The Combined Effect of Birth Weight and Lifestyle on Clustered Cardio-Metabolic Risk Factors in Children and Adolescents: A National School-Based Cross-Sectional Survey
Abstract
1. Introduction
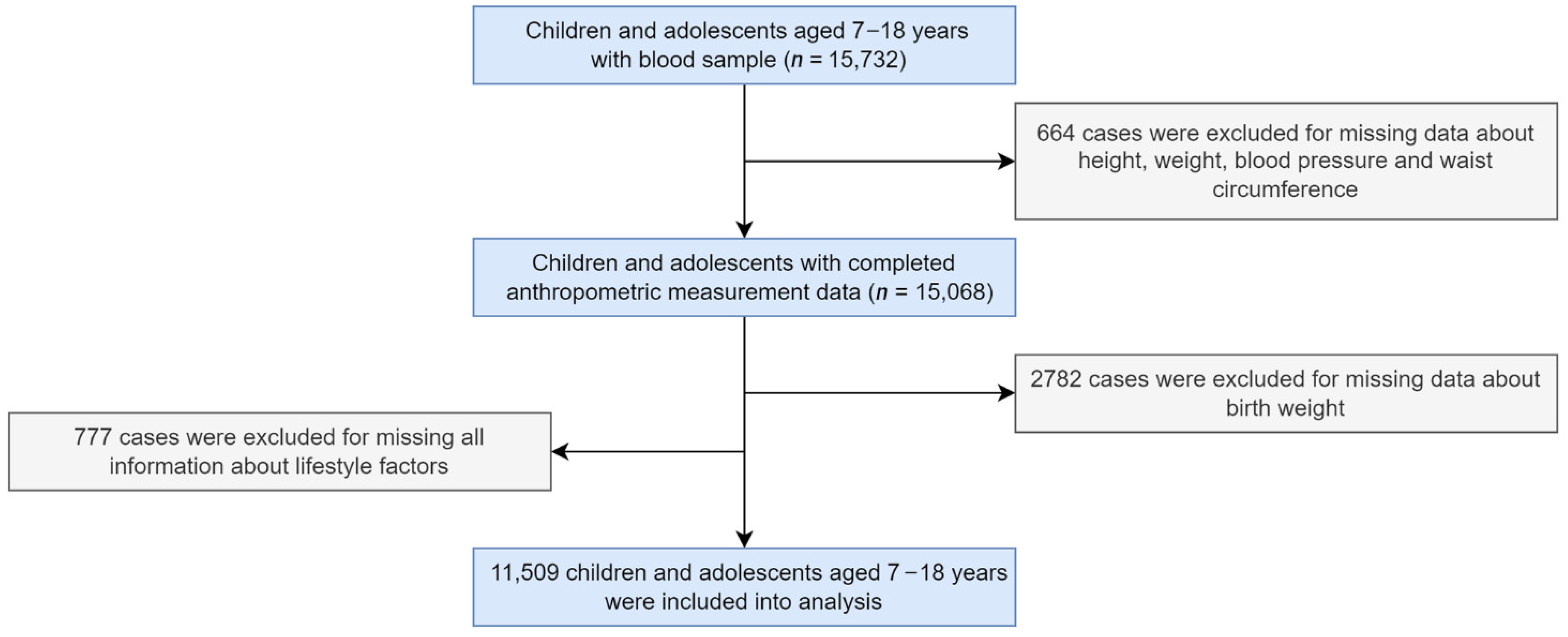
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Measurements
2.2.1. Questionnaire Survey
2.2.2. Anthropometric Measurement and Blood Sample Detection
2.3. Definition and Classification
2.3.1. Birth Weight
2.3.2. Lifestyle
2.3.3. Combination of Birth Weight and Lifestyle
2.3.4. Clustered CMRFs
2.3.5. Confounding Variables
2.4. Statistics Analysis
3. Results
3.1. The Characteristics of Participants
3.2. CMRFs and Clustered CMRFs in Different Groups
3.3. Multivariate Associations between CMRFs, Clustered CMRFs and Its Risk Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Du, A.; Liu, H.; Wang, Z.; Hu, J. Systematic Analysis of the Global, Regional and National Burden of Cardiovascular from 1990 to 2017. J. Epidemiol. Glob. Health 2022, 12, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Lung Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 125), S213–S256. [Google Scholar] [CrossRef]
- Jackson, S.L.; Zhang, Z.; Wiltz, J.L.; Loustalot, F.; Ritchey, M.D.; Goodman, A.B.; Yang, Q. Hypertension among Youths—United States, 2001–2016. MMWR Morb. Mortal. Wkly Rep. 2018, 67, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Kit, B.K.; Kuklina, E.; Carroll, M.D.; Ostchega, Y.; Freedman, D.S.; Ogden, C.L. Prevalence of and Trends in Dyslipidemia and Blood Pressure among US Children and Adolescents, 1999–2012. JAMA Pediatr. 2015, 169, 272–279. [Google Scholar] [CrossRef] [PubMed]
- May, A.L.; Kuklina, E.V.; Yoon, P.W. Prevalence of Cardiovascular Disease Risk Factors among US Adolescents, 1999−2008. Pediatrics 2012, 129, 1035–1041. [Google Scholar] [CrossRef]
- Perak, A.M.; Ning, H.; Kit, B.K.; de Ferranti, S.D.; Van Horn, L.V.; Wilkins, J.T.; Lloyd-Jones, D.M. Trends in Levels of Lipids and Apolipoprotein B in US Youths Aged 6 to 19 Years, 1999–2016. JAMA 2019, 321, 1895–1905. [Google Scholar] [CrossRef]
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P., 3rd; Tracy, R.E.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef]
- Juonala, M.; Viikari, J.S.; Kähönen, M.; Taittonen, L.; Laitinen, T.; Hutri-Kähönen, N.; Lehtimäki, T.; Jula, A.; Pietikäinen, M.; Jokinen, E.; et al. Life-time risk factors and progression of carotid atherosclerosis in young adults: The Cardiovascular Risk in Young Finns study. Eur. Heart J. 2010, 31, 1745–1751. [Google Scholar] [CrossRef]
- Li, S.; Chen, W.; Srinivasan, S.R.; Bond, M.G.; Tang, R.; Urbina, E.M.; Berenson, G.S. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA 2003, 290, 2271–2276. [Google Scholar] [CrossRef]
- Phillips, D.I.W.; Barker, D.J.P.; Hales, C.N.; Hirst, S.; Osmond, C. Thinness at birth and insulin resistance in adult life. Diabetologia 1994, 37, 150–154. [Google Scholar] [CrossRef]
- Davies, A.A.; Smith, G.D.; May, M.T.; Ben-Shlomo, Y. Association between Birth Weight and Blood Pressure Is Robust, Amplifies with Age, and May Be Underestimated. Hypertension 2006, 48, 431–436. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Hales, C.N.; Fall, C.H.D.; Osmond, C.; Phipps, K.; Clark, P.M.S. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): Relation to reduced fetal growth. Diabetologia 1993, 36, 62–67. [Google Scholar] [CrossRef]
- Stettler, N.; Stallings, V.A.; Troxel, A.B.; Zhao, J.; Schinnar, R.; Nelson, S.E.; Ziegler, E.E.; Strom, B.L. Weight gain in the first week of life and overweight in adulthood: A cohort study of European American subjects fed infant formula. Circulation 2005, 111, 1897–1903. [Google Scholar] [CrossRef]
- Vohr, B.R.; Allan, W.; Katz, K.H.; Schneider, K.C.; Ment, L.R. Early predictors of hypertension in prematurely born adolescents. Acta Paediatr. 2010, 99, 1812–1818. [Google Scholar] [CrossRef]
- Singhal, A.; Lucas, A. Early origins of cardiovascular disease: Is there a unifying hypothesis? Lancet 2004, 363, 1642–1645. [Google Scholar] [CrossRef]
- Fonseca, M.J.; Severo, M.; A Lawlor, D.; Barros, H.; Santos, A. Direct and BMI-mediated effect of birthweight on childhood cardio-metabolic health—A birth cohort study. Int. J. Obes. 2019, 43, 1923–1931. [Google Scholar] [CrossRef]
- Hovi, P.; Vohr, B.; Ment, L.R.; Doyle, L.W.; McGarvey, L.; Morrison, K.M.; Evensen, K.A.; van der Pal, S.; Grunau, R.E.; Brubakk, A.M.; et al. Blood Pressure in Young Adults Born at Very Low Birth Weight: Adults Born Preterm International Collaboration. Hypertension 2016, 68, 880–887. [Google Scholar] [CrossRef]
- Sørensen, H.T.; Sabroe, S.; Rothman, K.J.; Gillman, M.; Fischer, P.; Sørensen, T.I. Relation between weight and length at birth and body mass index in young adulthood: Cohort study. BMJ 1997, 315, 1137. [Google Scholar] [CrossRef]
- Seidman, D.S.; Laor, A.; Gale, R.; Stevenson, D.K.; Danon, Y.L. A longitudinal study of birth weight and being overweight in late adolescence. Am. J. Dis. Child. 1991, 145, 782–785. [Google Scholar] [CrossRef]
- Darendeliler, F.; Poyrazoglu, S.; Sancakli, O.; Bas, F.; Gokcay, G.; Aki, S.; Eskiyurt, N. Adiponectin is an indicator of insulin resistance in non-obese prepubertal children born large for gestational age (LGA) and is affected by birth weight. Clin. Endocrinol. 2009, 70, 710–716. [Google Scholar] [CrossRef]
- Giapros, V.; Evagelidou, E.; Challa, A.; Kiortsis, D.; Drougia, A.; Andronikou, S. Serum adiponectin and leptin levels and insulin resistance in children born large for gestational age are affected by the degree of overweight. Clin. Endocrinol. 2007, 66, 353–359. [Google Scholar]
- Dong, B.; Dong, Y.-H.; Yang, Z.-G.; Wang, X.-J.; Zou, Z.-Y.; Wang, Z.; Ma, J. Healthy Body Weight may Modify Effect of Abnormal Birth Weight on Metabolic Syndrome in Adolescents. Obesity 2019, 27, 462–469. [Google Scholar] [CrossRef]
- Fan, J.; Shi, X.; Jia, X.; Wang, Y.; Zhao, Y.; Bao, J.; Zhang, H.; Yang, Y. Birth weight, childhood obesity and risk of hypertension: A Mendelian randomization study. J. Hypertens. 2021, 39, 1876–1883. [Google Scholar] [CrossRef]
- Hirschler, V.; Edit, S.; Miorin, C.; Guntsche, Z.; Maldonado, N.; Garcia, C.; Lapertosa, S.; Gonzalez, C.D.; Calzia, V.; Di Firma, R.; et al. Association between High Birth Weight and Later Central Obesity in 9-Year-Old Schoolchildren. Metab. Syndr. Relat. Disord. 2021, 19, 213–217. [Google Scholar] [CrossRef]
- Kuciene, R.; Dulskiene, V.; Medzioniene, J. Associations between high birth weight, being large for gestational age, and high blood pressure among adolescents: A cross-sectional study. Eur. J. Nutr. 2018, 57, 373–381. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, S.-J.; Fu, G.-J.; Zhao, Y.; Xie, Y.J.; Zhang, Y.; Wang, Y.-X. The associations of high birth weight with blood pressure and hypertension in later life: A systematic review and meta-analysis. Hypertens. Res. 2013, 36, 725–735. [Google Scholar] [CrossRef]
- Pocobelli, G.; Dublin, S.; Enquobahrie, D.A.; Mueller, B.A. Birth Weight and Birth Weight for Gestational Age in Relation to Risk of Hospitalization with Primary Hypertension in Children and Young Adults. Matern. Child Health J. 2016, 20, 1415–1423. [Google Scholar] [CrossRef]
- Luetic, G.G.; Menichini, M.L.; Deri, N.; Steinberg, J.; Carrá, A.; Cristiano, E.; Patrucco, L.; Curbelo, M.C.; Rojas, J.I. High birth weight and risk of multiple sclerosis: A multicentre study in Argentina. Mult. Scler. Relat. Disord. 2021, 47, 102628. [Google Scholar] [CrossRef]
- Ekelund, U.; Luan, J.; Sherar, L.B.; Esliger, D.W.; Griew, P.; Cooper, A. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA 2012, 307, 704–712. [Google Scholar] [CrossRef]
- Danielsen, Y.; Júlíusson, P.; Nordhus, I.; Kleiven, M.; Meltzer, H.; Olsson, S.; Pallesen, S. The relationship between life-style and cardio-metabolic risk indicators in children: The importance of screen time. Acta Paediatr. 2010, 100, 253–259. [Google Scholar] [CrossRef]
- Owens, J.A.; Weiss, M.R. Insufficient sleep in adolescents: Causes and consequences. Minerva Pediatr. 2017, 69, 326–336. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Duan, Y.; Yang, Z. Prevalence of low birth weight and macrosomia estimates based on heaping adjustment method in China. Sci. Rep. 2021, 11, 15016. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, D.; Shen, H.; Yu, L.; Gao, Q.; Mao, L.; Jiang, F.; Luo, Y.; Xie, M.; Zhang, Y.; et al. Physical activity and health in Chinese children and adolescents: Expert consensus statement (2020). Br. J. Sports Med. 2020, 54, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Øglund, G.P.; Wells, J.; Ekelund, U. Prenatal, birth and early life predictors of sedentary behavior in young people: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ridgway, C.; Brage, S.; Sharp, S.J.; Corder, K.; Westgate, K.; van Sluijs, E.; Goodyer, I.M.; Hallal, P.C.; Anderssen, S.A.; Sardinha, L.; et al. Does Birth Weight Influence Physical Activity in Youth? A Combined Analysis of Four Studies Using Objectively Measured Physical Activity. PLoS ONE 2011, 6, e16125. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, L.; Ma, Y.; Wang, H.; Luo, J.; Zhang, X.; Luo, C.; Wang, H.; Zhao, H.; Pan, D.; et al. A national school-based health lifestyles interventions among Chinese children and adolescents against obesity: Rationale, design and methodology of a randomized controlled trial in China. BMC Public Health 2015, 15, 210. [Google Scholar] [CrossRef]
- Barth, J.R.; William, H.; Jackson, R. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet. Gynecol. 2020, 135, e118–e135. [Google Scholar]
- Dang, J.; Chen, T.; Ma, N.; Liu, Y.; Zhong, P.; Shi, D.; Dong, Y.; Zou, Z.; Ma, Y.; Song, Y.; et al. Associations between Breastfeeding Duration and Obesity Phenotypes and the Offsetting Effect of a Healthy Lifestyle. Nutrients 2022, 14, 1999. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Adams Hillard, P.J.; Katz, E.S.; et al. National Sleep Foundation’s updated sleep duration recommendations: Final report. Sleep Health. 2015, 1, 233–243. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M.; Blowey, D.; Carroll, A.E.; Daniels, S.R.; de Ferranti, S.D.; Dionne, J.M.; Falkner, B.; Flinn, S.K.; et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003, 26 (Suppl. 21), S5–S20. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Boundary Value of High Waist Circumference Screening for Children and Adolescents Aged 7–18 Years. Available online: http://www.nhc.gov.cn/wjw/pqt/201807/417de6982ab8493b91aba925b51a8a19.shtml (accessed on 13 June 2018).
- Dou, Y.; Jiang, Y.; Yan, Y.; Chen, H.; Zhang, Y.; Chen, X.; Wang, Y.; Cheng, H.; Zhao, X.; Hou, D.; et al. Waist-to-height ratio as a screening tool for cardiometabolic risk in children and adolescents: A nationwide cross-sectional study in China. BMJ Open 2020, 10, e037040. [Google Scholar] [CrossRef]
- Fernández-Alvira, J.M.; Mouratidou, T.; Bammann, K.; Hebestreit, A.; Barba, G.; Sieri, S.; Reisch, L.; Eiben, G.; Hadjigeorgiou, C.; Kovacs, E.; et al. Parental education and frequency of food consumption in European children: The IDEFICS study. Public Health Nutr. 2013, 16, 487–498. [Google Scholar] [CrossRef]
- Renom Espineira, A.; Fernandes-Rosa, F.L.; Bueno, A.C.; de Souza, R.M.; Moreira, A.C.; de Castro, M.; Barbieri, M.A.; Bettiol, H.; Antonini, S.R. Postnatal growth and cardiometabolic profile in young adults born large for gestational age. Clin. Endocrinol. 2011, 75, 335–341. [Google Scholar] [CrossRef]
- Araújo, J.; Severo, M.; Barros, H.; Mishra, G.D.; Guimarães, J.T.; Ramos, E. Developmental trajectories of adiposity from birth until early adulthood and association with cardiometabolic risk factors. Int. J. Obes. 2015, 39, 1443–1449. [Google Scholar] [CrossRef]
- Ornoy, A. Growth and neurodevelopmental outcome of children born to mothers with pregestational and gestational diabetes. Pediatr Endocrinol. Rev. 2005, 3, 104–113. [Google Scholar]
- Ornoy, A.; Wolf, A.; Ratzon, N.; Greenbaum, C.; Dulitzky, M. Neurodevelopmental outcome at early school age of children born to mothers with gestational diabetes. Arch. Dis. Child Fetal Neonatal Ed. 1999, 81, F10–F14. [Google Scholar] [CrossRef]
- Silverman, B.L.; Rizzo, T.A.; Cho, N.H.; Metzger, B.E. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care 1998, 21, B142. [Google Scholar]
- DeVader, S.R.; Neeley, H.L.; Myles, T.D.; Leet, T.L. Evaluation of gestational weight gain guidelines for women with normal prepregnancy body mass index. Obstet. Gynecol. 2007, 110, 745–751. [Google Scholar] [CrossRef]
- Groenendaal, F.; Elferink-Stinkens, P.M.; Registry, T.N.P. Hypoglycaemia and seizures in large-for-gestational-age (LGA) full-term neonates. Acta Paediatr. 2007, 95, 874–876. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Rossi, R.; Bührer, C.; Siebert, G.; Kjos, S.L.; Dudenhausen, J.W.; Vetter, K. Rate and risk factors of hypoglycemia in large-for-gestational-age newborn infants of nondiabetic mothers. Am. J. Obstet. Gynecol. 2002, 187, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Touger, L.; Looker, H.C.; Krakoff, J.; Lindsay, R.S.; Cook, V.; Knowler, W.C. Early growth in offspring of diabetic mothers. Diabetes Care 2005, 28, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.D.; Mathiesen, E.R.; Hansen, T.; Pedersen, O.; Jensen, D.M.; Lauenborg, J.; Schmidt, L.; Damm, P. Overweight and the Metabolic Syndrome in Adult Offspring of Women with Diet-Treated Gestational Diabetes Mellitus or Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2009, 94, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, V.; Marcovecchio, M.L.; De Giorgis, T.; Diesse, L.; Chiarelli, F.; Mohn, A. Progression of Cardio-Metabolic Risk Factors in Subjects Born Small and Large for Gestational Age. PLoS ONE 2014, 9, e104278. [Google Scholar] [CrossRef]
- Raaijmakers, A.; Zhang, Z.Y.; Claessens, J.; Cauwenberghs, N.; van Tienoven, T.P.; Wei, F.F.; Jacobs, L.; Levtchenko, E.; Pauwels, S.; Kuznetsova, T.; et al. Does Extremely Low Birth Weight Predispose to Low-Renin Hypertension? Hypertension 2017, 69, 443–449. [Google Scholar] [CrossRef]
- Hoy, W.E.; Hughson, M.D.; Bertram, J.F.; Douglas-Denton, R.; Amann, K. Nephron number, hypertension, renal disease, and renal failure. J. Am. Soc. Nephrol. 2005, 16, 2557–2564. [Google Scholar] [CrossRef]
- Samuel, T.; Hoy, W.E.; Douglas-Denton, R.; Hughson, M.D.; Bertram, J.F. Determinants of Glomerular Volume in Different Cortical Zones of the Human Kidney. J. Am. Soc. Nephrol. 2005, 16, 3102–3109. [Google Scholar] [CrossRef]
- Lopes, A.A.S.; Port, F.K. The low birth weight hypothesis as a plausible explanation for the black/white differences in hypertension, non-insulin-dependent diabetes, and end-stage renal disease. Am. J. Kidney Dis. 1995, 25, 350–356. [Google Scholar] [CrossRef]
- Eriksson, J.; Forsén, T.; Tuomilehto, J.; Osmond, C.; Barker, D. Fetal and Childhood Growth and Hypertension in Adult Life. Hypertension 2000, 36, 790–794. [Google Scholar] [CrossRef]
- Gomes, K.B.A.; Leal, V.S.; Oliveira, J.S.; Pereira, C.G.D.S.; Gonçalves, F.C.L.D.S.P.; Andrade, I.S.; Eickmann, S.H.; Lira, P.I.C.; Lima, M.C. Birth weight and overweight in adolescents: The erica project in the city of recife, pernambuco. Rev. Paul. Pediatr. 2021, 39, e2019380. [Google Scholar] [CrossRef]
- Skilton, M.R.; Siitonen, N.; Würtz, P.; Viikari, J.S.; Juonala, M.; Seppälä, I.; Laitinen, T.; Lehtimäki, T.; Taittonen, L.; Kähönen, M.; et al. High birth weight is associated with obesity and increased carotid wall thickness in young adults: The cardiovascular risk in young Finns study. Arterioscler. Thromb Vasc. Biol. 2014, 34, 1064–1068. [Google Scholar] [CrossRef]
- Andersen, L.B.; Riddoch, C.; Kriemler, S.; Hills, A.P. Physical activity and cardiovascular risk factors in children. Br. J. Sports Med. 2011, 45, 871–876. [Google Scholar] [CrossRef]
- Kalea, A.Z.; Drosatos, K.; Buxton, J.L. Nutriepigenetics and cardiovascular disease. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 252–259. [Google Scholar] [CrossRef]
- Lissak, G. Adverse physiological and psychological effects of screen time on children and adolescents: Literature review and case study. Environ. Res. 2018, 164, 149–157. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, T.; Liu, H.; Katzmarzyk, P.T.; Chaput, J.-P.; Fogelholm, M.; Johnson, W.D.; Kuriyan, R.; Kurpad, A.; Lambert, E.; et al. Joint association of birth weight and physical activity/sedentary behavior with obesity in children ages 9–11 years from 12 countries. Obesity 2017, 25, 1091–1097. [Google Scholar] [CrossRef]
- Deng, J.R.; Tan, W.Q.; Yang, S.Y.; Ao, L.P.; Liang, J.P.; Li, L.X.; Gao, Y.H.; Yang, Y.; Liu, L. High birth weight and its interaction with physical activity influence the risk of obesity in early school-aged children. World J. Pediatr. 2020, 16, 385–392. [Google Scholar] [CrossRef]
- Baker, J.L.; Olsen, L.W.; Sørensen, T.I. Childhood body mass index and the risk of coronary heart disease in adulthood. Ugeskr Laeger 2008, 170, 2434–2437. [Google Scholar] [CrossRef][Green Version]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Pan, X. Clustering of cardio-metabolic risk factors and pre-diabetes among U.S. adolescents. Sci. Rep. 2021, 11, 5015. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, B.; Zhao, Z.; Yang, L.; Zhang, M.; Jiang, Y.; Li, Y.; Zhou, M.; Wang, L.; Huang, Z.; et al. Body-mass index and obesity in urban and rural China: Findings from consecutive nationally representative surveys during 2004–18. Lancet 2021, 398, 53–63. [Google Scholar] [CrossRef]
| Variables | Total | Boys | Girls | χ2/F | p |
|---|---|---|---|---|---|
| Age | 8.908 | 0.003 | |||
| 7–12 years | 7090 | 3609 (63.0) | 3481 (60.3) | ||
| 13–18 years | 4419 | 2123 (37.0) | 2296 (39.7) | ||
| Residence | 0.936 | 0.333 | |||
| Rural | 4523 | 2278 (39.7) | 2245 (38.9) | ||
| Urban | 6986 | 3454 (60.3) | 3532 (61.1) | ||
| Single-child status | 82.888 | <0.001 | |||
| Yes | 7771 | 4099 (71.5) | 3672 (63.6) | ||
| No | 3738 | 1633 (28.5) | 2105 (36.4) | ||
| Parental education level | 3.841 | 0.050 | |||
| Junior high school and below | 4499 | 2292 (40.0) | 2207 (38.2) | ||
| Senior high school and above | 7010 | 3440 (60.0) | 3570 (61.8) | ||
| Birth weight | 58.484 | <0.001 | |||
| NBW | 10,044 | 4913 (85.7) | 5131 (88.8) | ||
| LBW | 432 | 191 (3.3) | 241 (4.2) | ||
| HBW | 1033 | 628 (11.0) | 405 (7.0) | ||
| Fruits intake | 1.465 | 0.226 | |||
| Inadequate | 10,482 | 5202 (90.8) | 5280 (91.4) | ||
| Adequate | 1027 | 530 (9.2) | 497 (8.6) | ||
| Vegetables intake | 7.865 | 0.005 | |||
| Inadequate | 10,533 | 5204 (90.8) | 5329 (92.2) | ||
| Adequate | 976 | 528 (9.2) | 448 (7.8) | ||
| Meat intake | 113.261 | <0.001 | |||
| Improper | 9543 | 4538 (79.2) | 5005 (86.6) | ||
| proper | 1966 | 1194 (20.8) | 772 (13.4) | ||
| Sugar-sweetened beverage | 168.492 | <0.001 | |||
| Excessive | 1316 | 877 (15.3) | 439 (7.6) | ||
| Proper | 10,193 | 4855 (84.7) | 5338 (92.4) | ||
| Dietary consumption | 30.410 | <0.001 | |||
| Unhealthy | 8667 | 4189 (73.1) | 4478 (77.5) | ||
| Healthy | 2842 | 1543 (26.9) | 1299 (22.5) | ||
| Physical activity | 33.352 | <0.001 | |||
| Inadequate | 6031 | 2849 (49.7) | 3182 (55.1) | ||
| Adequate | 5478 | 2883 (50.3) | 2595 (44.9) | ||
| Screen time | 70.682 | <0.001 | |||
| Excessive | 3212 | 1802 (31.4) | 1410 (24.4) | ||
| Proper | 8297 | 3930 (68.6) | 4367 (75.6) | ||
| Sleep duration | 1.951 | 0.163 | |||
| Inadequate | 9196 | 4550 (79.4) | 4646 (80.4) | ||
| Adequate | 2313 | 1182 (20.6) | 1131 (19.6) | ||
| Lifestyle | 7.888 | 0.005 | |||
| Poor lifestyle | 5317 | 2573 (44.9) | 2744 (47.5) | ||
| Ideal lifestyle | 6192 | 3159 (55.1) | 3033 (52.5) | ||
| Blood pressure | 55.597 | <0.001 | |||
| Normal blood pressure | 9478 | 4568 (79.7) | 4910 (85.0) | ||
| Hypertension | 2031 | 1164 (20.3) | 867 (15.0) | ||
| Fasting glucoses | 39.274 | <0.001 | |||
| Normal fasting glucoses | 11,285 | 5574 (97.2) | 5711 (98.9) | ||
| Impaired fasting glucose | 224 | 158 (2.8) | 66 (1.1) | ||
| Blood lipids | 8.605 | 0.003 | |||
| Normal blood lipids | 8222 | 4166 (72.7) | 4056 (70.2) | ||
| Dyslipidemia | 3287 | 1566 (27.3) | 1721 (29.8) | ||
| Waist circumference | 4.408 | 0.036 | |||
| Normal waist circumference | 8870 | 4465 (77.9) | 4405 (76.3) | ||
| Abdominal obesity | 2639 | 1267 (22.1) | 1372 (23.7) | ||
| CMRFs scores | <0.001 | ||||
| 0 | 5725 | 2868 (50.0) | 2857 (49.4) | ||
| 1 | 3815 | 1825 (31.8) | 1990 (34.5) | ||
| 2 | 1553 | 791 (13.8) | 762 (13.2) | ||
| 3 | 406 | 244 (4.3) | 162 (2.8) | ||
| 4 | 10 | 4 (0.1) | 6 (0.1) | ||
| Combinations of birth weight and lifestyle a | 13.794 | <0.001 | |||
| NBW/Ideal lifestyle | 5430 | 2720 (47.5) | 2710 (46.9) | Ref | |
| NBW/Poor lifestyle | 4614 | 2193 (38.3) | 2421 (41.9) | 0.050 | |
| LBW/Ideal lifestyle | 225 | 109 (1.9) | 116 (2.0) | 0.992 | |
| LBW/Poor lifestyle | 207 | 82 (1.4) | 125 (2.2) | 0.015 | |
| HBW/Ideal lifestyle | 537 | 330 (5.8) | 207 (3.6) | <0.001 | |
| HBW/Poor lifestyle | 496 | 298 (5.2) | 198 (3.4) | <0.001 | |
| Total | 11,509 | 5732 | 5777 |
| Variables | Total | Hypertension | Impaired Fasting Glucose | Dyslipidemia | Abdominal Obesity | Clustered CMRFs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | p | n (%) | p | n (%) | p | n (%) | p | n (%) | p | ||
| Sex | <0.001 | <0.001 | 0.003 | 0.036 | <0.001 | ||||||
| Boys | 5732 | 1164 (20.3) | 158 (2.8) | 1566 (27.3) | 1267 (22.1) | 248 (4.3) | |||||
| Girls | 5777 | 867 (15.0) | 66 (1.1) | 1721 (29.8) | 1372 (23.7) | 168 (2.9) | |||||
| Age | 0.020 | <0.001 | 0.027 | 0.011 | 0.022 | ||||||
| 7–12 years | 7090 | 1205 (17.0) | 105 (1.5) | 2077 (29.3) | 1570 (22.1) | 234 (3.3) | |||||
| 13–18 years | 4419 | 826 (18.7) | 119 (2.7) | 1210 (27.4) | 1069 (24.2) | 182 (4.1) | |||||
| Residence | <0.001 | 0.787 | <0.001 | <0.001 | 0.331 | ||||||
| Rural | 4523 | 963 (21.3) | 90 (2.0) | 1096 (24.2) | 930 (20.6) | 173 (3.8) | |||||
| Urban | 6986 | 1068 (15.3) | 134 (1.9) | 2191 (31.4) | 1709 (24.5) | 243 (3.5) | |||||
| Birth weight | 0.406 | 0.297 | 0.034 | <0.001 | 0.528 | ||||||
| NBW | 10,044 | 1756 (17.5) | 203 (2.0) | 2877 (28.6) | 2233 (22.2) | 356 (3.5) | |||||
| LBW | 432 | 85 (19.7) | 7 (1.6) | 141 (32.6) | 89 (20.6) | 19 (4.4) | |||||
| HBW | 1033 | 190 (18.4) | 14 (1.4) | 269 (26.0) | 317 (30.7) | 41 (4.0) | |||||
| Fruits intake | 0.428 | 0.479 | 0.79 | 0.293 | 0.614 | ||||||
| Inadequate | 10,482 | 1859 (17.7) | 207 (2.0) | 2990 (28.5) | 2390 (22.8) | 376 (3.6) | |||||
| Adequate | 1027 | 172 (16.7) | 17 (1.7) | 297 (28.9) | 249 (24.2) | 40 (3.9) | |||||
| Vegetables intake | 0.212 | 0.029 | 0.154 | 0.226 | 0.897 | ||||||
| Inadequate | 10,533 | 1873 (17.8) | 214 (2.0) | 2989 (28.4) | 2400 (22.8) | 380 (3.6) | |||||
| Adequate | 976 | 158 (16.2) | 10 (1.0) | 298 (30.5) | 239 (24.5) | 36 (3.7) | |||||
| Meat intake | <0.001 | 0.397 | 0.002 | 0.732 | 0.017 | ||||||
| Improper | 9543 | 1738 (18.2) | 181 (1.9) | 2781 (29.1) | 2194 (23.0) | 363 (3.8) | |||||
| proper | 1966 | 293 (14.9) | 43 (2.2) | 506 (25.7) | 445 (22.6) | 53 (2.7) | |||||
| Sugar-sweetened beverage | 0.149 | 0.001 | 0.117 | 0.035 | 0.006 | ||||||
| Excessive | 1316 | 251 (19.1) | 42 (3.2) | 400 (30.4) | 332 (25.2) | 65 (4.9) | |||||
| Proper | 10,193 | 1780 (17.5) | 182 (1.8) | 2887 (28.3) | 2307 (22.6) | 351 (3.4) | |||||
| Dietary consumption | <0.001 | 0.193 | 0.749 | 0.824 | 0.312 | ||||||
| Unhealthy | 8667 | 1603 (18.5) | 177 (2.0) | 2482 (28.6) | 1983 (22.9) | 322 (3.7) | |||||
| Healthy | 2842 | 428 (15.1) | 47 (1.7) | 805 (28.3) | 656 (23.1) | 94 (3.3) | |||||
| Physical activity | 0.166 | 0.724 | 0.066 | 0.531 | 0.424 | ||||||
| Inadequate | 6031 | 1036 (17.2) | 120 (2.0) | 1678 (27.8) | 1397 (23.2) | 210 (3.5) | |||||
| Adequate | 5478 | 995 (18.2) | 104 (1.9) | 1609 (29.4) | 1242 (22.7) | 206 (3.8) | |||||
| Screen time | 0.248 | 0.326 | <0.001 | 0.415 | 0.585 | ||||||
| Excessive | 3212 | 588 (18.3) | 56 (1.7) | 845 (26.3) | 753 (23.4) | 121 (3.8) | |||||
| Proper | 8297 | 1443 (17.4) | 168 (2.0) | 2442 (29.4) | 1886 (22.7) | 295 (3.6) | |||||
| Sleep duration | 0.010 | 0.004 | 0.075 | 0.003 | 0.028 | ||||||
| Inadequate | 9196 | 1665 (18.1) | 196 (2.1) | 2661 (28.9) | 2163 (23.5) | 350 (3.8) | |||||
| Adequate | 2313 | 366 (15.8) | 28 (1.2) | 626 (27.1) | 476 (20.6) | 66 (2.9) | |||||
| Lifestyle | 0.004 | 0.263 | 0.002 | 0.226 | 0.545 | ||||||
| Poor lifestyle | 5317 | 978 (18.4) | 113 (2.1) | 1474 (27.7) | 1258 (23.7) | 200 (3.8) | |||||
| Ideal lifestyle | 6192 | 1053 (17.0) | 111 (1.8) | 1813 (29.3) | 1381 (22.3) | 216 (3.5) | |||||
| Combinations of birth weight and lifestyle a | 0.277 | 0.414 | 0.046 | <0.001 | 0.077 | ||||||
| NBW/Ideal lifestyle | 5430 | 921 (17.0) | Ref | 102 (1.9) | Ref | 1589 (29.3) | Ref | 1183 (21.8) | Ref | 189 (3.5) | Ref |
| NBW/Poor lifestyle | 4614 | 835 (18.1) | 0.514 | 101 (2.2) | 0.776 | 1288 (27.9) | 0.512 | 1050 (22.8) | 0.754 | 167 (3.6) | 0.998 |
| LBW/Ideal lifestyle | 225 | 41 (18.2) | 0.992 | 2 (0.9) | 0.817 | 80 (35.6) | 0.185 | 43 (19.1) | 0.879 | 13 (5.8) | 0.302 |
| LBW/Poor lifestyle | 207 | 44 (21.3) | 0.441 | 5 (2.4) | 0.987 | 61 (29.5) | 1.000 | 46 (22.2) | 1.000 | 6 (2.9) | 0.995 |
| HBW/Ideal lifestyle | 537 | 91 (16.9) | 1.000 | 7 (1.3) | 0.886 | 144 (26.8) | 0.724 | 155 (28.9) | 0.001 | 14 (2.6) | 0.828 |
| HBW/Poor lifestyle | 496 | 99 (20.0) | 0.383 | 7 (1.4) | 0.956 | 125 (25.2) | 0.244 | 162 (32.7) | <0.001 | 27 (5.4) | 0.117 |
| Total | 11,509 | 2031 (17.6) | 224 (1.9) | 3287 (28.6) | 2639 (22.9) | 416 (3.6) | |||||
| Birth Weight | Hypertension | Impaired Fasting Glucose | Dyslipidemia | Abdominal Obesity | Clustered CMRFs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Total a | NBW | Ref | Ref | Ref | Ref | Ref | |||||
| LBW | 1.21 (0.92–1.60) | 0.176 | 0.99 (0.46–2.17) | 0.985 | 1.13 (0.88–1.44) | 0.348 | 0.98 (0.76–1.27) | 0.897 | 1.43 (0.87–2.36) | 0.155 | |
| HBW | 1.02 (0.85–1.22) | 0.864 | 0.68 (0.39–1.19) | 0.171 | 0.99 (0.84–1.17) | 0.898 | 1.66 (1.43–1.92) | <0.001 | 1.18 (0.84–1.67) | 0.333 | |
| 7–12 years b | NBW | Ref | Ref | Ref | Ref | Ref | |||||
| LBW | 0.94 (0.64–1.37) | 0.737 | 0.80 (0.25–2.59) | 0.706 | 1.21 (0.89–1.66) | 0.218 | 0.82 (0.58–1.17) | 0.281 | 1.24 (0.62–2.49) | 0.541 | |
| HBW | 1.08 (0.85–1.36) | 0.537 | 0.60 (0.26–1.41) | 0.244 | 1.06 (0.86–1.30) | 0.585 | 1.74 (1.44–2.10) | <0.001 | 1.70 (1.12–2.58) | 0.013 | |
| 13–18 years b | NBW | Ref | Ref | Ref | Ref | Ref | |||||
| LBW | 1.81 (1.19–2.75) | 0.006 | 1.27 (0.44–3.65) | 0.652 | 0.96 (0.63–1.45) | 0.843 | 1.25 (0.86–1.83) | 0.245 | 1.82 (0.88–3.75) | 0.106 | |
| HBW | 0.92 (0.69–1.23) | 0.567 | 0.74 (0.35–1.57) | 0.434 | 0.84 (0.63–1.12) | 0.235 | 1.54 (1.21–1.96) | <0.001 | 0.65 (0.35–1.21) | 0.176 | |
| Boys c | NBW | Ref | Ref | Ref | Ref | Ref | |||||
| LBW | 1.54 (1.06–2.25) | 0.024 | 1.07 (0.42–2.73) | 0.883 | 1.18 (0.82–1.71) | 0.369 | 1.13 (0.77–1.65) | 0.534 | 1.47 (0.75–2.89) | 0.257 | |
| HBW | 0.98 (0.78–1.23) | 0.851 | 0.81 (0.45–1.48) | 0.496 | 0.98 (0.79–1.22) | 0.852 | 1.48 (1.21–1.81) | <0.001 | 0.92 (0.58–1.45) | 0.713 | |
| Girls c | NBW | Ref | Ref | Ref | Ref | Ref | |||||
| LBW | 0.96 (0.63–1.46) | 0.833 | 0.95 (0.45–2.01) | 0.892 | 1.07 (0.76–1.49) | 0.700 | 0.87 (0.62–1.23) | 0.442 | 1.44 (0.68–3.03) | 0.342 | |
| HBW | 1.02 (0.75–1.38) | 0.894 | 0.85 (0.47–1.54) | 0.591 | 0.99 (0.77–1.28) | 0.943 | 1.99 (1.59–2.48) | <0.001 | 1.83 (1.19–3.08) | 0.022 | |
| Lifestyles | Hypertension | Impaired Fasting Glucose | Dyslipidemia | Abdominal Obesity | Clustered CMRFs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Total a | Unhealthy dietary consumption | 1.10 (0.97–1.25) | 0.132 | 1.30 (0.93–1.82) | 0.129 | 0.95 (0.85–1.06) | 0.360 | 0.98 (0.88–1.09) | 0.695 | 1.02 (0.80–1.29) | 0.905 |
| Inadequate physical activity | 1.01 (0.91–1.12) | 0.874 | 1.06 (0.80–1.40) | 0.683 | 1.00 (0.91–1.10) | 0.953 | 0.99 (0.91–1.09) | 0.882 | 1.01 (0.82–1.24) | 0.940 | |
| Excessive screen time | 1.04 (0.92–1.17) | 0.572 | 0.99 (0.72–1.36) | 0.945 | 0.98 (0.88–1.09) | 0.758 | 1.17 (1.06–1.30) | 0.003 | 1.16 (0.92–1.45) | 0.209 | |
| Inadequate sleep duration | 1.18 (1.02–1.36) | 0.027 | 1.11 (0.72–1.71) | 0.651 | 0.97 (0.86–1.10) | 0.673 | 1.14 (1.01–1.29) | 0.035 | 1.17 (0.87–1.56) | 0.293 | |
| Poor lifestyle | 1.08 (0.97–1.20) | 0.181 | 1.14 (0.86–1.50) | 0.368 | 0.97 (0.88–1.06) | 0.500 | 1.08 (0.99–1.19) | 0.085 | 1.09 (0.89–1.33) | 0.431 | |
| 7–12 years b | Unhealthy dietary consumption | 1.00 (0.72–1.37) | 0.980 | 1.51 (0.90–2.54) | 0.116 | 0.94 (0.83–1.08) | 0.401 | 1.02 (0.89–1.17) | 0.756 | 1.02 (0.80–1.29) | 0.905 |
| Inadequate physical activity | 1.00 (0.88–1.15) | 0.970 | 0.95 (0.63–1.41) | 0.782 | 1.02 (0.91–1.15) | 0.711 | 1.02 (0.90–1.14) | 0.793 | 1.05 (0.80–1.38) | 0.727 | |
| Excessive screen time | 1.01 (0.87–1.18) | 0.859 | 1.08 (0.69–1.68) | 0.747 | 0.98 (0.86–1.12) | 0.798 | 1.17 (1.03–1.34) | 0.018 | 1.07 (0.79–1.44) | 0.682 | |
| Inadequate sleep duration | 1.25 (1.07–1.45) | 0.005 | 1.27 (0.78–2.09) | 0.335 | 0.99 (0.87–1.12) | 0.824 | 1.19 (1.04–1.36) | 0.010 | 1.24 (0.90–1.70) | 0.181 | |
| Poor lifestyle | 1.12 (0.98–1.29) | 0.098 | 1.05 (0.70–1.58) | 0.807 | 0.95 (0.84–1.07) | 0.353 | 1.15 (1.02–1.29) | 0.023 | 1.03 (0.78–1.35) | 0.852 | |
| 13–18 years b | Unhealthy dietary consumption | 1.07 (0.87–1.31) | 0.542 | 1.15 (0.74–1.81) | 0.532 | 0.96 (0.80–1.15) | 0.675 | 0.97 (0.82–1.15) | 0.743 | 1.10 (0.75–1.60) | 0.636 |
| Inadequate physical activity | 1.04 (0.88–1.24) | 0.639 | 1.18 (0.79–1.76) | 0.417 | 0.97 (0.83–1.13) | 0.665 | 0.96 (0.83–1.11) | 0.600 | 0.98 (0.71–1.34) | 0.879 | |
| Excessive screen time | 1.03 (0.85–1.25) | 0.764 | 0.83 (0.51–1.33) | 0.429 | 0.97 (0.81–1.16) | 0.708 | 1.16 (0.99–1.37) | 0.075 | 1.18 (0.83–1.67) | 0.364 | |
| Inadequate sleep duration | 1.01 (0.68–1.49) | 0.973 | 0.75 (0.31–1.83) | 0.532 | 0.95 (0.65–1.38) | 0.776 | 0.96 (0.68–1.37) | 0.822 | 1.10 (0.54–2.27) | 0.792 | |
| Poor lifestyle | 1.03 (0.87–1.22) | 0.721 | 1.19 (0.81–1.75) | 0.384 | 1.01 (0.86–1.18) | 0.937 | 1.02 (0.88–1.18) | 0.784 | 1.17 (0.85–1.60) | 0.338 | |
| Boys c | Unhealthy dietary consumption | 1.11 (0.94–1.31) | 0.213 | 1.31 (0.88–1.96) | 0.184 | 1.00 (0.86–1.16) | 0.999 | 1.03 (0.89–1.20) | 0.667 | 1.40 (1.01–1.94) | 0.046 |
| Inadequate physical activity | 1.00 (0.87–1.15) | 0.988 | 1.07 (0.76–1.49) | 0.704 | 0.95 (0.83–1.09) | 0.422 | 0.96 (0.84–1.10) | 0.551 | 1.12 (0.86–1.46) | 0.411 | |
| Excessive screen time | 1.05 (0.90–1.23) | 0.507 | 1.06 (0.73–1.53) | 0.769 | 0.94 (0.81–1.09) | 0.435 | 1.17 (1.01–1.35) | 0.034 | 1.10 (0.83–1.48) | 0.504 | |
| Inadequate sleep duration | 1.12 (0.93–1.36) | 0.236 | 1.08 (0.65–1.79) | 0.764 | 1.05 (0.88–1.25) | 0.613 | 1.15 (0.97–1.37) | 0.113 | 1.20 (0.81–1.76) | 0.369 | |
| Poor lifestyle | 1.08 (0.93–1.24) | 0.304 | 1.22 (0.87–1.70) | 0.244 | 0.97 (0.85–1.11) | 0.677 | 1.09 (0.95–1.24) | 0.213 | 1.28 (0.98–1.67) | 0.070 | |
| Girls c | Unhealthy dietary consumption | 1.11 (0.91–1.35) | 0.304 | 1.04 (0.74–1.47) | 0.817 | 0.90 (0.77–1.05) | 0.192 | 0.96 (0.82–1.11) | 0.551 | 0.68 (0.47–0.97) | 0.034 |
| Inadequate physical activity | 1.02 (0.87–1.20) | 0.803 | 0.99 (0.74–1.32) | 0.929 | 1.04 (0.91–1.19) | 0.558 | 1.02 (0.89–1.16) | 0.801 | 0.85 (0.62–1.17) | 0.327 | |
| Excessive screen time | 1.00 (0.83–1.21) | 0.973 | 0.93 (0.66–1.31) | 0.676 | 1.03 (0.88–1.20) | 0.738 | 1.18 (1.02–1.36) | 0.031 | 1.21 (0.84–1.74) | 0.313 | |
| Inadequate sleep duration | 1.28 (1.03–1.59) | 0.028 | 1.06 (0.71–1.57) | 0.789 | 0.91 (0.77–1.08) | 0.270 | 1.11 (0.93–1.32) | 0.263 | 1.18 (0.76–1.82) | 0.456 | |
| Poor lifestyle | 1.09 (0.93–1.28) | 0.304 | 0.97 (0.73–1.30) | 0.857 | 0.95 (0.84–1.09) | 0.486 | 1.08 (0.95–1.23) | 0.237 | 0.85 (0.61–1.17) | 0.323 | |
| Combinations | Hypertension | Impaired Fasting Glucose | Dyslipidemia | Abdominal Obesity | Clustered CMRFs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| Total a | NBW/Ideal lifestyle | Ref | Ref | Ref | Ref * | Ref | |||||
| NBW/Poor lifestyle | 1.06 (0.95–1.19) | 0.302 | 1.12 (0.83–1.49) | 0.459 | 0.98 (0.89–1.08) | 0.683 | 1.06 (0.96–1.17) * | 0.237 | 1.04 (0.83–1.29) | 0.754 | |
| LBW/Ideal lifestyle | 1.25 (0.84–1.85) | 0.269 | 0.56 (0.14–2.34) | 0.428 | 1.35 (0.97–1.88) | 0.074 | 0.94 (0.66–1.36) | 0.759 | 2.00 (1.07–3.72) | 0.029 | |
| LBW/Poor lifestyle | 1.25 (0.84–1.84) | 0.274 | 1.59 (0.62–4.10) | 0.333 | 0.89 (0.61–1.29) | 0.540 | 1.08 (0.76–1.54) | 0.669 | 0.95 (0.41–2.19) | 0.896 | |
| HBW/Ideal lifestyle | 0.94 (0.73–1.22) | 0.652 | 0.70 (0.32–1.55) | 0.384 | 0.98 (0.78–1.22) | 0.841 | 1.54 (1.26–1.90) | <0.001 | 0.76 (0.43–1.33) | 0.327 | |
| HBW/Poor lifestyle | 1.16 (0.90–1.49) | 0.249 | 0.72 (0.33–1.58) | 0.411 | 0.99 (0.78–1.25) | 0.898 | 1.89 (1.53–2.33) | <0.001 | 1.74 (1.13–2.68) | 0.012 | |
| 7–12 years b | NBW/Ideal lifestyle | Ref | Ref | Ref | Ref * | Ref | |||||
| NBW/Poor lifestyle | 1.11 (0.96–1.29) | 0.159 | 1.12 (0.74–1.71) | 0.592 | 0.92 (0.81–1.05) | 0.222 | 1.10 (0.97–1.25) * | 0.134 | 0.89 (0.66–1.21) | 0.470 | |
| LBW/Ideal lifestyle | 0.83 (0.49–1.43) | 0.504 | 1.03 (0.24–4.37) | 0.974 | 1.21 (0.80–1.83) | 0.367 | 0.74 (0.45–1.21) | 0.228 | 1.02 (0.36–2.84) | 0.977 | |
| LBW/Poor lifestyle | 1.16 (0.68–1.99) | 0.580 | 0.61 (0.08–4.59) | 0.632 | 1.14 (0.72–1.80) | 0.583 | 1.01 (0.62–1.66) | 0.963 | 1.38 (0.54–3.53) | 0.506 | |
| HBW/Ideal lifestyle | 1.06 (0.78–1.44) | 0.720 | 0.79 (0.28–2.24) | 0.653 | 0.97 (0.74–1.27) | 0.798 | 1.55 (1.20–2.00) * | 0.001 | 1.07 (0.56–2.04) | 0.838 | |
| HBW/Poor lifestyle | 1.21 (0.86–1.72) | 0.273 | 0.46 (0.11–1.92) | 0.284 | 1.11 (0.82–1.49) | 0.508 | 2.20 (1.67–2.88) | <0.001 | 2.37 (1.39–4.05) | 0.002 | |
| 13–18 years b | NBW/Ideal lifestyle | Ref | Ref | Ref | Ref * | Ref | |||||
| NBW/Poor lifestyle | 1.10 (0.86–1.39) | 0.458 | 1.26 (0.78–2.05) | 0.343 | 1.07 (0.87–1.31) | 0.550 | 0.90 (0.74–1.10) * | 0.316 | 1.18 (0.77–1.81) | 0.442 | |
| LBW/Ideal lifestyle | 2.43 (1.15–5.17) | 0.021 | 1.00 (0.66–1.62) | 0.981 | 1.69 (0.86–3.31) # | 0.129 | 1.38 (0.72–2.65) | 0.338 | 3.57 (1.23–10.37) | 0.019 | |
| LBW/Poor lifestyle | 1.32 (0.58–2.99) | 0.506 | 2.03 (0.44–9.43) | 0.366 | 0.51 (0.21–1.24) | 0.137 | 0.98 (0.49–1.96) | 0.950 | 1.00 (0.62–1.48) | 0.970 | |
| HBW/Ideal lifestyle | 0.64 (0.34–1.19) | 0.157 | 1.00 (0.64–1.52) | 0.970 | 1.04 (0.64–1.69) | 0.879 | 1.76 (1.16–2.67) | 0.008 | 0.36 (0.08–1.58) | 0.177 | |
| HBW/Poor lifestyle | 1.32 (0.78–2.23) | 0.296 | 1.58 (0.58–4.34) | 0.372 | 0.64 (0.35–1.16) | 0.139 | 1.70 (1.09–2.65) | 0.019 | 1.06 (0.40–2.83) | 0.907 | |
| Boys c | NBW/Ideal lifestyle | Ref | Ref | Ref | Ref * | Ref | |||||
| NBW/Poor lifestyle | 1.15 (0.92–1.43) | 0.215 | 1.65 (1.02–2.67) | 0.043 | 1.03 (0.85–1.24) | 0.757 | 1.07 (0.90–1.28) * | 0.451 | 1.33 (0.91–1.95) | 0.135 | |
| LBW/Ideal lifestyle | 2.45 (1.25–4.79) | 0.009 | 0.92 (0.12–7.10) | 0.935 | 1.14 (0.61–2.12) | 0.682 | 1.03 (0.55–1.95) | 0.920 | 2.78 (1.02–7.62) | 0.047 | |
| LBW/Poor lifestyle | 1.36 (0.64–2.91) | 0.428 | 1.02 (0.13–7.99) | 0.988 | 0.68 (0.31–1.50) | 0.342 | 1.08 (0.54–2.17) | 0.829 | 0.56 (0.08–4.23) | 0.576 | |
| HBW/Ideal lifestyle | 0.93 (0.58–1.50) | 0.775 | 0.31 (0.04–2.31) | 0.251 | 1.31 (0.90–1.90) | 0.155 | 1.56 (1.10–2.19) | 0.012 | 0.76 (0.29–1.98) | 0.574 | |
| HBW/Poor lifestyle | 1.03 (0.63–1.67) | 0.911 | 2.03 (0.79–5.21) | 0.139 | 0.88 (0.57–1.37) | 0.569 | 2.49 (1.73–3.57) | <0.001 | 1.19 (0.51–2.74) | 0.689 | |
| Girls c | NBW/Ideal lifestyle | Ref | Ref | Ref | Ref * | Ref | |||||
| NBW/Poor lifestyle | 1.01 (0.79–1.29) | 0.928 | 1.00 (0.68–1.49) | 0.991 | 0.98 (0.81–1.17) | 0.788 | 0.96 (0.81–1.15) * | 0.689 | 0.68 (0.41–1.13) * | 0.135 | |
| LBW/Ideal lifestyle | 1.24 (0.59–2.62) | 0.572 | 0.80 (0.20–3.30) | 0.758 | 1.40 (0.80–2.46) | 0.244 | 0.91 (0.50–1.68) | 0.771 | 1.60 (0.46–5.55) | 0.462 | |
| LBW/Poor lifestyle | 1.36 (0.58–3.20) | 0.477 | 1.04 (0.27–4.08) | 0.956 | 1.12 (0.59–2.15) | 0.723 | 0.90 (0.47–1.73) | 0.753 | 1.19 (0.26–5.41) | 0.818 | |
| HBW/Ideal lifestyle | 0.86 (0.48–1.54) | 0.600 | 0.91 (0.33–2.47) | 0.848 | 1.06 (0.68–1.65) | 0.789 | 2.00 (1.37–2.93) | <0.001 | 1.38 (0.52–3.68) | 0.520 | |
| HBW/Poor lifestyle | 1.53 (0.86–2.72) | 0.151 | 0.80 (0.24–2.70) | 0.715 | 1.02 (0.61–1.70) | 0.950 | 2.08 (1.34–3.20) | 0.001 | 1.92 (0.72–5.10) | 0.191 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, D.; Dang, J.; Ma, N.; Liu, Y.; Zhong, P.; Cai, S.; Ma, Y.; Zou, Z.; Dong, Y.; Song, Y.; et al. The Combined Effect of Birth Weight and Lifestyle on Clustered Cardio-Metabolic Risk Factors in Children and Adolescents: A National School-Based Cross-Sectional Survey. Nutrients 2022, 14, 3131. https://doi.org/10.3390/nu14153131
Shi D, Dang J, Ma N, Liu Y, Zhong P, Cai S, Ma Y, Zou Z, Dong Y, Song Y, et al. The Combined Effect of Birth Weight and Lifestyle on Clustered Cardio-Metabolic Risk Factors in Children and Adolescents: A National School-Based Cross-Sectional Survey. Nutrients. 2022; 14(15):3131. https://doi.org/10.3390/nu14153131
Chicago/Turabian StyleShi, Di, Jiajia Dang, Ning Ma, Yunfei Liu, Panliang Zhong, Shan Cai, Yinghua Ma, Zhiyong Zou, Yanhui Dong, Yi Song, and et al. 2022. "The Combined Effect of Birth Weight and Lifestyle on Clustered Cardio-Metabolic Risk Factors in Children and Adolescents: A National School-Based Cross-Sectional Survey" Nutrients 14, no. 15: 3131. https://doi.org/10.3390/nu14153131
APA StyleShi, D., Dang, J., Ma, N., Liu, Y., Zhong, P., Cai, S., Ma, Y., Zou, Z., Dong, Y., Song, Y., & Ma, J. (2022). The Combined Effect of Birth Weight and Lifestyle on Clustered Cardio-Metabolic Risk Factors in Children and Adolescents: A National School-Based Cross-Sectional Survey. Nutrients, 14(15), 3131. https://doi.org/10.3390/nu14153131









