Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions
Abstract
:1. Introduction: Life, Health, and Circadian Rhythms
2. Nutrition: Not Only Light Acts as (De)Synchronizer
3. Chrononutrition and Food Components Affecting Circadian Clocks
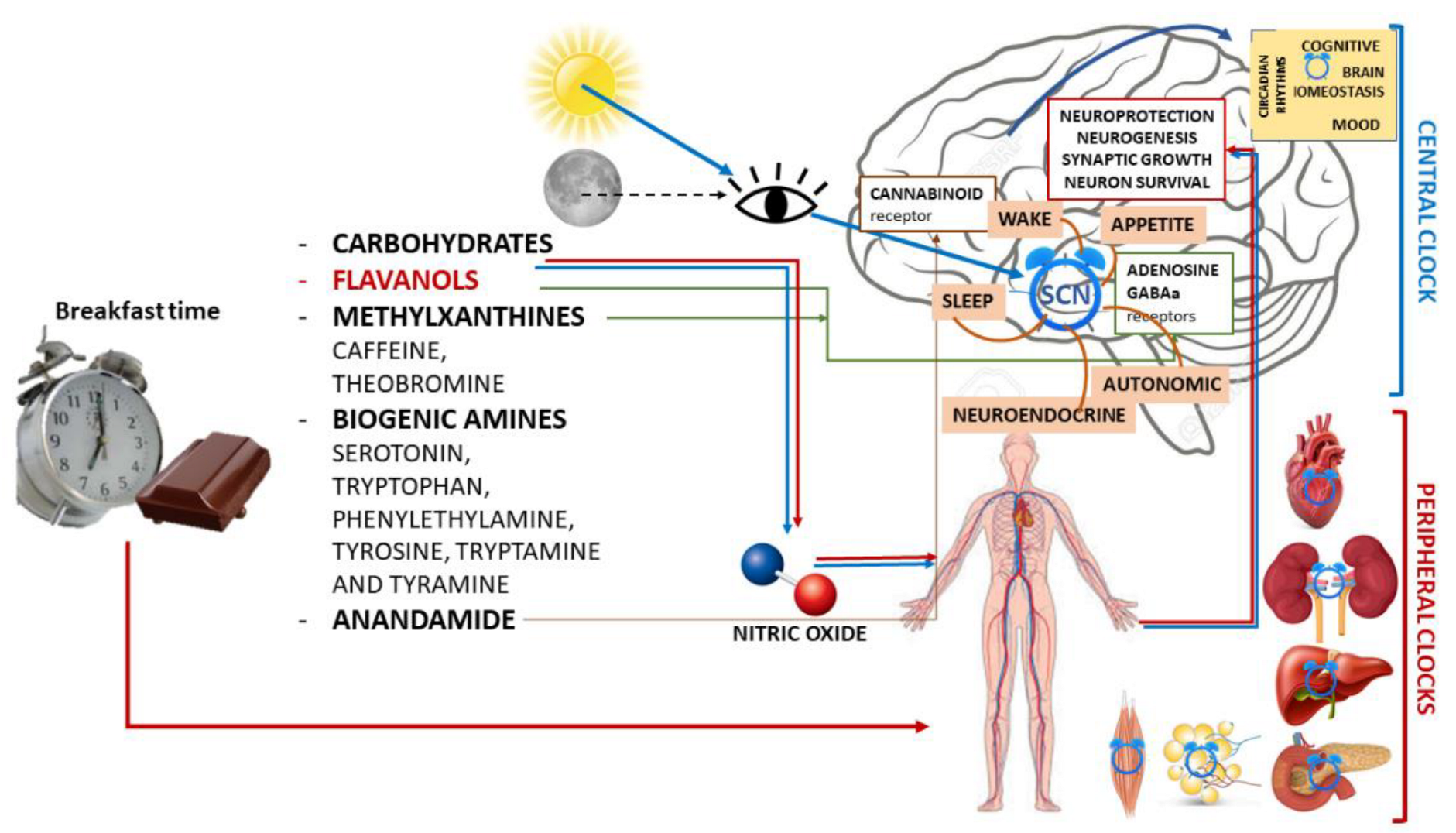
3.1. Chocolate
3.1.1. Biochemical Components and Neurobiological Impact
- -
- Carbohydrates, which have known behavioral effects.
- -
- Flavanols, which are ubiquitous in the plant kingdom. In foods normally consumed in the diet, high levels of flavonoids can be found in green and black tea, grapes, red wine, apples, and especially in cocoa and cocoa-containing products. In fact, cocoa is particularly rich in flavonoids and contains a distinct complement of flavanols (a subclass of flavonoids), flavan-3-ols, mainly present in the form of epicatechin and catechin [50], and their derivatives in high concentrations [56]. Flavan-3-ols are the building blocks for polymeric procyanidin type B-2.
- -
- Methylxanthines (MX), such as caffeine and its highly fat-soluble derivative and metabolite theobromine, which have peak plasma levels 60–120 min after ingestion.Like caffeine, theobromine binds to adenosine receptors, exhibiting its psychoactive potential similar to that of caffeine. However, these two MX have distinct functional binding properties.
- -
- Biogenic amines, such as serotonin, tryptophan, phenylethylamine, tyrosine, tryptamine, and tyramine, have a concentration that increases during fermentation and decreases during roasting and alkalinization.
- -
- Anandamide, an endogenous ligand for the cannabinoid receptor that is found in low quantities, such as 0.5 mg g−1, salsolinol, and tetrahydro-b-carboline.
3.1.2. Chocolate and Brain Functions
3.1.3. Chocolate and Mood
3.1.4. Chocolate, Sleep and Circadian Rhythms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeGates, T.; Fernandez, D.C.; Hattar, S. Light as a central modulator of circadian rhythms, sleep and affect. Nat. Rev. Neurosci. 2014, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Oike, H.; Oishi, K.; Kobori, M. Nutrients, Clock Genes, and Chrononutrition. Curr. Nutr. Rep. 2014, 3, 204–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef]
- Ospri, L.L.; Prusky, G.; Hattar, S. Mood, the Circadian System, and Melanopsin Retinal Ganglion Cells. Annu. Rev. Neurosci. 2017, 40, 539–556. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Ramsey, K.M.; Marcheva, B.; Bass, J. Circadian rhythms, sleep, and metabolism. J. Clin. Investig. 2011, 121, 2133–2141. [Google Scholar] [CrossRef]
- Noh, J.-Y.; Han, D.-H.; Yoon, J.-A.; Kim, M.-H.; Kim, S.-E.; Ko, I.-G.; Kim, K.-H.; Kim, C.-J.; Cho, S. Circadian Rhythms in Urinary Functions: Possible Roles of Circadian Clocks? Int. Neurourol. J. 2011, 15, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Pickard, G.E. Circadian rhythm of nociception in the golden hamster. Brain Res. 1987, 425, 395–400. [Google Scholar] [CrossRef]
- Refinetti, R. Rhythms of temperature selection and body temperature are out of phase in the golden hamster. Behav. Neurosci. 1995, 109, 523–527. [Google Scholar] [CrossRef]
- Lockley, S.W.; Evans, E.E.; Scheer, F.; Brainard, G.C.; Czeisler, C.A.; Aeschbach, D. Short-Wavelength Sensitivity for the Direct Effects of Light on Alertness, Vigilance, and the Waking Electroencephalogram in Humans. Sleep 2006, 29, 161–168. [Google Scholar] [CrossRef]
- Johnson, M.P.; Duffy, J.F.; Dijk, D.J.; Ronda, J.M.; Dyal, C.M.; Czeisler, C.A. Short-term memory, alertness and performance: A reappraisal of their relationship to body temperature. J. Sleep Res. 1992, 1, 24–29. [Google Scholar] [CrossRef]
- Foster, R.G.; Kreitzman, L. The rhythms of life: What your body clock means to you! Exp. Physiol. 2013, 99, 599–606. [Google Scholar] [CrossRef]
- Boivin, D.B.; Czeisler, C.A.; Dijk, D.-J.; Duffy, J.F.; Folkard, S.; Minors, D.S.; Totterdell, P.; Waterhouse, J.M. Complex Interaction of the Sleep-Wake Cycle and Circadian Phase Modulates Mood in Healthy Subjects. Arch. Gen. Psychiatry 1997, 54, 145–152. [Google Scholar] [CrossRef]
- Birchler-Pedross, A.; Schröder, C.M.; Münch, M.; Knoblauch, V.; Blatter, K.; Schnitzler-Sack, C.; Wirz-Justice, A.; Cajochen, C. Subjective Well-Being Is Modulated by Circadian Phase, Sleep Pressure, Age, and Gender. J. Biol. Rhythm. 2009, 24, 232–242. [Google Scholar] [CrossRef]
- Murray, G.; Allen, N.B.; Trinder, J. Mood and the circadian system: Investigation of a circadian component in positive affect. Chronobiol. Int. 2002, 19, 1151–1169. [Google Scholar] [CrossRef]
- Tahara, Y.; Shibata, S. Chronobiology and nutrition. Neuroscience 2013, 253, 78–88. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Yang, C.; Tong, X.; Sun, H.; Cong, Y.; Yin, X.; Li, L.; Cao, S.; Dong, X.; Gong, Y.; et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 2014, 72, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.J. Lifestyle and Circadian Health: Where the Challenges Lie? Nutr. Metab. Insights 2019, 12. [Google Scholar] [CrossRef]
- Hughes, M.E.; DiTacchio, L.; Hayes, K.R.; Vollmers, C.; Pulivarthy, S.; Baggs, J.E.; Panda, S.; HogenEsch, J.B. Harmonics of Circadian Gene Transcription in Mammals. PLoS Genet. 2009, 5, e1000442. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, H.; Tahara, Y.; Saito, K.; Ohnishi, N.; Kubo, Y.; Seo, Y.; Otsuka, M.; Fuse, Y.; Ohura, Y.; Hirao, A.; et al. Meal frequency patterns determine the phase of mouse peripheral circadian clocks. Sci. Rep. 2012, 2, 711. [Google Scholar] [CrossRef] [Green Version]
- Ángeles-Castellanos, M.; Salgado-Delgado, R.; Rodríguez, K.; Buijs, R.; Escobar, C. Expectancy for food or expectancy for chocolate reveals timing systems for metabolism and reward. Neuroscience 2008, 155, 297–307. [Google Scholar] [CrossRef]
- Feillet, C.A.; Albrecht, U.; Challet, E. “Feeding time” for the brain: A matter of clocks. J. Physiol. Paris 2006, 100, 252–260. [Google Scholar] [CrossRef]
- Verwey, M.; Amir, S. Food-entrainable circadian oscillators in the brain. Eur. J. Neurosci. 2009, 30, 1650–1657. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Jin, Y.; Ni, Y.; Zhang, D.; Kato, H.; Fu, Z. Effects of light cues on re-entrainment of the food-dominated peripheral clocks in mammals. Gene 2008, 419, 27–34. [Google Scholar] [CrossRef]
- Hara, R.; Wan, K.; Wakamatsu, H.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells 2001, 6, 269–278. [Google Scholar] [CrossRef]
- Stokkan, K.-A.; Yamazaki, S.; Tei, H.; Sakaki, Y.; Menaker, M. Entrainment of the Circadian Clock in the Liver by Feeding. Science 2001, 291, 490–493. [Google Scholar] [CrossRef] [Green Version]
- Damiola, F.; Minh, N.L.; Preitner, N.; Kornmann, B.; Fleury-Olela, F.; Schibler, U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [Green Version]
- Hirao, A.; Nagahama, H.; Tsuboi, T.; Hirao, M.; Tahara, Y.; Shibata, S. Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. Am. J. Physiol. Liver Physiol. 2010, 299, G1045–G1053. [Google Scholar] [CrossRef] [Green Version]
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.B.; Patterson, R.E.; Ang, A.; Emond, J.A.; Shetty, N.; Arab, L. Timing of energy intake during the day is associated with the risk of obesity in adults. J. Hum. Nutr. Diet. 2013, 27, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Hirao, A.; Tahara, Y.; Kimura, I.; Shibata, S. A Balanced Diet Is Necessary for Proper Entrainment Signals of the Mouse Liver Clock. PLoS ONE 2009, 4, e6909. [Google Scholar] [CrossRef] [Green Version]
- Itokawa, M.; Hirao, A.; Nagahama, H.; Otsuka, M.; Ohtsu, T.; Furutani, N.; Hirao, K.; Hatta, T.; Shibata, S. Time-restricted feeding of rapidly digested starches causes stronger entrainment of the liver clock in PER2::LUCIFERASE knock-in mice. Nutr. Res. 2013, 33, 109–119. [Google Scholar] [CrossRef]
- Micó, V.; Díez-Ricote, L.; Daimiel, L. Nutrigenetics and Nutrimiromics of the Circadian System: The Time for Human Health. Int. J. Mol. Sci. 2016, 17, 299. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Yao, C.; Huang, L.; Mao, Y.; Zhang, W.; Jiang, J.; Fu, Z. Nutrients and Circadian Rhythms in Mammals. J. Nutr. Sci. Vitaminol. 2015, 61, S89–S91. [Google Scholar] [CrossRef] [Green Version]
- Oosterman, J.E.; Kalsbeek, A.; la Fleur, S.E.; Belsham, D.D. Impact of nutrients on circadian rhythmicity. Am. J. Physiol. Integr. Comp. Physiol. 2015, 308, R337–R350. [Google Scholar] [CrossRef] [Green Version]
- Eckel-Mahan, K.L.; Patel, V.R.; Mohney, R.P.; Vignola, K.S.; Baldi, P.; Sassone-Corsi, P. Coordination of the transcriptome and metabolome by the circadian clock. Proc. Natl. Acad. Sci. USA 2012, 109, 5541–5546. [Google Scholar] [CrossRef] [Green Version]
- Oishi, K.; Uchida, D.; Ohkura, N.; Doi, R.; Ishida, N.; Kadota, K.; Horie, S. Ketogenic Diet Disrupts the Circadian Clock and Increases Hypofibrinolytic Risk by Inducing Expression of Plasminogen Activator Inhibitor-1. Arter. Thromb. Vasc. Biol. 2009, 29, 1571–1577. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.N.; Ho, K.; Crocker, A.; Yue, Z.; Koh, K.; Sehgal, A. The Effects of Caffeine on Sleep in Drosophila Require PKA Activity, But Not the Adenosine Receptor. J. Neurosci. 2009, 29, 11029–11037. [Google Scholar] [CrossRef] [Green Version]
- Oike, H.; Kobori, M.; Suzuki, T.; Ishida, N. Caffeine lengthens circadian rhythms in mice. Biochem. Biophys. Res. Commun. 2011, 410, 654–658. [Google Scholar] [CrossRef]
- Feldman, J.F. Circadian Periodicity in Neurospora: Alteration by Inhibitors of Cyclic AMP Phosphodiesterase. Science 1975, 190, 789–790. [Google Scholar] [CrossRef]
- Goodenough, J.E.; Bruce, V.G. The effects of caffeine and theophylline on the phototactic rhythm of Chlamydomonas Reinhardtii. Biol. Bull. 1980, 159, 649–655. [Google Scholar] [CrossRef]
- Bollig, I.; Mayer, K.; Mayer, W.-E.; Engelmann, W. Effects of cAMP, theophylline, imidazole, and 4-(3,4-dimethoxybenzyl)-2-imidazolidone on the leaf movement rhythm of Trifolium repens?a test of the cAMP-hypothesis of circadian rhythms. Planta 1978, 141, 225–230. [Google Scholar] [CrossRef]
- Socci, V.; Tempesta, D.; Desideri, G.; De Gennaro, L.; Ferrara, M. Enhancing Human Cognition with Cocoa Flavonoids. Front. Nutr. 2017, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.J.; Spencer, J.P. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radic. Biol. Med. 2012, 52, 35–45. [Google Scholar] [CrossRef]
- Hurst, W.J.; Jr, S.M.T.; Powis, T.G.; Valdez, F.; Hester, T.R. Cacao usage by the earliest Maya civilization. Nature 2002, 418, 289–290. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation as referred to in Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3654. [Google Scholar]
- Whiting, D.A. Natural phenolic compounds 1900–2000: A bird’s eye view of a century’s chemistry. Nat. Prod. Rep. 2001, 18, 583–606. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Pavlova, M.A.; Klosterhalfen, S.; Enck, P. Chocolate and the brain: Neurobiological impact of cocoa flavanols on cognition and behavior. Neurosci. Biobehav. Rev. 2013, 37 Pt 2, 2445–2453. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and Chocolate in Human Health and Disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef] [Green Version]
- Chemistry Behind the Sweet Lure of Chocolate. Available online: https://www.worldofchemicals.com/113/chemistry-articles/chemistry-behind-the-sweet-lure-of-chocolate.html (accessed on 22 December 2021).
- Scholey, A.; Owen, L. Effects of chocolate on cognitive function and mood: A systematic review. Nutr. Rev. 2013, 71, 665–681. [Google Scholar] [CrossRef]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Richelle, M.; Tavazzi, I.; Enslen, M.; Offord, E. Plasma kinetics in man of epicatechin from black chocolate. Eur. J. Clin. Nutr. 1999, 53, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.-O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2010, 2, 39–44. [Google Scholar] [CrossRef]
- Ghosh, D.; Scheepens, A. Vascular action of polyphenols. Mol. Nutr. Food Res. 2009, 53, 322–331. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. NeuroReport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Spencer, J.P.E. Food for thought: The role of dietary flavonoids in enhancing human memory, learning and neuro-cognitive performance. Proc. Nutr. Soc. 2008, 67, 238–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelleher, R.J.; Govindarajan, A.; Jung, H.-Y.; Kang, H.; Tonegawa, S. Translational Control by MAPK Signaling in Long-Term Synaptic Plasticity and Memory. Cell 2004, 116, 467–479. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Jeon, S.J.; Son, K.H.; Jung, J.W.; Lee, S.; Yoon, B.H.; Choi, J.W.; Cheong, J.H.; Ko, K.H.; Ryu, J.H. Effect of the flavonoid, oroxylin A, on transient cerebral hypoperfusion-induced memory impairment in mice. Pharmacol. Biochem. Behav. 2006, 85, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.; Hidalgo, J.; Bolea, I.; Ramirez, B.; Anglés, N.; Reguant, J.; Morelló, J.R.; Gutiérrez, C.; Boada, M.; Unzeta, M. A Diet Enriched in Polyphenols and Polyunsaturated Fatty Acids, LMN Diet, Induces Neurogenesis in the Subventricular Zone and Hippocampus of Adult Mouse Brain. J. Alzheimer’s Dis. 2009, 18, 849–865. [Google Scholar] [CrossRef]
- González-Gallego, J.; Sánchez-Campos, S.; Tuñón, M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007, 22, 287–293. [Google Scholar]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Flammer, A.J.; Hermann, F.; Sudano, I.; Spieker, L.; Hermann, M.; Cooper, K.A.; Serafini, M.; Lüscher, T.F.; Ruschitzka, F.; Noll, G.; et al. Dark Chocolate Improves Coronary Vasomotion and Reduces Platelet Reactivity. Circulation 2007, 116, 2376–2382. [Google Scholar] [CrossRef] [Green Version]
- Grassi, D.; Necozione, S.; Lippi, C.; Croce, G.; Valeri, L.; Pasqualetti, P.; Desideri, G.; Blumberg, J.B.; Ferri, C. Cocoa Reduces Blood Pressure and Insulin Resistance and Improves Endothelium-Dependent Vasodilation in Hypertensives. Hypertension 2005, 46, 398–405. [Google Scholar] [CrossRef] [Green Version]
- Grassi, D.; Desideri, G.; Necozione, S.; Lippi, C.; Casale, R.; Properzi, G.; Blumberg, J.B.; Ferri, C. Blood Pressure Is Reduced and Insulin Sensitivity Increased in Glucose-Intolerant, Hypertensive Subjects after 15 Days of Consuming High-Polyphenol Dark Chocolate. J. Nutr. 2008, 138, 1671–1676. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Ferri, C. Protective effects of dark chocolate on endothelial function and diabetes. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 662–668. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Necozione, S.; di Giosia, P.; Barnabei, R.; Allegaert, L.; Bernaert, H.; Ferri, C. Cocoa consumption dose-dependently improves flow-mediated dilation and arterial stiffness decreasing blood pressure in healthy individuals. J. Hypertens. 2015, 33, 294–303. [Google Scholar] [CrossRef]
- Shrime, M.G.; Bauer, S.R.; McDonald, A.C.; Chowdhury, N.H.; Coltart, C.E.M.; Ding, E.L. Flavonoid-Rich Cocoa Consumption Affects Multiple Cardiovascular Risk Factors in a Meta-Analysis of Short-Term Studies. J. Nutr. 2011, 141, 1982–1988. [Google Scholar] [CrossRef]
- Hollenberg, N.K.; Fisher, N.D.; McCullough, M.L. Flavanols, the Kuna, cocoa consumption, and nitric oxide. J. Am. Soc. Hypertens. 2009, 3, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent Research on Polyphenolics in Vision and Eye Health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef]
- Grassi, D.; Ferri, C.; Desideri, G. Brain Protection and Cognitive Function: Cocoa Flavonoids as Nutraceuticals. Curr. Pharm. Des. 2015, 22, 145–151. [Google Scholar] [CrossRef]
- Gage, F.H. Mammalian Neural Stem Cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef]
- Van Praag, H.; Lucero, M.J.; Yeo, E.; Stecker, K.; Heivand, N.; Zhao, C.; Yip, E.; Afanador, M.; Schroeter, H.; Hammerstone, J.; et al. Plant-Derived Flavanol (-)Epicatechin Enhances Angiogenesis and Retention of Spatial Memory in Mice. J. Neurosci. 2007, 27, 5869–5878, Erratum in J. Neurosci. 2007, 27. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Crichton, G.E.; Elias, M.F.; Alkerwi, A. Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study. Appetite 2016, 100, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Commenges, D.; Scotet, V.; Renaud, S.; Jacqmin-Gadda, H.; Barberger-Gateau, P.; Dartigues, J.-F. Intake of flavonoids and risk of dementia. Eur. J. Epidemiol. 2000, 16, 357–363. [Google Scholar] [CrossRef]
- Moreira, A.; Diógenes, M.J.; de Mendonça, A.; Lunet, N.; Barros, H. Chocolate Consumption is Associated with a Lower Risk of Cognitive Decline. J. Alzheimer’s Dis. 2016, 53, 85–93. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hozawa, A.; Ohmori, K.; Shimazu, T.; Matsui, T.; Ebihara, S.; Awata, S.; Nagatomi, R.; Arai, H.; Tsuji, I. Green tea consumption and cognitive function: A cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006, 83, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid Intake and Cognitive Decline over a 10-Year Period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef]
- Nurk, E.; Refsum, H.; Drevon, C.A.; Tell, G.S.; Nygaard, H.A.; Engedal, K.; Smith, A.D. Intake of Flavonoid-Rich Wine, Tea, and Chocolate by Elderly Men and Women Is Associated with Better Cognitive Test Performance. J. Nutr. 2008, 139, 120–127. [Google Scholar] [CrossRef]
- Smit, H.J.; Rogers, P.J. Effects of low doses of caffeine on cognitive performance, mood and thirst in low and higher caffeine consumers. Psychopharmacology 2000, 152, 167–173. [Google Scholar] [CrossRef]
- Smit, H.J.; Gaffan, E.A.; Rogers, P.J. Methylxanthines are the psycho-pharmacologically active constituents of chocolate. Psychopharmacology 2004, 176, 412–419. [Google Scholar] [CrossRef]
- Smit, H.J.; Blackburn, R.J. Reinforcing effects of caffeine and theobromine as found in chocolate. Psychopharmacology 2005, 181, 101–106. [Google Scholar] [CrossRef]
- Nehlig, A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010, 20, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Parker, G.; Roy, K.; Mitchell, P.; Wilhelm, K.; Malhi, G.; Hadzi-Pavlovic, D. Atypical Depression: A Reappraisal. Am. J. Psychiatry 2002, 159, 1470–1479. [Google Scholar] [CrossRef]
- Messaoudi, M.; Bisson, J.-F.; Nejdi, A.; Rozan, P.; Javelot, H. Antidepressant-like effects of a cocoa polyphenolic extract in Wistar–Unilever rats. Nutr. Neurosci. 2008, 11, 269–276. [Google Scholar] [CrossRef]
- Benton, D.; Donohoe, R.T. The effects of nutrients on mood. Public Health Nutr. 1999, 2, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reid, L.D. Endogenous opioid peptides and regulation of drinking and feeding. Am. J. Clin. Nutr. 1985, 42, 1099–1132. [Google Scholar] [CrossRef] [PubMed]
- Giraudo, S.; Grace, M.; Welch, C.; Billington, C.; Levine, A. Naloxone’s anorectic effect is dependant upon the relative palatability of food. Pharmacol. Biochem. Behav. 1993, 46, 917–921. [Google Scholar] [CrossRef]
- Young, S.N.; Smith, S.E.; Pihl, R.O.; Ervin, F.R. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology 1985, 87, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Si, E.; Bryant, H.; Yim, G. Opioid and non-opioid components of insulin-induced feeding. Pharmacol. Biochem. Behav. 1986, 24, 899–903. [Google Scholar] [CrossRef]
- Ottley, C. Food and mood. Nurs. Stand. 2000, 15, 46–52, quiz 54–55. [Google Scholar]
- Parker, G.; Parker, I.; Brotchie, H. Mood state effects of chocolate. J. Affect. Disord. 2006, 92, 149–159. [Google Scholar] [CrossRef]
- Fullerton, D.T.; Getto, C.J.; Swift, W.J.; Carlson, I.H. Sugar, opioids and binge eating. Brain Res. Bull. 1985, 14, 673–680. [Google Scholar] [CrossRef]
- Willner, P.; Benton, D.; Brown, E.; Cheeta, S.; Roderique-Davies, G.; Morgan, J.; Morgan, M. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacology 1998, 136, 272–283. [Google Scholar] [CrossRef]
- Hetherington, M.M.; MacDiarmid, J.I. “Chocolate Addiction”: A Preliminary Study of its Description and its Relationship to Problem Eating. Appetite 1993, 21, 233–246. [Google Scholar] [CrossRef]
- Rozin, P.; Levine, E.; Stoess, C. Chocolate craving and liking. Appetite 1991, 17, 199–212. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; De Graaf, C.; Stafleu, A.; Van Osch, M.J.P.; Nievelstein, R.A.J.; Van Der Grond, J. Effect of satiety on brain activation during chocolate tasting in men and women. Am. J. Clin. Nutr. 2006, 83, 1297–1305. [Google Scholar] [CrossRef]
- Michener, W.; Rozin, P. Pharmacological versus sensory factors in the satiation of chocolate craving. Physiol. Behav. 1994, 56, 419–422. [Google Scholar] [CrossRef]
- Smits, M.; Peeters, R.R.; Van Hecke, P.; Sunaert, S. A 3 T event-related functional magnetic resonance imaging (fMRI) study of primary and secondary gustatory cortex localization using natural tastants. Neuroradiology 2006, 49, 61–71. [Google Scholar] [CrossRef]
- Beaver, J.D.; Lawrence, A.D.; van Ditzhuijzen, J.; Davis, M.; Woods, A.; Calder, A.J. Individual Differences in Reward Drive Predict Neural Responses to Images of Food. J. Neurosci. 2006, 26, 5160–5166. [Google Scholar] [CrossRef] [Green Version]
- Martin, G. Human electroencephalographic (EEG) response to olfactory stimulation: Two experiments using the aroma of food. Int. J. Psychophysiol. 1998, 30, 287–302. [Google Scholar] [CrossRef]
- Rolls, E.T.; McCabe, C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur. J. Neurosci. 2007, 26, 1067–1076. [Google Scholar] [CrossRef]
- Nehlig, A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav. Rev. 1999, 23, 563–576. [Google Scholar] [CrossRef]
- Di Chiara, G. Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behav. Brain Res. 2002, 137, 75–114. [Google Scholar] [CrossRef]
- Schroeder, B.; Binzak, J.; Kelley, A. A common profile of prefrontal cortical activation following exposure to nicotine- or chocolate-associated contextual cues. Neuroscience 2001, 105, 535–545. [Google Scholar] [CrossRef]
- Medina, J.H.; Viola, H.; Wolfman, C.; Marder, M.; Wasowski, C.; Calvo, D.; Paladini, A.C. Overview—Flavonoids: A New Family of Benzodiazepine Receptor Ligands. Neurochem. Res. 1997, 22, 419–425. [Google Scholar] [CrossRef]
- Vignes, M.; Maurice, T.; Lanté, F.; Nedjar, M.; Thethi, K.; Guiramand, J.; Récasens, M. Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG). Brain Res. 2006, 1110, 102–115. [Google Scholar] [CrossRef]
- Sathyapalan, T.; Beckett, S.; Rigby, A.S.; Mellor, D.D.; Atkin, S.L. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr. J. 2010, 9, 55. [Google Scholar] [CrossRef] [Green Version]
- Pase, M.P.; Scholey, A.B.; Pipingas, A.; Kras, M.; Nolidin, K.; Gibbs, A.; Wesnes, K.; Stough, C. Cocoa polyphenols enhance positive mood states but not cognitive performance: A randomized, placebo-controlled trial. J. Psychopharmacol. 2013, 27, 451–458. [Google Scholar] [CrossRef]
- Buijs, F.N.; Leon, L.; Guzmán-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31, 170–181. [Google Scholar] [CrossRef]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Knutson, K.L.; Spiegel, K.; Penev, P.; Van Cauter, E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007, 11, 163–178. [Google Scholar] [CrossRef] [Green Version]
- Uehli, K.; Mehta, A.J.; Miedinger, D.; Hug, K.; Schindler, C.; Holsboer-Trachsler, E.; Leuppi, J.D.; Künzli, N. Sleep problems and work injuries: A systematic review and meta-analysis. Sleep Med. Rev. 2013, 18, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Kalmbach, D.A.; Anderson, J.R.; Drake, C.L. The impact of stress on sleep: Pathogenic sleep reactivity as a vulnerability to insomnia and circadian disorders. J. Sleep Res. 2018, 27, e12710. [Google Scholar] [CrossRef] [Green Version]
- Escobar, C.; Espitia-Bautista, E.; Guzmán-Ruiz, M.A.; Vargas, N.N.G.; Hernández-Navarrete, M.; Ángeles-Castellanos, M.; Morales-Pérez, B.; Buijs, R.M. Chocolate for breakfast prevents circadian desynchrony in experimental models of jet-lag and shift-work. Sci. Rep. 2020, 10, 6243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, J.; Angeles-Castellanos, M.; Escobar, C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur. J. Neurosci. 2005, 22, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Numano, R.; Abe, M.; Hida, A.; Takahashi, R.-I.; Ueda, M.; Block, G.D.; Sakaki, Y.; Menaker, M.; Tei, H. Resetting Central and Peripheral Circadian Oscillators in Transgenic Rats. Science 2000, 288, 682–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado-Delgado, R.C.; Saderi, N.; Basualdo, M.D.C.; Guerrero-Vargas, N.N.; Escobar, C.; Buijs, R.M. Shift Work or Food Intake during the Rest Phase Promotes Metabolic Disruption and Desynchrony of Liver Genes in Male Rats. PLoS ONE 2013, 8, e60052. [Google Scholar] [CrossRef] [Green Version]
- Schilperoort, M.; van den Berg, R.; Dollé, M.E.T.; Van Oostrom, C.T.M.; Wagner, K.; Tambyrajah, L.L.; Wackers, P.; DeBoer, T.; Hulsegge, G.; Proper, K.I.; et al. Time-restricted feeding improves adaptation to chronically alternating light-dark cycles. Sci. Rep. 2019, 9, 7874. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Damara, M.; Misra, N.; Meziane, H.; Champy, M.-F.; Chambon, P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl. Acad. Sci. USA 2015, 112, E6691–E6698. [Google Scholar] [CrossRef] [Green Version]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metabolism 2016, 65, 714–727. [Google Scholar] [CrossRef]
- Ekmekcioglu, C.; Touitou, Y. Chronobiological aspects of food intake and metabolism and their relevance on energy balance and weight regulation. Obes. Rev. 2010, 12, 14–25. [Google Scholar] [CrossRef]
- Wakamatsu, H.; Yoshinobu, Y.; Aida, R.; Moriya, T.; Akiyama, M.; Shibata, S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur. J. Neurosci. 2001, 13, 1190–1196. [Google Scholar] [CrossRef]
- Herrera, D.; Robertson, H. Activation of c-fos in the brain. Prog. Neurobiol. 1996, 50, 83–107. [Google Scholar] [CrossRef]
- Greco, C.; Sassone–Corsi, P. Circadian blueprint of metabolic pathways in the brain. Nat. Rev. Neurosci. 2018, 20, 71–82. [Google Scholar] [CrossRef]
- Acosta-Galvan, G.; Yi, C.-X.; van der Vliet, J.; Jhamandas, J.H.; Panula, P.; Angeles-Castellanos, M.; Del Basualdo, M.C.; Escobar, C.; Buijs, R.M. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 5813–5818. [Google Scholar] [CrossRef] [Green Version]
- Saderi, N.; Cazarez-Márquez, F.; Buijs, F.; Salgado-Delgado, R.; Guzman-Ruiz, M.; Basualdo, M.D.C.; Escobar, C.; Buijs, R. The NPY intergeniculate leaflet projections to the suprachiasmatic nucleus transmit metabolic conditions. Neuroscience 2013, 246, 291–300. [Google Scholar] [CrossRef]
- Grippo, R.M.; Purohit, A.M.; Zhang, Q.; Zweifel, L.S.; Güler, A.D. Direct Midbrain Dopamine Input to the Suprachiasmatic Nucleus Accelerates Circadian Entrainment. Curr. Biol. 2017, 27, 2465–2475.e3. [Google Scholar] [CrossRef]
- Ubaldo, L.; Buijs, R.; Escobar, C.; Angeles-Castellanos, M. Scheduled meal accelerates entrainment to a 6-h phase advance by shifting central and peripheral oscillations in rats. Eur. J. Neurosci. 2017, 46, 1875–1886. [Google Scholar] [CrossRef]
- Ángeles-Castellanos, M.; Amaya, J.M.; Salgado-Delgado, R.; Buijs, R.M.; Escobar, C. Scheduled Food Hastens Re-Entrainment More Than Melatonin Does after a 6-h Phase Advance of the Light-Dark Cycle in Rats. J. Biol. Rhythm. 2011, 26, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food Intake during the Normal Activity Phase Prevents Obesity and Circadian Desynchrony in a Rat Model of Night Work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef]
- Guerrero-Vargas, N.N.; Espitia-Bautista, E.; Buijs, R.M.; Escobar, C. Shift-work: Is time of eating determining metabolic health? Evidence from animal models. Proc. Nutr. Soc. 2018, 77, 199–215. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Chaix, A.; Manoogian, E.N.; Melkani, G.C.; Panda, S. Time-Restricted Eating to Prevent and Manage Chronic Metabolic Diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Oishi, K.; Okauchi, H.; Yamamoto, S.; Higo-Yamamoto, S. Dietary natural cocoa ameliorates disrupted circadian rhythms in locomotor activity and sleep-wake cycles in mice with chronic sleep disorders caused by psychophysiological stress. Nutrition 2020, 75–76, 110751. [Google Scholar] [CrossRef]
- Villafuerte, G.; Miguel-Puga, A.; Rodríguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrión, O. Sleep Deprivation and Oxidative Stress in Animal Models: A Systematic Review. Oxidative Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.S.; Dunbar, S.B.; Higgins, M.; Rogers, A.E.; Feeley, C.; Ioachimescu, O.; Euwer, K.; Eapen, D.; Quyyumi, A. Insomnia Symptoms Are Associated With Abnormal Endothelial Function. J. Cardiovasc. Nurs. 2017, 32, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.R.; Balkin, T.J.; Wesenten, N.J.; E Carson, R.; Varga, M.; Baldwin, P.; Selbie, S.; Belenky, G.; Herscovitch, P. Regional cerebral blood flow throughout the sleep-wake cycle. An H2(15)O PET study. Brain 1997, 120 Pt 7, 1173–1197. [Google Scholar] [CrossRef] [PubMed]
- Elvsåshagen, T.; Mutsaerts, H.J.; Zak, N.; Norbom, L.; Quraishi, S.H.; Pedersen, P.Ø.; Malt, U.F.; Westlye, L.T.; van Someren, E.J.; Bjørnerud, A.; et al. Cerebral blood flow changes after a day of wake, sleep, and sleep deprivation. NeuroImage 2018, 186, 497–509. [Google Scholar] [CrossRef]
- Pich, E.M.; Solfrini, V.; Biagini, G.; Fuxe, K.; Agnati, L.F. Effects of indole-pyruvic acid on sleep and food intake in the rat. Acta Physiol. Scand. 1990, 139, 583–589. [Google Scholar] [CrossRef]
- Biagini, G.; Pich, E.M.; Carani, C.; Marrama, P.; Gustafsson, J.; Fuxe, K.; Agnati, L.F. Indole-pyruvic acid, a tryptophan ketoanalogue, antagonizes the endocrine but not the behavioral effects of repeated stress in a model of depression. Biol. Psychiatry 1993, 33, 712–719. [Google Scholar] [CrossRef]
- Yamada, T.; Yamada, Y.; Okano, Y.; Terashima, T.; Yokogoshi, H. Anxiolytic effects of short- and long-term administration of cacao mass on rat elevated T-maze test. J. Nutr. Biochem. 2009, 20, 948–955. [Google Scholar] [CrossRef]
- McCarty, M. Vascular nitric oxide may lessen Alzheimer’s risk. Med. Hypotheses 1998, 51, 465–476. [Google Scholar] [CrossRef]
- Golomb, B.A.; Koperski, S.; White, H.L. Association between more frequent chocolate consumption and lower body mass index. Arch. Intern. Med. 2012, 172, 519–521. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Ahren, B.; Barnea, M.; Bar-Dayan, Y.; Froy, O. High-energy breakfast based on whey protein reduces body weight, postprandial glycemia and HbA 1C in Type 2 diabetes. J. Nutr. Biochem. 2017, 49, 1–7. [Google Scholar] [CrossRef]
- A Betts, J.; Richardson, J.D.; Chowdhury, E.; Holman, G.; Tsintzas, K.; Thompson, D. The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Romon, M.; Edme, J.L.; Boulenguez, C.; Lescroart, J.L.; Frimat, P. Circadian variation of diet-induced thermogenesis. Am. J. Clin. Nutr. 1993, 57, 476–480. [Google Scholar] [CrossRef]
- Webb, I.C.; Baltazar, R.M.; Lehman, M.N.; Coolen, L.M. Bidirectional interactions between the circadian and reward systems: Is restricted food access a unique zeitgeber? Eur. J. Neurosci. 2009, 30, 1739–1748. [Google Scholar] [CrossRef]
- Ángeles-Castellanos, M.; Mendoza, J.; Escobar, C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience 2007, 144, 344–355. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbarino, S.; Garbarino, E.; Lanteri, P. Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions. Nutrients 2022, 14, 3113. https://doi.org/10.3390/nu14153113
Garbarino S, Garbarino E, Lanteri P. Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions. Nutrients. 2022; 14(15):3113. https://doi.org/10.3390/nu14153113
Chicago/Turabian StyleGarbarino, Sergio, Emanuela Garbarino, and Paola Lanteri. 2022. "Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions" Nutrients 14, no. 15: 3113. https://doi.org/10.3390/nu14153113
APA StyleGarbarino, S., Garbarino, E., & Lanteri, P. (2022). Cyrcadian Rhythm, Mood, and Temporal Patterns of Eating Chocolate: A Scoping Review of Physiology, Findings, and Future Directions. Nutrients, 14(15), 3113. https://doi.org/10.3390/nu14153113







