopathy-friend or foe? Pharmacol. Res. 2011, 64, 187–194. [Google Scholar] [CrossRef]
- Pollard, A.E.; Martins, L.; Muckett, P.J.; Khadayate, S.; Bornot, A.; Clausen, M.; Admyre, T.; Bjursell, M.; Fiadeiro, R.; Wilson, L.; et al. Ampk activation protects against diet-induced obesity through ucp1-independent thermogenesis in subcutaneous white adipose tissue. Nat. Metab. 2019, 1, 340–349. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Kola, B.; Grossman, A.B.; Korbonits, M. The role of amp-activated protein kinase in obesity. Front. Horm. Res. 2008, 36, 198–211. [Google Scholar] [PubMed]
- Wu, Z.; Ma, Q.; Cai, S.; Sun, Y.; Zhang, Y.; Yi, J. Rhus chinensis mill. Fruits ameliorate hepatic glycolipid metabolism disorder in rats induced by high fat/high sugar diet. Nutrients 2021, 13, 4480. [Google Scholar] [CrossRef]
- Ranaweera, S.S.; Natraj, P.; Rajan, P.; Dayarathne, L.A.; Mihindukulasooriya, S.P.; Dinh, D.T.T.; Jee, Y.; Han, C.H. Anti-obesity effect of sulforaphane in broccoli leaf extract on 3t3-l1 adipocytes and ob/ob mice. J. Nutr. Biochem. 2022, 100, 108885. [Google Scholar] [CrossRef] [PubMed]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef]
- Choe, S.Y.; Seo, Y.; Bang, C.Y.; Woo, S.H.; Kang, M. Protective effects of gymnaster koraiensis extract on high fat diet-induced fatty liver in mice. Adv. Tradit. Med. 2021, 21, 361–369. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Spiegelman, B.M. Tumor necrosis factor alpha: A key component of the obesity-diabetes link. Diabetes 1994, 43, 1271–1278. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. Tnf-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, F.; Hu, J.; Zhang, L.; Xue, C.; Zhang, Z.; Li, B. Antithrombotic activity of oral administered low molecular weight fucoidan from laminaria japonica. Thromb. Res. 2016, 144, 46–52. [Google Scholar] [CrossRef]
- Sun, N.; Sun, B.; Li, C.; Zhang, J.; Yang, W. Effects of different pretreatment methods and dietary factors on the form and bioavailability of iodine in laminaria japonica. J. Aquat. Food Prod. Technol. 2022, 31, 154–169. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Tocaciu, S.; Oliver, L.J.; Lowenthal, R.M.; Peterson, G.M.; Patel, R.; Shastri, M.; McGuinness, G.; Olesen, I.; Fitton, J.H. The effect of undaria pinnatifida fucoidan on the pharmacokinetics of letrozole and tamoxifen in patients with breast cancer. Integr. Cancer Ther. 2018, 17, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef] [PubMed]

| Animal Model | Diabetes-Inducer | Positive Control | Active Compounds | Administration Route | Dosage | Treatment Duration | Mechanisms | Lab Test | Efficacy | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Kunming mice | Alloxan | Glibenclamide | Polysaccharide | Oral | 50, 100, 200 mg/kg | 28 days | ↑ Glucose utilization, ↓ Hormone-sensitive lipase, free fatty acids mobilization | ↑ Insulin, HDL-C, ↓ FBG, TC, TG, LDL-C | Hypoglycemic, hypolipidemic effect | [3] |
| Kunming mice | Alloxan | None | Polysaccharide | Oral | 75, 150, 300 mg/mL | 2 weeks | Recovery of the secretary function of islet cells | ↑ Insulin, amylin, ↓ FBG | Hypoglycemic effect | [18] |
| Kunming mice | Streptozocin | Ethyl acetate fraction, acarbose | Butyl-isobutyl-phthalate | Intragastric | 25, 50, 100 mg/kg | 3 days | ↓ α-glucosidase | ↓ Glucose | Hypoglycemic effect | [19] |
| Sprague-Dawley rats | Streptozocin | None | NR | Oral | 100 mg/kg | 5 days | ↑ Utilization efficiency of GSH, ↓ ROS | ↑ GSH, GSH reductase, GSH peroxidase, XD ↓ Glucose, XO | Anti-hyperglycemic, antioxidant effect | [20] |
| Sprague-Dawley rats | Streptozocin | Probucol | Low molecular weight fucoidan | Intragastric | 50, 100 mg/kg | 12 weeks | ↓ Oxidative stress, prostanoid production, hyper-responsiveness of aortic smooth muscles | ↑ GSH, SOD, 6-keto-PGF1α, ↓ BP, TC, TG, LDL-C, MF, COX-2 expression, TXAS | Hypolipidemic, hypotensive, antioxidant effect | [21] |
| Sprague-Dawley rats | Streptozocin | None | NR | Ad libitum | 4, 15% w/w | 13 weeks | ↑ Bile acid synthesis, lipid excretion, ↓ Lipid absorption | ↑ Insulin, fecal TC, fecal TG, fecal TL, ↓ Glucose, TC, TG, LDL-C, Hepatic TG | Hypoglycemic, Hypolipidemic effect | [22] |
| Wister rats | Streptozocin | PKC inhibitor | Sulfated polysaccharide | Intragastric | 200 mg/kg | 80 days | Downregulation of PKC, modulation of NF-κB signaling pathway | ↓ RI, Urinary volume, BUN, urinary protein/Cr, serum Cr, histopathological score, PKC-αPKC-β, NF-κB, p65, P-selectin | The effect of mitigating diabetic nephropathy | [23] |
| Wister rats | Alloxan | None | NR | Oral | 1.25, 5.0, 12.5 g/kg | 2 weeks | ↑ Anti-oxidation, Recovery of the pancreatic islet cell secreting function | ↑ Insulin, SOD, GSH-Px, B cell index, ↓ FBG, MDA, NO, pancreatic SOD, pancreatic iNOS | Hypoglycemic, antioxidant effect | [24] |
| Type 2 diabetic Goto-Kakizaki rats | Sodium laurate | Cilostazol | Low molecular weight fucoidan | Intragastric | 20, 40, 80 mg/kg | 4 weeks | ↑ VEGF expression, eNOS phosphorylation, NO production | ↑ HDL-C, NO, plantar capillary density, neovascularization around femoral artery, gastrocnemius size, weight, ↓ TG, TG, LDL-C, ulceration score, claudication score, vascular plaques rate, intimal hyperplasia thickness, ICAM-1, IL-1β, ADP | Anti-inflammation, anti-thrombosis, enhancing revascularization effect | [25] |
| C57BL/6N mice | High-fat diet | None | Total Polyphenol | Oral | 5% | 16 weeks | Regulation of α-glucose homeostasis, ↑ muscle glucose uptake, activation of insulin-signaling-related proteins | ↑ IL-6, IL-10, p-Akt, p-AMPK, ↓ α-glucosidase activity, TNF-α | Antidiabetic effect | [8] |
| Animal Model | Obesity-Inducer | Active Compounds | Administration Route | Dosage | Treatment Duration | Positive Control | Mechanisms | Lab Test | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| C57BL/6N mice | HFD | Oral | supplementing 5% of the diet | 16 weeks | None | ↓ IL-1β, Il-6 | ↓ blood glucose, leptin | Anti-obesity effect Anti-inflammatory effect | [13] | |
| C57BL/6J mice | HFD | Fucoidan | Oral | 200 mg/kg | 16 weeks | None | ↓ TNF-α, IL-1β, MCP-1 | ↓ TC, TAG, fasting blood glucose, serum LBP | Beneficial effect on MetS | [26] |
| SD rats | HFD | Ethanol extract | Oral | 400 mg/kg | 6 weeks | None | ↑ p-AMPK/AMPK, p-ACC/ACC ↓ TNF-α | ↓ serum TG, TC, LDL-C, FFA, leptin, glucose, insulin ↑ HDL-C and HDL-C/TC ratio, adiponectin | Anti-obesity effect | [27] |
| C57BL/6 mice | HFD | Insoluble dietary fiber | Oral | supplementing 5% of the diet | 8 weeks | None | regulation of SREBP-1c/FAS signaling | ↓ serum glucose, TC, HDL-C, LDL-C, ALT, AST ↑ acetate, propionate, cecal SCFA | Anti-obesity effect Gut microbiota dysbiosis | [28] |
| C57BL/6J mice | HFD | Polysaccharide | Oral | HFD plus 2 g/kg SP | 8 weeks | None | ↑ p-AMPK ↓ FAS, TNF-α | ↑ adiponectin secretion ↓ TG, TC, FFA, leptin secretion, Hepatic TG, cholesterol content in the liver, serum LDL-C | Hypoglycemic effect, improved serum lipid profiles, ameliorated intestinal damage | [29] |
| BALB/c mice | high-fat diet | Polysaccharide | Oral | 0.25% LJPs solution as drinking water | 10 weeks (not specified) | None | ↑ ratio of HDL-C/LDL-C, SCFAs ↓ levels of serum lipids, | Gut microbiota normalization Anti-obesity effect | [30] | |
| C57BL/6N mice | High-fat diet | N/A | Oral | supplementing 5% of the diet | 16 weeks | None | ↑ p-AMPK | ↑ Fecal BA ↓ Fasting blood glucose levels, Plasma TG levels, hepatic lipid accumulation | Hypotriglyceridemic effect | [31] |
| Animal Model | Atherosclerosis Inducer | Active Compounds | Administration Route | Dosage | Treatment Duration | Positive Control | Mechanisms | Lab Test | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Guangdong mice | ROS/RNS | Polysaccharide | Oral | 200 mg/kg/body mass/day | 4 weeks | None | ↑ ABTS | ↓ TC, HDL-C, TG, LDL-C/HDL-C ratio | Anti-cardiovascular diseases, hypolipidemic, antioxidative effects | [14] |
| C57BL/6 mice | HFD | Polysaccharide | Oral | 200 mg/kg/body mass/day | 8 weeks | None | ↑ intestinal goblet cells ↓ ACC1, FAS, PPAR-γ, TNF-α, MAPKs, p-ERK, p-JNK, Akkermansia | ↓ glycemia, glucose, fasting insulin/glucose, HOMA-IR, inflammation, Firmicutes/Bacteroidetes ratio | Anti-insulin resistance, anti-obesity, anti-inflammation effects | [32] |
| LDLr−/− mice | HFD | Polysaccharide | Oral | 200 mg/kg/body mass/day | 14 weeks | None | ↑ SOD ↓ MAPKs, TNF-α, p- ERK1/2, p-JNK1/2 | ↓ atherosclerotic plaque, TC, TG, LDL-C/HDL-C, MDA | Anti-atherosclerotic, hypolipidemic, antioxidative effects | [33] |
| Sprague-Dawley rats | HFD | FPS | Oral | 0.4 g/kg | 8 weeks | None | ↑ LPL, LCAT | ↑ HDL-C ↓ TG, TC, LDL-C, synthesis of endogenous lipids | Hypoglycemic, anti-atherosclerotic cardiovascular diseases effects | [34] |
| BALB/c mice | HFD | L-LJA | Oral | 0.3% | 11 weeks | None | ↑ GPR41, GPR43, CPT-1A ↓ PPAR-γ, TNF-α | ↑ HDL-C, SOD, CAT, SCFAs ↓ TC, TG, LDL-C | Anti-obesity effect | [35] |
| Kunming mice | Hyperlipidemic diets | Polysaccharides | Oral | 100, 200, 400 mg/kg/day | 12 weeks | None | ↑ SOD, CAT, GST | ↓ TC, TG, HDL-C, LDL-C, MDA | hypolipidemic, enhancing antioxidant enzyme effects | [36] |
| LDL receptor-deficient C57BL6J mice | HCD | Polysaccharide (Fucoidan) | Oral | 50, 100 mg/kg/day | 16 weeks | None | ↓ LOX-1, IL-1b, IL-6, TNF-α, ICAM-1, VCAM-1 | ↓ TG, TC, LDL-C, HDL-C, atherosclerotic plaque formation, macrophage infiltration, smooth muscle cell proliferation, ROS generation | Anti-atherosclerotic, hypolipidemic, anti-inflammatory effects | [37] |
| LDL receptor-deficient C57BL6J mice | HCD | Polysaccharide (Fucoidan) | Oral | 50, 100 mg/kg/day | 14 weeks | Simvastatin (5 mg/kg/day) | ↓ VLDL, SREBP-1c, ACC1, FAS, p-IRS-1, p-IRS-2, PI3K, AKT, P70S6K, nuclear Foxo1 | ↑ Apolipoprotein A1, HDL, Sortilin ↓ insulin resistance, fat accumulation, plaque, HDL-C, FFA, hepatic cholesterol/TC, VLDL-CE/FC/TG/apolipoprotein B | Anti-atherosclerotic, hypolipidemic, insulin signaling regulating effects | [38] |
| Animal Model | Obesity-Inducer | Active Compounds | Administration Route | Dosage | Treatment Duration | Positive Control | Mechanisms | Lab Test | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| SD rats | HFD | N/A | Oral | 2.5 g/kg | 8 weeks | None | ↑ SOD, GSH-Px | ↓ TG, TC, NEFA | Hypolipidemic effect | [39] |
| SPF male rats | HFD | N/A | Oral | 1.0 mL | 8 weeks | None | ↓ HMGCR, SREBP-1c, CD36 | ↓ serum TC, TG, NEFA ↑ fecal acid acetate, propionate, isobutyrate | Anti-hyperlipidemia effect | [40] |
| ICR mice | Non-alcoholic fatty liver/high fat-diet | None | Oral | 50 mg/kg | 4 weeks | None | ↑ AMPK and regulation of its downstream proteins | ↑ p-AMPK, PPAR-α, APT-1, ↓ body weight, liver index, visceral fat index, plasma, and hepatic TC, TG, HDL, LDL, hepatic steatosis, accumulation of hepatic lipids, hepatocellular swelling, vacuoles (normal diet + LJP group) | Hypolipidemic effect | [41] |
| Patient /Inclusion Criteria | Intervention (n) | Control (n) | Treatment Period | Outcome | Main Results | Adverse Effect | Reference/Research Design |
|---|---|---|---|---|---|---|---|
| Healthy, female (n = 22) /NR | Sea tangle (20 g/day) | - | 8 weeks | Body composition, dietary intakes, QOL | ↓ body weight/fat, BMI, intake of energy/protein/fat ↑ balanced diet/mealtime, intake of fiber improvement: QOL | NR | [42]/CT |
| Healthy, high GGT/aged 25–60 year | Fermented sea tangle (250 mg×6) | Placebo | 4 weeks | Oxidative stress | ↓ GGT, MDA ↑ SOD, CAT activities | No | [47]/RCT |
| Healthy (n = 40) /aged 18–75 year | LJP (625 mg) | LJP + probiotics (lactic acid bacteria) | 4 weeks (+2 weeks follow-up) | Gastrointestinal symptom, QOL, microbiome | No significant changes | No | [48] /RCT |
| Healthy, female (n = 21) /middle aged | γ-aminobutyric acid-enriched fermented sea tangle (1000 mg/day) | Placebo (sucrose) | 8 weeks | Body composition, muscular strength | ↓ fat, TG ↑ BDNF, ACE, HGH, IGF-1, total lean mass improvement: total work, muscle strength | No | [50] /RCT |
| Healthy (n = 70) /LDL-C 120~160 mg/dL, BMI 22~30 | Dried kombu powder (2.0 g/day) | Placebo (dextrin powder) | 6 weeks | Liver/renal function, body composition, lipid/glucose profiles | ↓ fat ↑ adiponectin | diarrhea, variation in LDH/γ-GTP/UA | [43] /RCT |
| Healthy (n = 48) /NR | Roasted kombu (6 g/day) | - | 4 weeks | Liver/renal function, lipid/glucose profiles, insulin, gastrointestinal symptoms | ↓ UA ↓ serum triglyceride (only in subjects with high serum triglyceride levels) ↑ TC, CPR, abdominal distension | No | [44] /RCT, cross-over |
| 1) Healthy (n = 48) 2) high TG (n = 9) | Roasted kombu (6 g/day, first 4 weeks) | Roasted kombu (6 g/day, last 4 weeks) | 4 weeks | Lipid metabolomics | ↑ Plasmanyl/plasmenyl forms of PC, PE ↓ LPC, LPE improvement: PC/PE with DL, LPC/LPE with AL, FFA (high TG subjects) | NR | [45] /RCT, cross-over (re-analysis) |
| Healthy (n = 50) /BMI < 30 kg/m2 | iodine-reduced boiled kelp powder (3 g alginate/day) | Placebo | 8 weeks | Lipids, thyroid hormone | ↓ body fat (male subjects) | No | [46] /RCT |
| Author, Year /Research Design | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Period & Carryover Effects | Overall |
|---|---|---|---|---|---|---|---|
| [47]/RCT |  |  |  |  |  | - |  |
| [48] /RCT |  |  |  |  |  | - |  |
| [50] /RCT |  |  |  |  |  | - |  |
| [43]/RCT |  |  |  |  |  | - |  |
| [46] /RCT |  |  |  |  |  | - |  |
| * [44]/RCT, cross-over |  |  |  |  |  |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.-S.; Ko, S.-J.; Lee, Y.N.; Lee, G.; Rahman, M.H.; Kim, B. The Effect of Laminaria japonica on Metabolic Syndrome: A Systematic Review of Its Efficacy and Mechanism of Action. Nutrients 2022, 14, 3046. https://doi.org/10.3390/nu14153046
Lee I-S, Ko S-J, Lee YN, Lee G, Rahman MH, Kim B. The Effect of Laminaria japonica on Metabolic Syndrome: A Systematic Review of Its Efficacy and Mechanism of Action. Nutrients. 2022; 14(15):3046. https://doi.org/10.3390/nu14153046
Chicago/Turabian StyleLee, In-Seon, Seok-Jae Ko, Yu Na Lee, Gahyun Lee, Md. Hasanur Rahman, and Bonglee Kim. 2022. "The Effect of Laminaria japonica on Metabolic Syndrome: A Systematic Review of Its Efficacy and Mechanism of Action" Nutrients 14, no. 15: 3046. https://doi.org/10.3390/nu14153046
APA StyleLee, I.-S., Ko, S.-J., Lee, Y. N., Lee, G., Rahman, M. H., & Kim, B. (2022). The Effect of Laminaria japonica on Metabolic Syndrome: A Systematic Review of Its Efficacy and Mechanism of Action. Nutrients, 14(15), 3046. https://doi.org/10.3390/nu14153046








