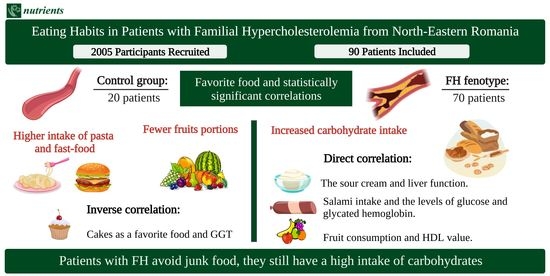
Eating Habits in Patients with Familial Hypercholesterolemia from North-Eastern Romania
Abstract
Share and Cite
Maștaleru, A.; Cojocariu, A.S.; Oancea, A.; Leon-Constantin, M.-M.; Roca, M.; Zota, I.M.; Abdulan, I.M.; Rusu, C.; Trandafir, L.M.; Costache, A.D.; et al. Eating Habits in Patients with Familial Hypercholesterolemia from North-Eastern Romania. Nutrients 2022, 14, 3124. https://doi.org/10.3390/nu14153124
Maștaleru A, Cojocariu AS, Oancea A, Leon-Constantin M-M, Roca M, Zota IM, Abdulan IM, Rusu C, Trandafir LM, Costache AD, et al. Eating Habits in Patients with Familial Hypercholesterolemia from North-Eastern Romania. Nutrients. 2022; 14(15):3124. https://doi.org/10.3390/nu14153124
Chicago/Turabian StyleMaștaleru, Alexandra, Alexandra Sabina Cojocariu, Andra Oancea, Maria-Magdalena Leon-Constantin, Mihai Roca, Ioana Mădălina Zota, Irina Mihaela Abdulan, Cristina Rusu, Laura Mihaela Trandafir, Alexandru Dan Costache, and et al. 2022. "Eating Habits in Patients with Familial Hypercholesterolemia from North-Eastern Romania" Nutrients 14, no. 15: 3124. https://doi.org/10.3390/nu14153124
APA StyleMaștaleru, A., Cojocariu, A. S., Oancea, A., Leon-Constantin, M.-M., Roca, M., Zota, I. M., Abdulan, I. M., Rusu, C., Trandafir, L. M., Costache, A. D., Cojocaru, E., Roca, I. C., & Mitu, F. (2022). Eating Habits in Patients with Familial Hypercholesterolemia from North-Eastern Romania. Nutrients, 14(15), 3124. https://doi.org/10.3390/nu14153124