The Interplay between Dietary Phosphorous, Protein Intake, and Mortality in a Prospective Hemodialysis Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Ascertainment
2.3. Socio-Demographic, Comorbidity, and Dialysis Treatment Data
2.4. Body Anthropometry and Nutritional Measures
2.5. Outcome Ascertainment
2.6. Statistical Methods
3. Results
3.1. Cohort Description
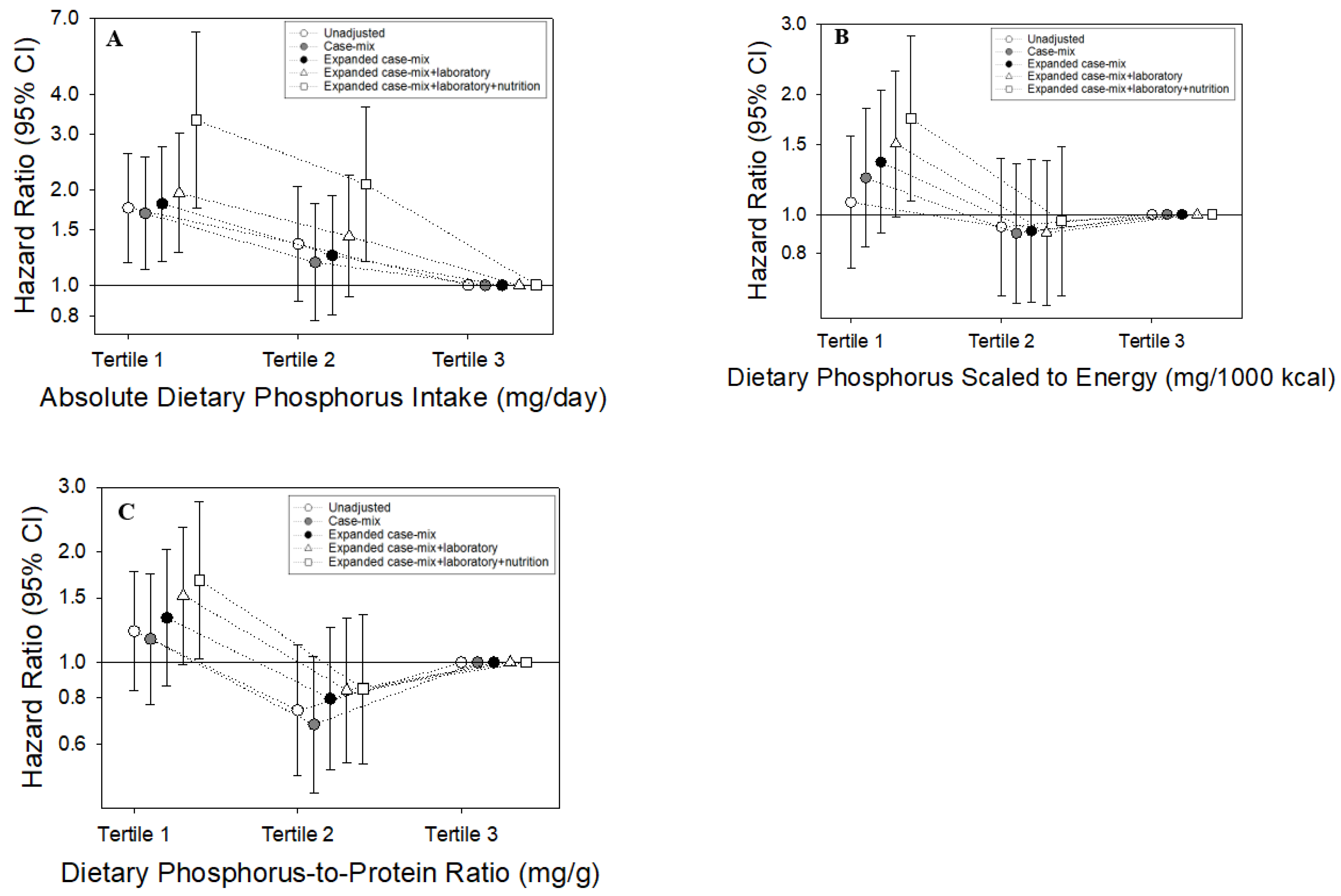
3.2. Absolute Dietary Phosphorus Intake and Mortality
3.3. Dietary Phosphorus Intake Scaled to Energy Intake and Mortality
3.4. Dietary Phosphorus-to-Protein Ratio and Mortality
3.5. Absolute Dietary Phosphorus Intake and Mortality across Clinically Relevant Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleh, T.; Sumida, K.; Molnar, M.Z.; Potukuchi, P.K.; Thomas, F.; Lu, J.L.; Gyamlani, G.G.; Streja, E.; Kalantar-Zadeh, K.; Kovesdy, C.P. Effect of Age on the Association of Vascular Access Type with Mortality in a Cohort of Incident End-Stage Renal Disease Patients. Nephron 2017, 137, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Tentori, F.; Blayney, M.J.; Albert, J.M.; Gillespie, B.W.; Kerr, P.G.; Bommer, J.; Young, E.W.; Akizawa, T.; Akiba, T.; Pisoni, R.L.; et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am. J. Kidney Dis. 2008, 52, 519–530. [Google Scholar] [CrossRef]
- Young, E.W.; Albert, J.M.; Satayathum, S.; Goodkin, D.A.; Pisoni, R.L.; Akiba, T.; Akizawa, T.; Kurokawa, K.; Bommer, J.; Piera, L.; et al. Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005, 67, 1179–1187. [Google Scholar] [CrossRef] [Green Version]
- Silver, J.; Naveh-Many, T. FGF-23 and secondary hyperparathyroidism in chronic kidney disease. Nat. Rev. Nephrol. 2013, 9, 641–649. [Google Scholar] [CrossRef]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelii, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef] [Green Version]
- Amann, K.; Tornig, J.; Kugel, B.; Gross, M.L.; Tyralla, K.; El-Shakmak, A.; Szabo, A.; Ritz, E. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int. 2003, 63, 1296–1301. [Google Scholar] [CrossRef] [Green Version]
- Pun, P.H.; Horton, J.R.; Middleton, J.P. Dialysate Calcium Concentration and the Risk of Sudden Cardiac Arrest in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Lowrie, E.G.; Lew, N.L. Death risk in hemodialysis patients: The predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am. J. Kidney Dis. 1990, 15, 458–482. [Google Scholar] [CrossRef]
- Narasaki, Y.; Rhee, C.M. Dietary Therapy for Managing Hyperphosphatemia. Clin. J. Am. Soc. Nephrol. 2020, 16, 9–11. [Google Scholar] [CrossRef]
- Slinin, Y.; Foley, R.N.; Collins, A.J. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J. Am. Soc. Nephrol. 2005, 16, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.P.; Kelepouris, E. New Directions in Phosphorus Management in Dialysis. J. Renal Nutr. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.N.; Nigwekar, S. Phosphate Absorption and Hyperphosphatemia Management in Kidney Disease: A Physiology-Based Review. Kidney Med. 2021, 3, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2009, 113, S1–S130. [Google Scholar]
- Lynch, K.E.; Lynch, R.; Curhan, G.C.; Brunelli, S.M. Prescribed Dietary Phosphate Restriction and Survival among Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Bross, R.; Benner, D.; Kopple, J.D. Association of Dietary Phosphorus to Protein Ratio with Mortality in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 638–692. [Google Scholar] [CrossRef] [Green Version]
- Khoueiry, G.; Waked, A.; Goldman, M.; El-Charabaty, E.; Dunne, E.; Smith, M.; Kleiner, M.; Lafferty, J.; Kalantar-Zadeh, K.; El-Sayegh, S. Dietary intake in hemodialysis patients does not reflect a heart healthy diet. J. Ren. Nutr. 2011, 21, 438–447. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Deepak, S.; Block, D.; Block, G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J. Ren. Nutr. 2002, 12, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Shinaberger, C.S.; Greenland, S.; Kopple, J.D.; Van Wyck, D.; Mehrotra, R.; Kovesdy, C.P.; Kalantar-Zadeh, K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am. J. Clin. Nutr. 2008, 88, 1511–1518. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Kalantar-Zadeh, K.; Kopple, J.D. Frailty and Protein-Energy Wasting in Elderly Patients with End Stage Kidney Disease. J. Am. Soc. Nephrol. 2013, 24, 337–351. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Tortorici, A.R.; Chen, J.L.; Kamgar, M.; Lau, W.L.; Moradi, H.; Rhee, C.M.; Streja, E.; Kovesdy, C.P. Dietary restrictions in dialysis patients: Is there anything left to eat? Semin. Dialysis. 2015, 28, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, L. An Update on Phosphate Binders: A Dietitian’s Perspective. J. Ren. Nutr. 2016, 26, 209–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forfang, D.; Edwards, D.P.; Kalantar-Zadeh, K. The Impact of Phosphorus Management Today on Quality of Life: Patient Perspectives. Kidney Med. 2022, 4, 100437. [Google Scholar] [CrossRef]
- You, A.S.; Kalantar, S.S.; Norris, K.C.; Peralta, R.A.; Narasaki, Y.; Fischman, R.; Fischman, M.; Semerjian, A.; Nakata, T.; Azadbadi, Z.; et al. Dialysis symptom index burden and symptom clusters in a prospective cohort of dialysis patients. J. Nephrol. 2022, 35, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.R.; Alvestrand, A.; Danielsson, A.; Divino, J.C.; Gutierrez, A.; Lindholm, B.; Bergström, J. Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int. 1998, 53, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cupisti, A.; Kalantar-Zadeh, K. Management of Natural and Added Dietary Phosphorus Burden in Kidney Disease. Semin Nephrol. 2013, 33, 180–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winger, R.J.; Uribarri, J.; Lloyd, L. Phosphorus-containing food additives: An insidious danger for people with chronic kidney disease. Trends Food Sci. Technol. 2012, 24, 92–102. [Google Scholar] [CrossRef]


| Overall | Dietary Phosphorus Intake (mg/Day) | |||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | ||
| N (%) | 415 | 138 | 137 | 140 |
| Age (mean ± SD) | 55 ± 15 | 57 ± 15 | 57 ± 13 | 53 ± 15 |
| Male (%) | 55 | 41 | 58 | 66 |
| Black race (%) | 36 | 35 | 34 | 40 |
| Hispanic ethnicity (%) | 48 | 46 | 47 | 52 |
| Vintage (years, mean ± SD) | 5 ± 4 | 4 ± 3 | 5 ± 4 | 5 ± 5 |
| BMI (kg/m2, mean ± SD) | 27.6 ± 6.6 | 27.4 ± 6.6 | 27.5 ± 6.0 | 27.9 ± 7.0 |
| spKt/V | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.3 | 1.7 ± 0.4 |
| Dialysis access | ||||
| AV Fistula/Graft | 47 | 39 | 50 | 53 |
| Catheter | 11 | 11 | 12 | 10 |
| Unknown | 41 | 50 | 37 | 37 |
| Insurance | ||||
| Medicare/Medicaid | 75 | 76 | 77 | 73 |
| Private | 11 | 13 | 12 | 9 |
| Other | 14 | 11 | 12 | 19 |
| COMORBIDITIES | ||||
| Diabetes (%) | 55 | 59 | 50 | 55 |
| CHF (%) | 8 | 9 | 7 | 9 |
| CAD (%) | 9 | 8 | 9 | 10 |
| Combined CV disease (%) | 17 | 16 | 15 | 19 |
| LABORATORY RESULTS | ||||
| Serum phosphorus (mg/dL) | 5.1 ± 1.5 | 5.1 ± 1.4 | 5.0 ± 1.3 | 5.1 ± 1.6 |
| Serum albumin (g/dL) | 4.0 ± 0.4 | 4.0 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.4 |
| nPCR (g/kg/day) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 |
| Serum creatinine (mg/dL) | 9.7 ± 3.0 | 9.5 ± 3.1 | 9.6 ± 2.6 | 10.2 ± 3.1 |
| DIETARY INTAKE | ||||
| Energy (kcal/day) | 998 (566, 1527) | 446 (302, 596) | 1006 (842, 1252) | 1790 (1398, 2373) |
| Protein (g/day) | 45 (25, 73) | 20 (14, 25) | 45 (36, 55) | 84 (69, 124) |
| MEDICATIONS | ||||
| Phosphate binder use (%) | 93 | 92 | 94 | 93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown-Tortorici, A.R.; Narasaki, Y.; You, A.S.; Norris, K.C.; Streja, E.; Peralta, R.A.; Guerrero, Y.; Daza, A.; Arora, R.; Lo, R.; et al. The Interplay between Dietary Phosphorous, Protein Intake, and Mortality in a Prospective Hemodialysis Cohort. Nutrients 2022, 14, 3070. https://doi.org/10.3390/nu14153070
Brown-Tortorici AR, Narasaki Y, You AS, Norris KC, Streja E, Peralta RA, Guerrero Y, Daza A, Arora R, Lo R, et al. The Interplay between Dietary Phosphorous, Protein Intake, and Mortality in a Prospective Hemodialysis Cohort. Nutrients. 2022; 14(15):3070. https://doi.org/10.3390/nu14153070
Chicago/Turabian StyleBrown-Tortorici, Amanda R., Yoko Narasaki, Amy S. You, Keith C. Norris, Elani Streja, Rene Amel Peralta, Yalitzi Guerrero, Andrea Daza, Ria Arora, Robin Lo, and et al. 2022. "The Interplay between Dietary Phosphorous, Protein Intake, and Mortality in a Prospective Hemodialysis Cohort" Nutrients 14, no. 15: 3070. https://doi.org/10.3390/nu14153070







