Selenium Status and Supplementation Effects in Pregnancy—A Study on Mother–Child Pairs from a Single-Center Cohort
Abstract
1. Introduction
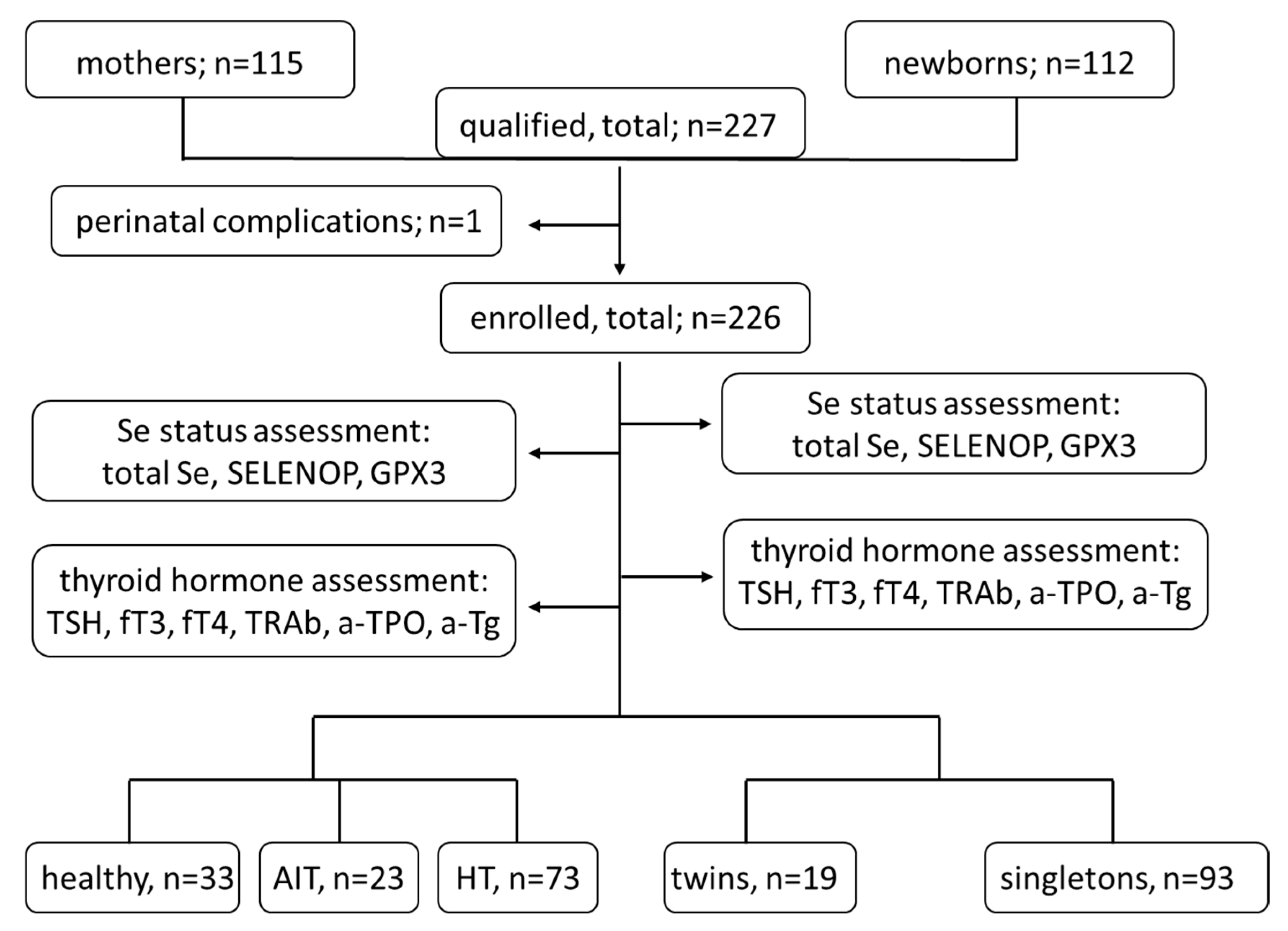
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Statistical Analysis
3. Results
3.1. Group Characteristics
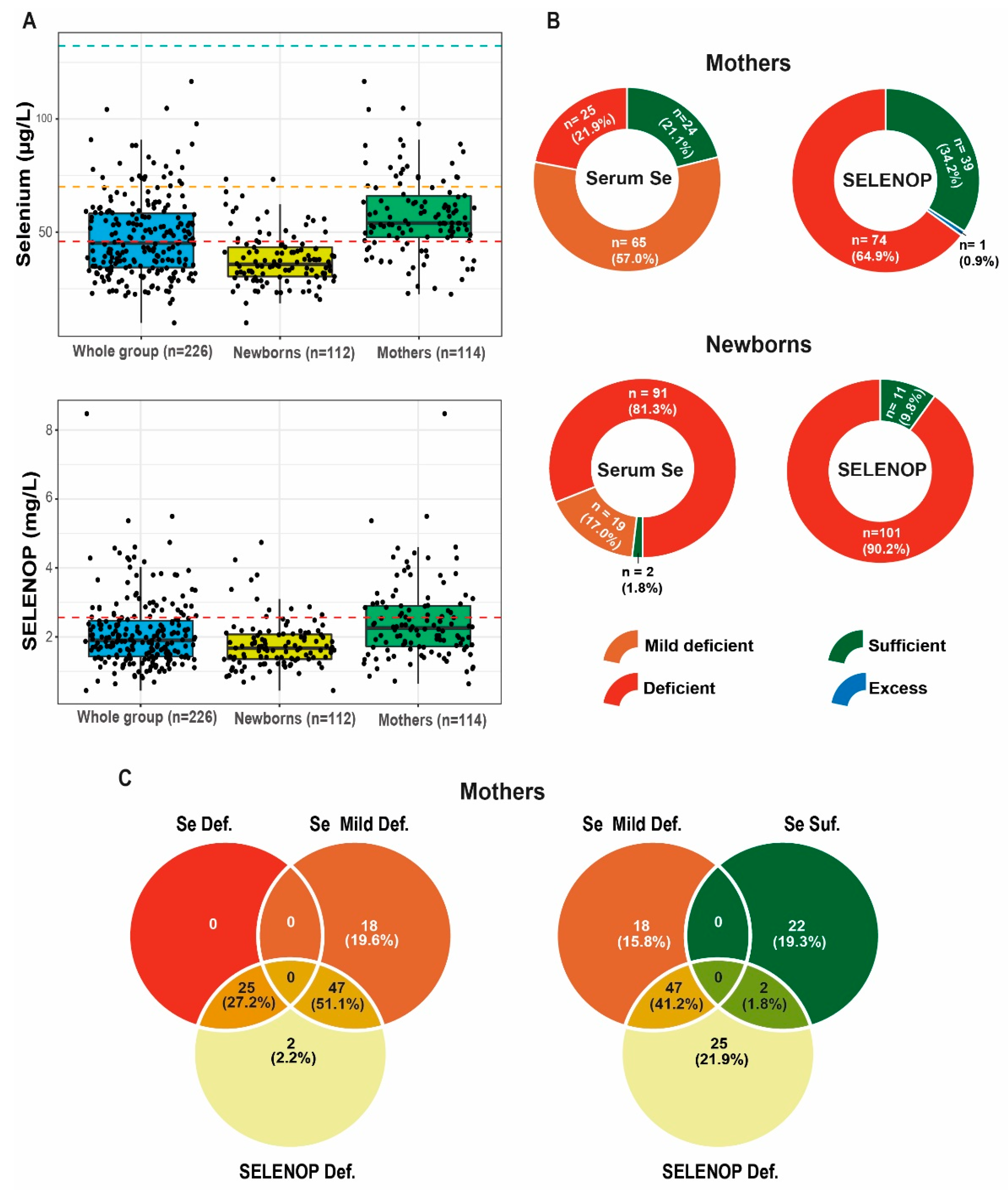
3.2. Correlation Analysis of Se Status Biomarkers in the Mothers and the Newborns
3.3. Selenium Status in the Mothers and the Newborns
3.4. Effects of Se Supplementation
3.5. Relationship between the Status and Supplementation of Se and the Parameters of Thyroid Diseases
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schomburg, L.; Köhrle, J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol. Nutr. Food Res. 2008, 52, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.W.; Hoffmann, P.R.; Schomburg, L. Editorial: Selenium and Selenoproteins in Brain Development, Function, and Disease. Front. Neurosci. 2022, 15, 821140. [Google Scholar] [CrossRef] [PubMed]
- Hubalewska-Dydejczyk, A.; Duntas, L.; Gilis-Januszewska, A. Pregnancy, thyroid, and the potential use of selenium. Horm. Athens Greece 2020, 19, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Majdi, M.R.; Tavallaie, S.; Azimi-Nezhad, M.; et al. Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Neonatal Med. 2011, 24, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, E.; Pearce, E.N. Iodine status and supplementation before, during, and after pregnancy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101430. [Google Scholar] [CrossRef] [PubMed]
- Berbel, P.; Mestre, J.L.; Santamaría, A.; Palazón, I.; Franco, A.; Graells, M.; González-Torga, A.; De Escobar, G.M. Delayed Neurobehavioral Development in Children Born to Pregnant Women with Mild Hypothyroxinemia During the First Month of Gestation: The Importance of Early Iodine Supplementation. Thyroid 2009, 19, 511–519. [Google Scholar] [CrossRef]
- Levie, D.; Korevaar, T.I.M.; Bath, S.; Dalmau-Bueno, A.; Murcia, M.; Espada, M.; Dineva, M.; Ibarluzea, J.M.; Sunyer, J.; Tiemeier, H.; et al. Thyroid Function in Early Pregnancy, Child IQ, and Autistic Traits: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab. 2018, 103, 2967–2979. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The Importance of Adequate Iodine during Pregnancy and Infancy. World Rev. Nutr. Diet 2016, 115, 118–124. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Clinical Relevance of Environmental Factors in the Pathogenesis of Autoimmune Thyroid Disease. Endocrinol. Metab. 2016, 31, 213–222. [Google Scholar] [CrossRef]
- Reyes, M.R.M.; Glinoer, D.; Van Oyen, H.; Vandevijvere, S. High Prevalence of Thyroid Disorders in Pregnant Women in a Mildly Iodine-deficient Country: A Population-Based Study. J. Clin. Endocrinol. Metab. 2013, 98, 3694–3701. [Google Scholar] [CrossRef]
- Trofimiuk-Müldner, M.; Konopka, J.; Sokołowski, G.; Dubiel, A.; Kieć-Klimczak, M.; Kluczyński, Ł.; Motyka, M.; Rzepka, E.; Walczyk, J.; Sokołowska, M.; et al. Current iodine nutrition status in Poland (2017): Is the Polish model of obligatory iodine prophylaxis able to eliminate iodine deficiency in the population? Public Health Nutr. 2020, 23, 2467–2477. [Google Scholar] [CrossRef]
- Ambroziak, U.; Hybsier, S.; Shahnazaryan, U.; Krasnodębska-Kiljańska, M.; Rijntjes, E.; Bartoszewicz, Z.; Bednarczuk, T.; Schomburg, L. Severe selenium deficits in pregnant women irrespective of autoimmune thyroid disease in an area with marginal selenium intake. J. Trace Elem. Med. Biol. 2017, 44, 186–191. [Google Scholar] [CrossRef]
- Hubalewska-Dydejczyk, A.; Lewiński, A.; Milewicz, A.; Radowicki, S.; Poręba, R.; Karbownik-Lewińska, M.; Kostecka-Matyja, M.; Trofimiuk-Müldner, M.; Pach, R.; Zygmunt, A.; et al. Management of thyroid diseases during pregnancy. Endokrynol. Pol. 2011, 62, 362–381. [Google Scholar]
- Lazarus, J.; Brown, R.S.; Daumerie, C.; Hubalewska-Dydejczyk, A.; Negro, R.; Vaidya, B. 2014 European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and in Children. Eur. Thyroid J. 2014, 3, 76–94. [Google Scholar] [CrossRef]
- Hubalewska-Dydejczyk, A.; Trofimiuk-Müldner, M.; Ruchala, M.; Lewiński, A.; Bednarczuk, T.; Zgliczyński, W.; Syrenicz, A.; Kos-Kudla, B.; Jarząb, B.; Gietka-Czernel, M.; et al. Thyroid diseases in pregnancy: Guidelines of the Polish Society of Endocrinology [Choroby tarczycy w ciąży: Zalecenia postępowania Polskiego Towarzystwa Endokrynologicznego]. Endokrynol. Pol. 2021, 72, 425–488. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef]
- Negro, R.; Attanasio, R.; Grimaldi, F.; Marcocci, C.; Guglielmi, R.; Papini, E.; on behalf of AME (Associazione Medici Endocrinologi) and AACE (American Association of Clinical Endocrinologists) Italian Chapter. A 2016 Italian Survey about the Clinical Use of Selenium in Thyroid Disease. Eur. Thyroid J. 2016, 5, 164–170. [Google Scholar] [CrossRef]
- Filipowicz, D.; Majewska, K.; Kalantarova, A.; Szczepanek-Parulska, E.; Ruchała, M. The rationale for selenium supplementation in patients with autoimmune thyroiditis, according to the current state of knowledge. Endokrynol. Pol. 2021, 72, 153–162. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19—A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Mullan, K.R.; McMullan, P.; Hunter, A.; McCance, D.R.; Smyth, P.; Bath, S.C.; Rayman, M.; Woodside, J.V. Selenium status in a Northern Irish pregnant cohort with iodine deficiency. Eur. J. Clin. Nutr. 2021, 75, 403–405. [Google Scholar] [CrossRef]
- Jin, Y.; Coad, J.; Weber, J.L.; Thomson, J.S.; Brough, L. Selenium Intake in Iodine-Deficient Pregnant and Breastfeeding Women in New Zealand. Nutrients 2019, 11, 69. [Google Scholar] [CrossRef]
- Hoeflich, J.; Hollenbach, B.; Behrends, T.; Hoeg, A.; Stosnach, H.; Schomburg, L. The choice of biomarkers determines the selenium status in young German vegans and vegetarians. Br. J. Nutr. 2010, 104, 1601–1604. [Google Scholar] [CrossRef]
- Hollenbach, B.; Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A.; Köhrle, J.; Schomburg, L. New assay for the measurement of selenoprotein P as a sepsis biomarker from serum. J. Trace Elem. Med. Biol. 2008, 22, 24–32. [Google Scholar] [CrossRef]
- Schomburg, L.; Schweizer, U.; Holtmann, B.; Flohé, L.; Sendtner, M.; Köhrle, J. Gene disruption discloses role of selenoprotein P in selenium delivery to target tissues. Biochem. J. 2003, 370 Pt 2, 397–402. [Google Scholar] [CrossRef]
- Hughes, D.J.; Fedirko, V.; Jenab, M.; Schomburg, L.; Méplan, C.; Freisling, H.; Bueno-De-Mesquita, H.; Hybsier, S.; Becker, N.-P.; Czuban, M.; et al. Selenium status is associated with colorectal cancer risk in the European prospective investigation of cancer and nutrition cohort. Int. J. Cancer 2014, 136, 1149–1161. [Google Scholar] [CrossRef]
- Combs, J.G.F. Biomarkers of Selenium Status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef]
- Wasowicz, W.; Gromadzinska, J.; Rydzynski, K.; Tomczak, J. Selenium status of low-selenium area residents: Polish experience. Toxicol. Lett. 2002, 137, 95–101. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academies Press (US), 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK225483/ (accessed on 7 July 2022).
- Scientific Opinion on Dietary Reference Values for Selenium|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/3846 (accessed on 7 July 2022).
- Kipp, A.P.; Strohm, D.; Brigelius-Flohé, R.; Schomburg, L.; Bechthold, A.; Leschik-Bonnet, E.; Heseker, H. Revised reference values for selenium intake. J. Trace Elem. Med. Biol. Organ. Soc. Miner. Trace Elem. GMS 2015, 32, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Loui, A.; Raab, A.; Braetter, P.; Obladen, M.; de Braetter, V.N. Selenium status in term and preterm infants during the first months of life. Eur. J. Clin. Nutr. 2007, 62, 349–355. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bomer, N.; Beverborg, N.G.; Hoes, M.F.; Streng, K.W.; Vermeer, M.; Dokter, M.M.; Ijmker, J.; Anker, S.D.; Cleland, J.G.F.; Hillege, H.L.; et al. Selenium and outcome in heart failure. Eur. J. Heart Fail. 2020, 22, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Sluis, K.B.; Darlow, B.A.; George, P.M.; Mogridge, N.; Dolamore, B.A.; Winterbourn, C.C. Selenium and Glutathione Peroxidase Levels in Premature Infants in a Low Selenium Community (Christchurch, New Zealand). Pediatr. Res. 1992, 32, 189–194. [Google Scholar] [CrossRef][Green Version]
- Wiehe, L.; Cremer, M.; Wisniewska, M.; Becker, N.-P.; Rijntjes, E.; Martitz, J.; Hybsier, S.; Renko, K.; Bührer, C.; Schomburg, L. Selenium status in neonates with connatal infection. Br. J. Nutr. 2016, 116, 504–513. [Google Scholar] [CrossRef]
- Handy, D.E.; Hang, G.; Scolaro, J.; Metes, N.; Razaq, N.; Yang, Y.; Loscalzo, J. Aminoglycosides Decrease Glutathione Peroxidase-1 Activity by Interfering with Selenocysteine Incorporation. J. Biol. Chem. 2006, 281, 3382–3388. [Google Scholar] [CrossRef]
- Renko, K.; Martitz, J.; Hybsier, S.; Heynisch, B.; Voss, L.; Everley, R.A.; Gygi, S.P.; Stoedter, M.; Wisniewska, M.; Köhrle, J.; et al. Aminoglycoside-driven biosynthesis of selenium-deficient Selenoprotein P. Sci. Rep. 2017, 7, 4391. [Google Scholar] [CrossRef]
- Brodin, O.; Hackler, J.; Misra, S.; Wendt, S.; Sun, Q.; Laaf, E.; Stoppe, C.; Björnstedt, M.; Schomburg, L. Selenoprotein P as Biomarker of Selenium Status in Clinical Trials with Therapeutic Dosages of Selenite. Nutrients 2020, 12, 1067. [Google Scholar] [CrossRef]
- Biswas, K.; McLay, J.; Campbell, F.M. Selenium Supplementation in Pregnancy-Maternal and Newborn Outcomes. J. Nutr. Metab. 2022, 2022, 4715965. [Google Scholar] [CrossRef]
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The Influence of Selenium Supplementation on Postpartum Thyroid Status in Pregnant Women with Thyroid Peroxidase Autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268. [Google Scholar] [CrossRef]
- Anan, Y.; Ogra, Y.; Somekawa, L.; Suzuki, K.T. Effects of chemical species of selenium on maternal transfer during pregnancy and lactation. Life Sci. 2009, 84, 888–893. [Google Scholar] [CrossRef]
- Schweizer, U.; Michaelis, M.; Köhrle, J.; Schomburg, L. Efficient selenium transfer from mother to offspring in selenoprotein-P-deficient mice enables dose-dependent rescue of phenotypes associated with selenium deficiency. Biochem. J. 2004, 378 Pt 1, 21–26. [Google Scholar] [CrossRef]
- Hill, K.E.; Motley, A.K.; Winfrey, V.P.; Burk, R.F. Selenoprotein P Is the Major Selenium Transport Protein in Mouse Milk. PLoS ONE 2014, 9, e103486. [Google Scholar] [CrossRef]
- Stråvik, M.; Gustin, K.; Barman, M.; Skröder, H.; Sandin, A.; Wold, A.E.; Sandberg, A.-S.; Kippler, M.; Vahter, M. Infant Iodine and Selenium Status in Relation to Maternal Status and Diet During Pregnancy and Lactation. Front. Nutr. 2021, 8, 733602. [Google Scholar] [CrossRef]
- Bartalena, L.; Kahaly, G.J.; Baldeschi, L.; Dayan, C.M.; Eckstein, A.; Marcocci, C.; Marinò, M.; Vaidya, B.; Wiersinga, W.M.; Ayvaz, G.; et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021, 185, G43–G67. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Adams, J.; Sorenson, J.; Pollard, E.; Kirby, J.; Audhya, T. Evidence-Based Recommendations for an Optimal Prenatal Supplement for Women in the U.S., Part Two: Minerals. Nutrients 2021, 13, 1849. [Google Scholar] [CrossRef]
- Veisa, V.; Kalere, I.; Zake, T.; Strele, I.; Makrecka-Kuka, M.; Upmale-Engela, S.; Skesters, A.; Rezeberga, D.; Lejnieks, A.; Pudule, I.; et al. Assessment of Iodine and Selenium Nutritional Status in Women of Reproductive Age in Latvia. Medicina 2021, 57, 1211. [Google Scholar] [CrossRef] [PubMed]


| Subgroup | |||||||
|---|---|---|---|---|---|---|---|
| Whole Group | AIT (+) | AIT (−) | TSH > 2.5 [mIU/L] | TSH 0.27–2.5 [mIU/L] | Healthy Group | ||
| Me [Q1–Q3] | Me [Q1–Q3] | Me [Q1–Q3] | Me [Q1–Q3] | Me [Q1–Q3] | Me [Q1–Q3] | ||
| Mother | TSH [mIU/L] | 2.09 [1.31 –2.86] | 1.65 [1.23–2.46] | 2.15 [1.31–2.93] | 3.21 * [2.93–4.34] | 1.56 * [1.11–2.1] | 1.79 [1.17–2.19] |
| ft3 [pmol/L] | 4.03 [3.62–4.38] | 3.71 ** [3.39–4.14] | 4.17 ** [3.73–4.43] | 3.96 *** [3.44–4.36] | 4.05 [3.71–4.42] | 4.33 *** [3.92–4.59] | |
| ft4 [pmol/L] | 13.6 [12.2–15.4] | 13.8 [12.2–16.3] | 13.6 [12.2–15.1] | 13.7 [12.6–16.3] | 13.5 [12.1–14.8] | 13.4 [11.4–14.5] | |
| a-TPO [IU/mL] | 12 [10–20] | 82 * [46–173] | 11 * [10–14] | 12 [10–20] | 12 [10–17] | 11 [10–13] | |
| a-Tg [IU/mL] | 14 [11–19] | 38 * [22–104] | 13 * [11–15] | 14 [10–17] | 14 [11–19] | 13 [11–15] | |
| TRAb [IU/L] | 0.46 [0.3–0.71] | 0.65 [0.3–1.08] | 0.35 [0.3–0.68] | 0.48 [0.3–0.68] | 0.33 [0.29–0.87] | 0.47 [0.27–0.86] | |
| Child | TSH [mIU/L] | 7.87 [5.77–11.15] | 6.59 [5.37–9.89] | 8.44 [5.85–11.55] | 9.22 *** [7.25–11] | 7.08 [5.49–10.8] | 6.43 *** [4.77–9.1] |
| ft3 [pmol/L] | 2.07 [1.8–2.37] | 2.02 [1.65–2.22] | 2.07 [1.83–2.43] | 2.1 [1.8–2.42] | 2.01 [1.75–2.35] | 2.07 [1.83–2.85] | |
| ft4 [pmol/L] | 16.4 [15.1–17.6] | 16.45 [14.7–17.4] | 16.4 [15.2–17.9] | 16.4 [15–17.9] | 16.3 [15.1–17.4] | 16.2 [15.1–17.9] | |
| a-TPO [IU/mL] | 9 [9–11] | 60.5 * [28–105] | 9 * [9–9] | 9 [9–9] | 9 [9–13] | 9 [9–9] | |
| a-Tg [IU/mL] | 13 [10–14] | 18 * [14–47] | 12 * [10–14] | 13 [10–14] | 13 [10–16] | 12 [10–14] | |
| TRAb [IU/L] | 0.3 [0.23–0.43] | 0.4 [0.3–0.9] | 0.3 [0.23–0.43] | 0.3 [0.23–0.41] | 0.3 [0.26–0.53] | 0.3 [0.12–0.57] | |
| Group | U | p * | T | p ** | ||
|---|---|---|---|---|---|---|
| Mother | Child | |||||
| Me [Q1–Q3] | Me [Q1–Q3] | |||||
| Se [µg/L] | 54 [48–66] | 36 [30–43] | 2113 | <0.001 | 226 | <0.001 |
| GPx [U/L] | 199 [167–232] | 137 [112–164] | 1926 | <0.001 | 426.5 | <0.001 |
| SELENOP [mg/L] | 2.3 [1.7–2.9] | 1.7 [1.4–2.1] | 3647 | <0.001 | 1114 | <0.001 |
| Percentage of Women on Various Selenium Supplements (Number from Total n = 35) | Formula | Dose [µg/per Day] |
|---|---|---|
| 51% (20) | sodium selenate | 55 |
| 40% (14) | sodium selenate | 26/30 |
| 3% (1) | L-selenomethionine | 6.25 |
| Se Supplementation | U | p | ||
|---|---|---|---|---|
| No | Yes | |||
| Me [Q1–Q3] | Me [Q1–Q3] | |||
| Se M [µg/L] | 54 [46–64] | 58 [50–71] | 1040 | 0.42 |
| GPX3 M [U/L] | 199 [170–225] | 208.5 [179–254] | 1049 | 0.46 |
| SELENOP M [mg/L] | 2.2 [1.7–2.6] | 2.3 [1.7–3.6] | 986 | 0.23 |
| Se C [µg/L] | 35 [30–43] | 37 [32–45] | 950.5 | 0.15 |
| GPX3 C [U/L] | 137 [113–161] | 139 [117–180] | 1047 | 0.45 |
| SELENOP C [mg/L] | 1.6 [1.3–2] | 1.7 [1.4–2.2] | 986 | 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipowicz, D.; Szczepanek-Parulska, E.; Kłobus, M.; Szymanowski, K.; Chillon, T.S.; Asaad, S.; Sun, Q.; Mikulska-Sauermann, A.A.; Karaźniewicz-Łada, M.; Główka, F.K.; et al. Selenium Status and Supplementation Effects in Pregnancy—A Study on Mother–Child Pairs from a Single-Center Cohort. Nutrients 2022, 14, 3082. https://doi.org/10.3390/nu14153082
Filipowicz D, Szczepanek-Parulska E, Kłobus M, Szymanowski K, Chillon TS, Asaad S, Sun Q, Mikulska-Sauermann AA, Karaźniewicz-Łada M, Główka FK, et al. Selenium Status and Supplementation Effects in Pregnancy—A Study on Mother–Child Pairs from a Single-Center Cohort. Nutrients. 2022; 14(15):3082. https://doi.org/10.3390/nu14153082
Chicago/Turabian StyleFilipowicz, Dorota, Ewelina Szczepanek-Parulska, Małgorzata Kłobus, Krzysztof Szymanowski, Thilo Samson Chillon, Sabrina Asaad, Qian Sun, Aniceta A. Mikulska-Sauermann, Marta Karaźniewicz-Łada, Franciszek K. Główka, and et al. 2022. "Selenium Status and Supplementation Effects in Pregnancy—A Study on Mother–Child Pairs from a Single-Center Cohort" Nutrients 14, no. 15: 3082. https://doi.org/10.3390/nu14153082
APA StyleFilipowicz, D., Szczepanek-Parulska, E., Kłobus, M., Szymanowski, K., Chillon, T. S., Asaad, S., Sun, Q., Mikulska-Sauermann, A. A., Karaźniewicz-Łada, M., Główka, F. K., Wietrzyk, D., Schomburg, L., & Ruchała, M. (2022). Selenium Status and Supplementation Effects in Pregnancy—A Study on Mother–Child Pairs from a Single-Center Cohort. Nutrients, 14(15), 3082. https://doi.org/10.3390/nu14153082









