Sperm as a Carrier of Genome Instability in Relation to Paternal Lifestyle and Nutritional Conditions
Abstract
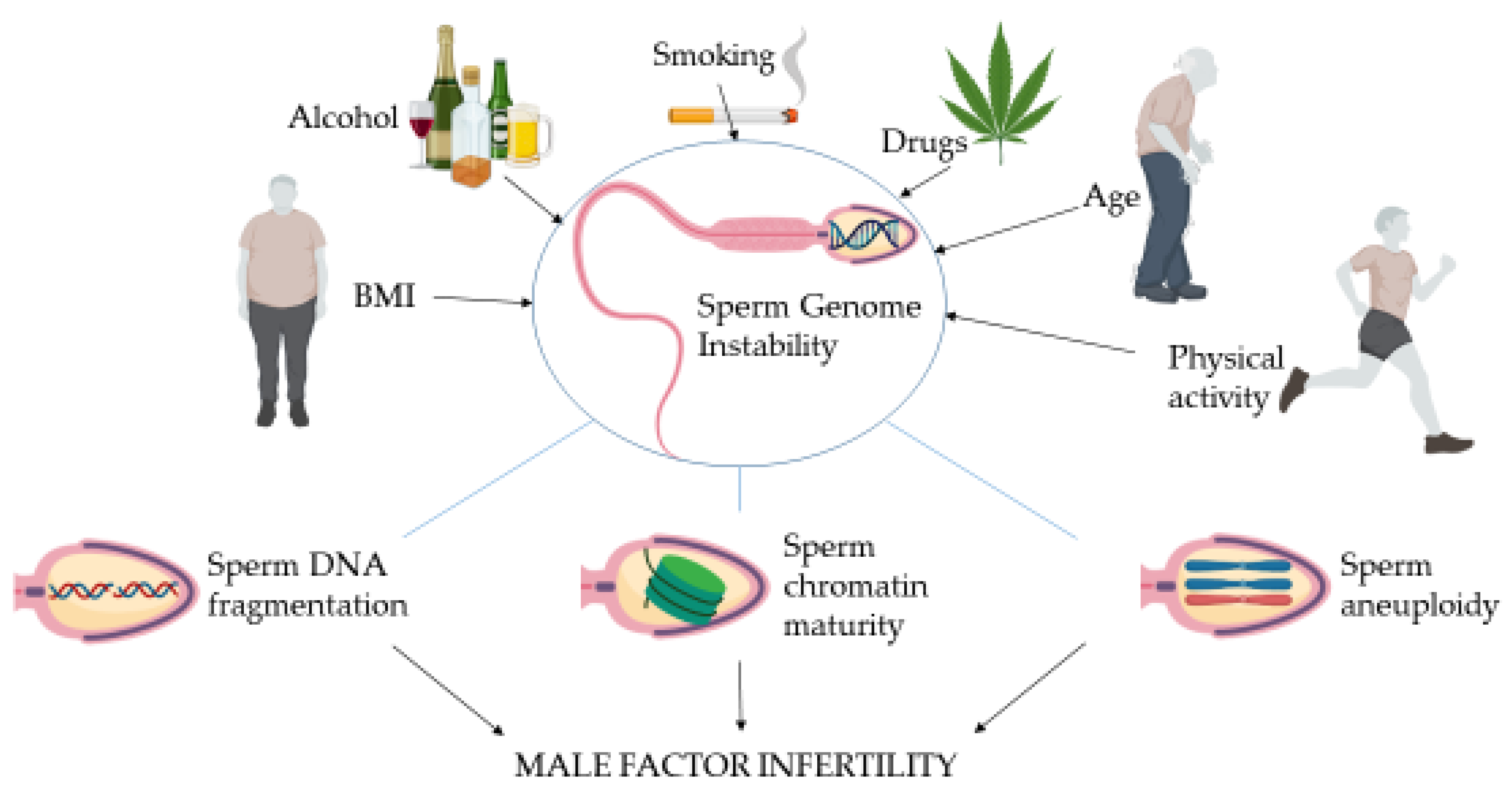
:1. Introduction
2. Materials and Methods
2.1. Study Protocol
- Sperm DNA fragmentation in a fertile population conducted between October 2017 and October 2020, approved on 26 June 2017, ref. no: 17/24/285 (Belgian registration no: B300201732872);
- Sperm DNA fragmentation in an infertile population conducted between October 2017 and October 2020, approved on 11 August 2017 (Belgian registration no: B300201733352).
- OS conducted between January 2017 and March 2018, approved on 31 July 2017, ref. no: 17/29/321 (Belgian registration no: B300201733042).
- Chromatin maturity and stability conducted between January 2020 and March 2020, approved on 13 January 2020, ref. no: 19/51/629.
- Sperm aneuploidy retrospective data collection between January 2014 and December 2016, approved on 6 July 2020, ref. no: 20/26/350.
2.2. Participants
2.3. Procedure and Intervention
2.4. Semen Analysis
2.5. Oxidative Stress (OS)
2.6. Sperm DNA Fragmentation (SDF)
2.7. Sperm Nuclear Chromatin Condensation and Decondensation Assessment
- Condensed chromatin—histones replaced by protamines, transforming the nucleus into a highly compact structure;
- Hypocondensed chromatin—insufficient chromatin condensation or a potential condition of underprotamination rendering the paternal genome susceptible to damage;
- Decondensed chromatin ability of compacted chromatin to decondense in vitro after sodium dodecyl sulphate (SDS) + EDTA treatment;
- Hypercondensed chromatin—resistance to decondensation achieving a state of hyperstability making the paternal genome unavailable for further fertilization.
2.8. Fluorescence In Situ Hybridization (FISH) Analysis
2.9. Statistical Analysis
3. Results
3.1. Lifestyle Factors Affecting Sperm Genome Instability in the Infertile Group
3.2. Lifestyle Factors Affecting Sperm Genome Instability in a Fertile Group
3.3. Incidence of Lifestyle Risk Factors in the Fertile and Subfertile Groups
3.4. Nutritional Intervention and Genome Instability in the Infertile Group
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thoma, M.E.; McLain, A.; Louis, J.F.; King, R.B.; Trumble, A.C.; Sundaram, R.; Louis, G.B. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil. Steril. 2013, 99, 1324–1331.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jequier, A.M. Clinical andrology—still a major problem in the treatment of infertility. Hum. Reprod. 2004, 19, 1245–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Jensen, T.K.; Lindahl-Jacobsen, R.; Christensen, K.; Nielsen, N.C.; Bostofte, E. Good Semen Quality and Life Expectancy: A Cohort Study of 43,277 Men. Am. J. Epidemiol. 2009, 170, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, M.L.; Li, S.; Behr, B.; Cullen, M.R.; Galusha, D.; Lamb, D.J.; Lipshultz, L.I. Semen quality, infertility and mortality in the USA. Hum. Reprod. 2014, 29, 1567–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, M.L.; Li, S.; Behr, B.; Pera, R.R.; Cullen, M.R. Relationship between semen production and medical comorbidity. Fertil. Steril. 2015, 103, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Latif, T.; Jensen, T.K.; Mehlsen, J.; Holmboe, S.A.; Brinth, L.; Pors, K.; Skouby, S.O.; Jørgensen, N.; Lindahl-Jacobsen, R. Semen Quality as a Predictor of Subsequent Morbidity: A Danish Cohort Study of 4,712 Men with Long-Term Follow-up. Am. J. Epidemiol. 2017, 186, 910–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, N.; Auger, J.; Giwercman, A.; Irvine, D.S.; Jensen, T.K.; Jouannet, P.; Keiding, N.; Le Bon, C.; Macdonald, E.; Pekuri, A.-M.; et al. Semen analysis performed by different laboratory teams: An intervariation study. Int. J. Androl. 1997, 20, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.E.; Ernest, E.; Jensen, T.K.; Hjolland, N.; Kolstad, H.; Henriksen, T.; Scheike, T.; Giwercman, A.; Olsen, J.; Skakkebæk, N. Relation between semen quality and fertility: A population-based study of 430 first-pregnancy planners. Lancet 1998, 352, 1172–1177. [Google Scholar] [CrossRef]
- Auger, J.; Eustache, F.; Ducot, B.; Blandin, T.; Daudin, M.; Diaz, I.; Matribi, S.E.; Gony, B.; Keskes, L.; Kolbezen, M.; et al. Intra- and inter-individual variability in human sperm concentration, motility and vitality assessment during a workshop involving ten laboratories. Hum. Reprod. 2000, 15, 2360–2368. [Google Scholar] [CrossRef] [Green Version]
- Guzick, D.S.; Overstreet, J.W.; Factor-Litvak, P.; Brazil, C.K.; Nakajima, S.T.; Coutifaris, C.; Carson, S.A.; Cisneros, P.; Steinkampf, M.P.; Hill, J.A.; et al. Sperm Morphology, Motility, and Concentration in Fertile and Infertile Men. N. Engl. J. Med. 2001, 345, 1388–1393. [Google Scholar] [CrossRef]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm Chromatin Structure Assay: Its Clinical Use for Detecting Sperm DNA Fragmentation in Male Infertility and Comparisons with Other Techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Chohan, K.R.; Griffin, J.T.; Lafromboise, M.; de Jonge, C.J.; Carell, D.T. Comparison of chromatin assays for DNA fragmentation evaluation in human sperm. J. Androl. 2006, 27, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Mesbah, M.; Huber, C.; Dadoune, J.P. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int. J. Androl. 1990, 13, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Urner, F.; Bianchi, P.; Bizzaro, D.; Wagner, I.; Jaquenoud, N.; Manicardi, G.; Campana, A. Sperm chromatin anomalies can influence decondensation after intracytoplasmic sperm injection. Hum. Reprod. 1996, 11, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kvist, U.; Kjellberg, S.; Björndahl, L.; Hammar, M.; Roomans, G.M. Zinc in Sperm Chromatin and Chromatin Stability in Fertile Men and Men in Barren Unions. Scand. J. Urol. Nephrol. 1988, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Libman, J. Sperm DNA damage: Clinical significance in the era of assisted reproduction. CMAJ 2006, 175, 495–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrell, D.T.; Liu, L.; Peterson, C.M.; Jones, K.P.; Hatasaka, H.H.; Erickson, L.; Campbell, B. SDF is increased in couples with unexplained recurrent pregnancy loss. Arch. Androl. 2003, 49, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Bungum, M.; Humaidan, P.; Spano, M.; Jepson, K.; Bungum, L.; Giwercman, A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum. Reprod. 2004, 19, 1401–1408. [Google Scholar] [CrossRef]
- Virro, M.R.; Larson-Cook, K.L.; Evenson, D.P. Sperm chromatin structure assay (scsa) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil. Steril. 2004, 81, 1289–1295. [Google Scholar] [CrossRef]
- Lewis, S.E.M.; Aitken, R.J. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res. 2005, 322, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; de Iuliis, G.N.; McLachlan, R.I. Biological and clinical significance of DNA damage in the male germ line. Int. J. Androl. 2009, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Barratt, C.L.; Aitken, R.J.; Björndahl, L.; Carrell, D.T.; de Boer, P.; Kvist, U.; Lewis, S.E.; Perreault, S.D.; Perry, M.J.; Ramos, L.; et al. Sperm DNA: Organization, protection and vulnerability: From basic science to clinical applications—A position report. Hum. Reprod. 2010, 25, 824–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zini, A. Are sperm chromatin and DNA defects relevant in the clinic? Syst. Biol. Reprod. Med. 2011, 57, 78–85. [Google Scholar] [CrossRef]
- Ruixue, W.; Hongli, Z.; Zhihong, Z.; Rulin, D.; Dongfeng, G.; Ruizhi, L. The impact of semen quality, occupational exposure to environmental factors and lifestyle on recurrent pregnancy loss. J. Assist. Reprod. Genet. 2013, 30, 1513–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef] [PubMed]
- Selvam, M.K.P.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef]
- González-Marín, C.; Gosálvez, J.; Roy, R. Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells. Int. J. Mol. Sci. 2012, 13, 14026–14052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengual, L.; Ballescá, J.L.; Ascaso, C.; Oliva, R. Marked differences in protamine content and P1/P2 ratios in sperm cells from percoll fractions between patients and controls. J. Androl. 2003, 24, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Carrell, D.T.; Hammoud, S.S. The human sperm epigenome and its potential role in embryonic development. Mol. Hum. Reprod. 2010, 16, 37–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; San Gabriel, M.; Zini, A. Sperm nuclear histone to protamine ratio in fertile and infertile men: Evidence of heterogeneous subpopulations of spermatozoa in the ejaculate. J. Androl. 2006, 27, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Gabriel, M.S.; Zhang, X. The histone to protamine ratio in human spermatozoa: Comparative study of whole and processed semen. Fertil. Steril. 2007, 87, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Rousseaux, S.; Reynoird, N.; Escoffier, E.; Thevenon, J.; Caron, C.; Khochbin, S. Epigenetic reprogramming of the male genome during gametogenesis and in the zygote. Reprod. Biomed. Online 2008, 16, 492–503. [Google Scholar] [CrossRef]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2010, 16, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadoune, J.-P. Expression of mammalian spermatozoal nucleoproteins. Microsc. Res. Tech. 2003, 61, 56–75. [Google Scholar] [CrossRef]
- Seli, E.; Sakkas, D. Spermatozoal nuclear determinants of reproductive outcome: Implications for ART. Hum. Reprod. Updat. 2005, 11, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philpott, A.; Leno, G.H. Nucleoplasmin remodels sperm chromatin in Xenopus egg extracts. Cell 1992, 69, 759–767. [Google Scholar] [CrossRef]
- Ménézo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef]
- Menezo, Y.; Evenson, D.; Cohen, M.; Dale, B. Effect of antioxidants on sperm genetic damage. Adv. Exp. Med. Biol. 2014, 791, 173–189. [Google Scholar]
- Burrello, N.; Arcidiacono, G.; Vicari, E.S.D.; Asero, P.; Di Benedetto, D.; de Palma, A.; Romeo, R.; D’Agata, R.; Calogero, A.E. Morphologically normal spermatozoa of patients with secretory oligo-astheno-teratozoospermia have an increased aneuploidy rate. Hum. Reprod. 2004, 19, 2298–2302. [Google Scholar] [CrossRef] [Green Version]
- Vendrell, X.; Ferrer-Buitrago, M.; Mengual, E.G.; Muñoz, P.; Triviño, J.C.; Calatayud, C.; Rawe, V.Y.; Ruiz-Jorro, M. Correlation between aneuploidy, apoptotic markers and DNA fragmentation in spermatozoa from normozoospermic patients. Reprod. Biomed. Online 2013, 28, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Egozcue, J.; Blanco, J.; Anton, E.; Sarrate, Z.; Vidal, F. Genetic Analysis of Sperm and Implications of Severe Male Infertility—A Review. Placenta 2003, 24, S62–S65. [Google Scholar] [CrossRef]
- Braga, D.P.D.A.F.; Halpern, G.; Figueira, R.D.C.S.; Setti, A.S.; Iaconelli, A.; Borges, E. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil. Steril. 2012, 97, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, C.N.; Sharma, A.; Abbara, A.; Luo, R.; White, C.J.; Hoskin, S.G.; Khanjani, S.; Crawford, M.J.; Ramsay, J.W.; Minhas, S.; et al. Burdens and awareness of adverse self-reported lifestyle factors in men with sub-fertility: A cross-sectional study in 1149 men. Clin. Endocrinol. 2020, 93, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Pols, A.M.; Peeters, P.H.M.; Bueno-De-Mesquita, H.B.; Ocke, M.C.; Wentink, A.C.; Kemper, H.C.G.; Collette, A.H.J. Validity and Repeatability of a Modified Baecke Questionnaire on Physical Activity. Int. J. Epidemiol. 1995, 24, 381–388. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction, 5th ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Björndahl, L.; Barratt, C.L.; Mortimer, D.; Jouannet, P. ‘How to count sperm properly’: Checklist for acceptability of studies based on human semen analysis. Hum. Reprod. 2015, 31, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Punjabi, U.; Spiessens, C. Basic Semen Analysis Courses: Experience in Belgium. In Modern ART in the 2000′s–Andrology in the Nineties; Ombelet, W., Bosmans, E., Vandeput, H., Vereecken, A., Renier, M., Hoomans, E., Eds.; The Parthenon Publishing Group: London, UK, 1998; pp. 107–113. [Google Scholar]
- Bjorndahl, L.; Barratt, C.; Fraser, L.; Kvist, U.; Mortimer, D. ESHRE basic semen analysis courses 1995–1999: Immediate beneficial effects of standardized training. Hum. Reprod. 2002, 17, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Punjabi, U.; Wyns, C.; Mahmoud, A.; Vernelen, K.; China, B.; Verheyen, G. Fifteen years of Belgian experience with external quality assessment of semen analysis. Andrology 2016, 4, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Sharma, R.; Roychoudhury, S.; Du Plessis, S.; Sabanegh, E. MiOXSYS: A novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil. Steril. 2016, 106, 566–573.e10. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, L.A.; de Iuliis, G.; Aitken, R.J. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: Development of an improved methodology. Int. J. Androl. 2010, 34, 2–13. [Google Scholar] [CrossRef]
- Punjabi, U.; van Mulders, H.; Goovaerts, I.; Peeters, K.; Clasen, K.; Janssens, P.; Zemtsova, O.; de Neubourg, D. Sperm DNA fragmentation in the total and vital fractions before and after density gradient centrifugation: Significance in male fertility diagnosis. Clin. Biochem. 2018, 62, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, U.; van Mulders, H.; Goovaerts, I.; Peeters, K.; Roelant, E.; de Neubourg, D. DNA fragmentation in concert with the simultaneous assessment of cell viability in a subfertile population: Establishing thresholds of normality both before and after density gradient centrifugation. J. Assist. Reprod. Genet. 2019, 36, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Castilla, J.A.; Gil, T.; Hortas, M.L.; Vergara, F.; Herruzo, A. DNA and chromatin structure: Influence of incubation on the chromatin condensation and nuclear stability of human spermatozoa by flow cytometry. Hum. Reprod. 1995, 10, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, U.; Peeters, K.; de Neubourg, D. Sperm nuclear maturity and chromatin stability in subfertile patients: Density gradient centrifugation is fair but non-discriminative in selecting the right population. Reprod. Biol. 2019, 19, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Vegetti, W.; van Assche, E.; Frias, A.M.; Verheyen, G.; Bianchi, M.M.; Bonduelle, M.; Liebaers, I.; van Steirteghem, A. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum. Reprod. 2000, 15, 351–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; Electronic Version; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Lee, S.W. Methods for testing statistical differences between groups in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Tomlinson, M.; Moffatt, O.; Manicardi, G.C.; Bizzaro, D.; Afnan, M.; Sakkas, D. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: Implications for assisted conception. Hum. Reprod. 2001, 16, 2160–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zini, A.; Bielecki, R.; Phang, D.; Zenzes, M.T. Correlations between two markers of sperm DNA integrity, DNA denaturation and DNA fragmentation, in fertile and infertile men. Fertil. Steril. 2001, 75, 674–677. [Google Scholar] [CrossRef]
- Oleszczuk, K.; Augustinsson, L.; Bayat, N.; Giwercman, A.; Bungum, M. Prevalence of high DNA fragmentation index in male partners of unexplained infertile couples. Andrology 2013, 1, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Feijó, C.M.; Esteves, S.C. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil. Steril. 2014, 101, 58–63.e3. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.; Baker, H. Andrology: Seminal leukocytes: Passengers, terrorists or good Samaritans? Hum. Reprod. 1995, 10, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Whittington, K.; Harrison, S.C.; Williams, K.M.; Day, J.L.; McLaughlin, E.A.; Hull, M.G.R.; Ford, W.C.L. Reactive oxygen species (ROS) production and the outcome of diagnostic tests of sperm function. Int. J. Androl. 1999, 22, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Keskes-Ammar, L.; Feki-Chakroun, N.; Rebai, T.; Sahnoun, Z.; Ghozzi, H.; Hammami, S.; Zghal, K.; Fki, H.; Damak, J.; Bahloul, A. Sperm oxidative stress and the effect of an oral vitamin E and selenium supplement on semen quality in infertile men. Arch. Androl. 2003, 49, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.-H.; Chao, H.-T.; Chen, H.-W.; Hwang, T.I.; Liao, T.-L.; Wei, Y.-H. Increase of oxidative stress in human sperm with lower motility. Fertil. Steril. 2008, 89, 1183–1190. [Google Scholar] [CrossRef]
- Caroppo, E.; Dattilo, M. Sperm redox biology challenges the role of antioxidants as a treatment for male factor infertility. FS Rev. 2022, 3, 90–104. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Marchiani, S.; Cambi, M.; Olivito, B.; Azzari, C.; Forti, G.; Baldi, E. Investigation on the Origin of Sperm DNA Fragmentation: Role of Apoptosis, Immaturity and Oxidative Stress. Mol. Med. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Björndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010, 16, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef]
- Tempest, H.G.; Ko, E.; Rademaker, A.; Chan, P.; Robaire, B.; Martin, R.H. Intra-individual and inter-individual variations in sperm aneuploidy frequencies in normal men. Fertil. Steril. 2009, 91, 185–192. [Google Scholar] [CrossRef]
- Shi, Q.; Martin, R. Aneuploidy in human sperm: A review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet. Genome Res. 2000, 90, 219–226. [Google Scholar] [CrossRef]
- Tempest, H.; Griffin, D. The relationship between male infertility and increased levels of sperm disomy. Cytogenet. Genome Res. 2004, 107, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Templado, C.; Uroz, L.; Estop, A. New insights on the origin and relevance of aneuploidy in human spermatozoa. Mol. Hum. Reprod. 2013, 19, 634–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannou, D.; Fortun, J.; Tempest, H. Meiotic nondisjunction and sperm aneuploidy in humans. Reproduction 2019, 157, R15–R31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyrobek, A.J.; Eskenazi, B.; Young, S.; Arnheim, N.; Tiemann-Boege, I.; Jabs, E.W.; Glaser, R.L.; Pearson, F.S.; Evenson, D. Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc. Natl. Acad. Sci. USA 2006, 103, 9601–9606. [Google Scholar] [CrossRef] [Green Version]
- Plastira, K.; Msaouel, P.; Angelopoulou, R.; Zanioti, K.; Plastiras, A.; Pothos, A.; Bolaris, S.; Paparisteidis, N.; Mantas, D. The effects of age on DNA fragmentation, chromatin packaging and conventional semen parameters in spermatozoa of oligoasthenoteratozoospermic patients. J. Assist. Reprod. Genet. 2007, 24, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, A.D.; Tirado, E.; Garcia, D.; Datta, V.; Sakkas, D. DNA fragmentation of sperm: A radical examination of the contribution of oxidative stress and age in 16,945 semen samples. Hum. Reprod. 2020, 35, 2188–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.L.; Dunleavy, J.; Gemmell, N.J.; Nakagawa, S. Consistent age-dependent declines in human semen quality: A systematic review and meta-analysis. Ageing Res. Rev. 2015, 19, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Print, C.G.; Loveland, K.L. Germ cell suicide: New insights into apoptosis during spermatogenesis. Bioessays 2000, 22, 423–430. [Google Scholar] [CrossRef]
- Cocuzza, M.; Athayde, K.S.; Agarwal, A.; Sharma, R.; Pagani, R.; Lucon, A.M.; Srougi, M.; Hallak, J. Age-Related Increase of Reactive Oxygen Species in Neat Semen in Healthy Fertile Men. Urology 2008, 71, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef] [Green Version]
- García-Ferreyra, J.; Luna, D.; Villegas, L.; Romero, R.; Zavala, P.; Hilario, R.; Dueñas-Chacón, J. High Aneuploidy Rates Observed in Embryos Derived from Donated Oocytes are Related to Male Aging and High Percentages of Sperm DNA Fragmentation. Clin. Med. Insights: Reprod. Health 2015, 9, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Domyati, M.M.; Al-Din, A.-B.M.; Barakat, M.T.; El-Fakahany, H.M.; Xu, J.; Sakkas, D. Deoxyribonucleic acid repair and apoptosis in testicular germ cells of aging fertile men: The role of the poly(adenosine diphosphate-ribosyl)ation pathway. Fertil. Steril. 2009, 91, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Kaarouch, I.; Bouamoud, N.; Madkour, A.; Louanjli, N.; Saadani, B.; Assou, S.; Aboulmaouahib, S.; Amzazi, S.; Copin, H.; Benkhalifa, M.; et al. Paternal age: Negative impact on sperm genome decays and IVF outcomes after 40 years. Mol. Reprod. Dev. 2018, 85, 271–280. [Google Scholar] [CrossRef]
- Dakouane, M.; Bicchieray, L.; Bergere, M.; Albert, M.; Vialard, F.; Selva, J. A histomorphometric and cytogenetic study of testis from men 29–102 years old. Fertil. Steril. 2005, 83, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, A.O.; Wilde, N.; Gibson, M.; Parks, A.; Carrell, D.T.; Meikle, A.W. Male obesity and alteration in sperm parameters. Fertil. Steril. 2008, 90, 2222–2225. [Google Scholar] [CrossRef]
- Andersen, M.K.; Sandholt, C.H. Recent Progress in the Understanding of Obesity: Contributions of Genome-Wide Association Studies. Curr. Obes. Rep. 2015, 4, 401–410. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Chavarro, J.E.; Toth, T.L.; Wright, D.L.; Meeker, J.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef] [Green Version]
- Fariello, R.M.; Pariz, J.R.; Spaine, D.M.; Cedenho, A.P.; Bertolla, R.P.; Fraietta, R. Association between obesity and alteration of sperm DNA integrity and mitochondrial activity. Br. J. Urol. 2012, 110, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Bakos, H.W.; Tremellen, K. Impact of body mass index on seminal oxidative stress. Andrologia 2011, 43, 121–128. [Google Scholar] [CrossRef]
- Singer, G.; Granger, D.N. Inflammatory Responses Underlying the Microvascular Dysfunction Associated with Obesity and Insulin Resistance. Microcirculation 2007, 14, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Banks, S.; King, A.S.; Irvine, D.S.; Saunders, P. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction 2005, 129, 505–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybar, R.; Kopecka, V.; Prinosilova, P.; Markova, P.; Rubes, J. Male obesity and age in relationship to semen parameters and sperm chromatin integrity. Andrologia 2011, 43, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.B.A.; Petersen, C.G.; Mauri, A.L.; Vagnini, L.D.; Renzi, A.; Petersen, B.; Mattila, M.; Dieamant, F.; Baruffi, R.L.R.; Franco, J.G. Association between body mass index and sperm quality and sperm DNA integrity. A large population study. Andrologia 2018, 50, e12889. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Vicari, E.; Calogero, A.E. Negative Effect of Increased Body Weight on Sperm Conventional and Nonconventional Flow Cytometric Sperm Parameters. J. Androl. 2012, 33, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Petersen, G.L.; Schmidt, L.; Pinborg, A.; Kamper-Jørgensen, M. The influence of female and male body mass index on live births after assisted reproductive technology treatment: A nationwide register-based cohort study. Fertil. Steril. 2013, 99, 1654–1662. [Google Scholar] [CrossRef]
- Bakos, H.W.; Henshaw, R.C.; Mitchell, M.; Lane, M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil. Steril. 2011, 95, 1700–1704. [Google Scholar] [CrossRef]
- Practice Committee of American Society for Reproductive Medicine The clinical utility of sperm DNA integrity testing. Fertil. Steril. 2008, 90, S178–S180. [CrossRef] [PubMed]
- Li, Y.; Lin, H.; Li, Y.; Cao, J. Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertil. Steril. 2011, 95, 116–123. [Google Scholar] [CrossRef]
- Mitra, A.; Chakraborty, B.; Mukhopadhay, D.; Pal, M.; Mukherjee, S.; Banerjee, S.; Chaudhuri, K. Effect of smoking on semen quality, FSH, testosterone level, and CAG repeat length in androgen receptor gene of infertile men in an Indian city. Syst. Biol. Reprod. Med. 2012, 58, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.R.; Agarwal, A.; Sharma, R.K.; Said, T.M.; Sikka, S.C.; Thomas, A.J. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil. Steril. 2003, 80, 1431–1436. [Google Scholar] [CrossRef]
- Sepaniak, S.; Forges, T.; Gerard, H.; Foliguet, B.; Bene, M.-C.; Monnier-Barbarino, P. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology 2006, 223, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.R.; Agarwal, A.; Sharma, R.K.; Nelson, D.R.; Thomas, A.J. Effect of cigarette smoking on levels of seminal oxidative stress in infertile men: A prospective study. Fertil. Steril. 2002, 78, 491–499. [Google Scholar] [CrossRef]
- Lesgards, J.-F.; Durand, P.; Lassarre, M.; Stocker, P.; Lesgards, G.; Lanteaume, A.; Prost, M.; Lehucher-Michel, M.-P. Assessment of lifestyle effects on the overall antioxidant capacity of healthy subjects. Environ. Health Perspect. 2002, 110, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, R.M.; Nasrallah, Y.S.; Hassan, M.M.; Farrag, A.F.; Majzoub, A.; Agarwal, A. The effect of cigarette smoking on human seminal parameters, sperm chromatin structure and condensation. Andrologia 2018, 50, e12910. [Google Scholar] [CrossRef]
- Koch, O.R.; Pani, G.; Borrello, S.; Colavitti, R.; Cravero, A.; Farrè, S.; Galeotti, T. Oxidative stress and antioxidant defenses in ethanol-induced cell injury. Mol. Asp. Med. 2004, 25, 191–198. [Google Scholar] [CrossRef]
- Amory, J. Drug effects on spermatogenesis. Drugs Today 2007, 43, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Asgari, S.A.; Farshi, A.; Iravani, S.; Khoshdel, A.; Shekarchi, B. Opium Consumption Is Negatively Associated With Serum Prostate-Specific Antigen (PSA), Free PSA, and Percentage of Free PSA Levels. J. Addict. Med. 2013, 7, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Peugh, J.; Belenko, S. Alcohol, Drugs and Sexual Function: A Review. J. Psychoact. Drugs 2001, 33, 223–232. [Google Scholar] [CrossRef]
- Pourmasumi, S.; Sabeti, P.; Rahiminia, T.; Mangoli, E.; Tabibnejad, N.; Talebi, A.R. The etiologies of sperm DNA abnormalities in male infertility: An assessment and review. Int. J. Reprod. Biomed. 2017, 15, 331–344. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Coombes, J. The influence of antioxidant supplementation on markers of inflammation and the relationship to oxidative stress after exercise. J. Nutr. Biochem. 2007, 18, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Schuppe, H.-C. Influence of genital heat stress on semen quality in humans. Andrologia 2007, 39, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [Green Version]
- Dattilo, M.; Giuseppe, D.; Ettore, C.; Ménézo, Y. Improvement of gamete quality by stimulating and feeding the endogenous antioxidant system: Mechanisms, clinical results, insights on gene-environment interactions and the role of diet. J. Assist. Reprod. Genet. 2016, 33, 1633–1648. [Google Scholar] [CrossRef] [Green Version]
- Ménézo, Y.; Hazout, A.; Panteix, G.; Robert, F.; Rollet, J.; Cohen-Bacrie, P.; Chapuis, F.; Clément, P.; Benkhalifa, M. Antioxidants to reduce sperm DNA fragmentation: An unexpected adverse effect. Reprod. Biomed. Online 2007, 14, 418–421. [Google Scholar] [CrossRef]
- Dattilo, M.; Cornet, D.; Amar, E.; Cohen, M.; Menezo, Y. The importance of the one carbon cycle nutritional support in human male fertility: A preliminary clinical report. Reprod. Biol. Endocrinol. 2014, 12, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairo Consensus Workshop Group; Barratt, C.; Mortimer, D.; Amer, M.; Baldi, E.; de Jonge, C.; Fawzy, M.; Jørgensen, N.; Krausz, C.; Mahran, A.; et al. The current status and future of andrology: A consensus report from the Cairo workshop group. Andrology 2019, 8, 27–52. [Google Scholar] [CrossRef]
- De Ligny, W.; Smits, R.M.; Mackenzie-Proctor, R.; Jordan, V.; Fleischer, K.; de Bruin, J.P.; Showell, M.G. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2022, 12, CD007411. [Google Scholar] [CrossRef]
- Fraga, C.G.; Motchnik, P.A.; Shigenaga, M.K.; Helbock, H.J.; Jacob, R.A.; Ames, B.N. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. USA 1991, 88, 11003–11006. [Google Scholar] [CrossRef] [Green Version]
- Kodama, H.; Yamaguchi, R.; Fukuda, J.; Kasai, H.; Tanaka, T. Increased oxidative deoxyribonucleic acid damage in the spermatozoa of infertile male patients. Fertil. Steril. 1997, 68, 519–524. [Google Scholar] [CrossRef]
- Greco, E.; Romano, S.; Iacobelli, M.; Ferrero, S.; Baroni, E.; Minasi, M.G.; Ubaldi, F.; Rienzi, L.; Tesarik, J. ICSI in cases of sperm DNA damage: Beneficial effect of oral antioxidant treatment. Hum. Reprod. 2005, 20, 2590–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Miari, G.; Froiland, D.; Thompson, J. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Piomboni, P.; Gambera, L.; Serafini, F.; Campanella, G.; Morgante, G.; de Leo, V. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J. Androl. 2008, 10, 201–206. [Google Scholar] [CrossRef]
- Omu, A.; Al-Azemi, M.; Kehinde, E.; Anim, J.; Oriowo, M.; Mathew, T. Indications of the Mechanisms Involved in Improved Sperm Parameters by Zinc Therapy. Med. Princ. Pr. 2008, 17, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Thompson, J.; Tremellen, K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod. Biomed. Online 2009, 18, 761–768. [Google Scholar] [CrossRef]
- Vani, K.; Kurakula, M.; Syed, R.; Alharbi, K. Clinical Relevance of Vitamin C Among Lead-Exposed Infertile Men. Genet. Test. Mol. Biomark. 2012, 16, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Amengual, M.J.; Gosalvez, J.; Coward, K.; Hannaoui, N.; Benet, J.; García-Peiró, A.; Prats, J. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia 2013, 45, 211–216. [Google Scholar] [CrossRef]
- Gual-Frau, J.; Abad, C.; Amengual, M.J.; Hannaoui, N.; Checa, M.A.; Ribas-Maynou, J.; Lozano, I.; Nikolaou, A.; Benet, J.; García-Peiró, A.; et al. Oral antioxidant treatment partly improves integrity of human sperm DNA in infertile grade I varicocele patients. Hum. Fertil. 2015, 18, 225–229. [Google Scholar] [CrossRef]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Barekat, F.; Tavalaee, M.; Deemeh, M.R.; Bahreinian, M.; Azadi, L.; Abbasi, H.; Rozbahani, S.; Nasr-Esfahani, M.H. A Preliminary Study: N-acetyl-L-cysteine Improves Semen Quality following Varicocelectomy. Int. J. Fertil. Steril. 2016, 10, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Stenqvist, A.; Oleszczuk, K.; Leijonhufvud, I.; Giwercman, A. Impact of antioxidant treatment on DNA fragmentation index: A double-blind placebo-controlled randomized trial. Andrology 2018, 6, 811–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Parameters | Numbers | Mean ± SD (Range) |
|---|---|---|
| Demographic variables | ||
| Male age at diagnosis (years) | 580 | 33.8 ± 8.9 (18.0–64.5) |
| Body mass index (kg/m2) | 440 | 24.9 ± 3.9 (13.0–43.2) |
| Smoking | ||
| Non-smokers | 343 (77.6%) | |
| Smokers | 99 (22.4%) | |
| Alcohol | ||
| Abstainers | 128 (28.6%) | |
| Alcohol users | 319 (71.4%) | |
| Drugs | ||
| Abstainers | 389 (87.2%) | |
| Drug users | 57 (12.8%) | |
| Physical activity score | 173 | 8.0 ± 2.0 (0.0–12.4) |
| ORP (mV/M/mL) | 241 | 3.1 ± 11.9 (−3.7–163.2) |
| Non-Smokers | Smokers | p Value * | ||
|---|---|---|---|---|
| Current Smokers | Former Smokers | |||
| Male age (years) | 33.1 ± 9.2 (16.0–64.5) n = 291 | 36.6 ± 7.8 (21.0–61.3) n = 80 | 37.1 ± 3.6 (32.4–42.9) n = 6 | <0.001 |
| BMI (kg/m2) | 24.5 ± 3.7 (13.0–38.5) n = 265 | 26.1 ± 4.1 (19.0–37.4) n = 67 | 25.1 ± 3.2 (20.2–28.7) n = 6 | 0.021 |
| Sperm concentration (M/mL) | 70.7 ± 65.5 (0.6–512.5) n = 290 | 60.2 ± 62.7 (0.6–300.0) n = 81 | 40.7 ± 13.8 (26.3–60.0) n = 6 | 0.068 |
| Progressive sperm motility (%) | 51.1 ± 12.8 (4.0–89.0) n = 290 | 46.8 ± 16.3 (3.0–75.0) n = 81 | 52.8 ± 8.5 (44.0–68.0) n = 6 | 0.242 |
| Sperm morphology (%) | 5.2 ± 3.4 (0.0–18.0) n = 283 | 4.4 ± 3.6 (0.0–17.0) n = 79 | 6.0 ± 2.1 (4.0–9.0) n = 6 | 0.025 |
| Total SDF (%) | 10.4 ± 7.9 (0.0–63.0) n = 278 | 9.8 ± 8.4 (0.0–42.3) n = 72 | 9.8 ± 6.5 (4.0–21.0) n = 6 | 0.324 |
| Vital SDF (%) | 1.3 ± 1.7 (0.0–14.0) n = 278 | 1.0 ± 1.0 (0.0–4.2) n = 72 | 1.0 ± 0.6 (0.0–2.0) n = 6 | 0.797 |
| ORP (mV/M/mL) | 2.6 ± 7.6 (−3.7–57.3) n = 107 | 2.0 ± 3.6 (−0.2–18.2) n = 39 | 1.4 ± 0.8 (0.8–3.0) n = 6 | 0.212 |
| Parameters | Total SDF | Vital SDF | ||
|---|---|---|---|---|
| Coefficient (SE) | p Value | Coefficient (SE) | p Value | |
| Age (years) | 0.1950 (0.0564) | <0.001 | 0.0019 (0.0148) | 0.899 |
| BMI (kg/m2) | 0.1335 (0.1430) | 0.352 | 0.0044 (0.0375) | 0.907 |
| smoking | −1.9127 (1.4703) | 0.195 | −0.5473 (0.3856) | 0.158 |
| Alcohol | 0.1608 (1.2769) | 0.900 | 0.1617 (0.3349) | 0.629 |
| Drugs | −0.1544 (1.4262) | 0.914 | −0.0325 (0.3741) | 0.931 |
| Physical activity score | −0.3168 (0.3384) | 0.351 | −0.1275 (0.0888) | 0.153 |
| Parameters | ORP | |
|---|---|---|
| Coefficient (SE) | p Value | |
| Total SDF (%) | −0.2009 (0.1129) | 0.077 |
| Vital SDF (%) | 1.0999 (0.4291) | 0.011 |
| Parameters | Fertile Group | Subfertile Group | p Value |
|---|---|---|---|
| Semen parameters | (n = 44) | (n = 511) | |
| Sperm concentration (M/mL) | 82.3 ± 50.2 (16.7–263.8) | 68.8 ± 67.4 (0.6–512.5) | 0.006 |
| Total sperm count (M) | 288.9 ± 190.8 (21.7–767.3) | 233.8 ± 217.3 (1.0–1436.2) | 0.013 |
| Progressive motility (%) | 57.9 ± 9.1 (34.0–74.0) | 50.0 ± 14.4 (3.0–89.0) | <0.001 |
| Total motility (%) | 66.4 ± 8.5 (46.0–82.0) | 56.6 ± 14.6 (5.0–91.0) | <0.001 |
| Morphology (%) | 8.4 ± 4.2 (1.0–22.0) | 5.0 ± 3.5 (0.0–18.0) | <0.001 |
| SDF parameters | (n = 46) | (n = 501) | |
| Total SDF (%) | 10.6 ± 8.6 (1.4–54.6) | 10.7 ± 8.5 (0.0–68.6) | 0.976 |
| Vital SDF (%) | 1.4 ± 1.5 (0.0–6.6) | 1.4 ± 2.2 (0.0–25.0) | 0.508 |
| Chromatin parameters | (n = 10) | (n = 65) | |
| Chromatin condensation (%) | 84.5 ± 7.2 (67.0–92.0) | 68.0 ± 12.4 (28.9–90.0) | <0.001 |
| Chromatin decondensation (%) | 89.9 ± 2.6 (85.0–93.0) | 68.0 ± 17.8 (5.4–90.2) | <0.001 |
| Chromatin hypocondensation (%) | 7.8 ± 2.6 (5.0–14.0) | 9.2 ± 7.0 (1.4–54.6) | 0.601 |
| Chromatin hypercondensation (%) | 2.1 ± 0.9 (1.0–4.0) | 10.8 ± 7.8 (1.6–33.1) | <0.001 |
| Frequency of sperm aneuploidy | (n = 20) | (n = 203) | |
| Chromosome 13 | |||
| Nullisomy (%) | 0.11 ± 0.15 | 0.16 ± 0.26 | 0.282 |
| Disomy (%) | 0.14 ± 0.11 | 0.17 ± 0.30 | 0.294 |
| Chromosome 18 | |||
| Nullisomy (%) | 0.06 ± 0.09 | 0.19 ± 0.42 | 0.061 |
| Disomy (%) | 0.12 ± 0.16 | 0.20 ± 0.45 | 0.234 |
| Chromosome 21 | |||
| Nullisomy (%) | 0.08 ± 0.11 | 0.14 ± 0.17 | 0.072 |
| Disomy (%) | 0.08 ± 0.09 | 0.16 ± 0.35 | 0.605 |
| Chromosome X/Y | |||
| Nullisomy (%) | 0.30 ± 0.31 | 0.31 ± 0.48 | 0.422 |
| Disomy XX (%) | 0.15 ± 0.21 | 0.11 ± 0.16 | 0.814 |
| Disomy XY (%) | 0.10 ± 0.12 | 0.22 ± 0.66 | 0.32 |
| Disomy YY (%) | 0.07 ± 0.10 | 0.07 ± 0.15 | 0.833 |
| Autosomal aneuploidy (%) | 0.57 ± 0.34 | 1.03 ± 1.10 | 0.04 |
| Sex aneuploidy (%) | 0.61 ± 0.45 | 0.71 ± 0.99 | 0.779 |
| Diploidy (%) | 0.48 ± 0.39 | 1.17 ± 1.97 | 0.004 |
| Parameters | Before Intervention | After Intervention | % Difference | p Value * |
|---|---|---|---|---|
| Semen volume (mL) | 3.3 ± 1.3 | 3.4 ± 1.9 | 0.2 | 0.8457 |
| Sperm concentration (M/mL) | 57.9 ± 63.2 | 64.0 ± 67.1 | 6.1 | 0.7706 |
| Total count (M/ejaculate) | 179.4 ± 192.6 | 212.3 ± 291.2 | 32.8 | 0.9033 |
| Progressive motility (%) | 36.4 ± 18.9 | 46.5 ± 16.8 | 10.1 | 0.0844 |
| Total motility (%) | 46.1 ± 18.5 | 56.0 ± 17.3 | 9.9 | 0.0596 |
| Sperm morphology (%) | 3.6 ± 2.6 | 3.9 ± 2.9 | 0.3 | 0.7311 |
| Total SDF (%) | 18.7 ± 18.1 | 14.9 ± 12.9 | −3.8 | 0.4973 |
| Vital SDF (%) | 1.5 ± 1.5 | 1.6 ± 1.1 | 0.1 | 0.4666 |
| Study | Supplement/Day | Duration | Study Design and Patient Population | SDF Assay | Study Results |
|---|---|---|---|---|---|
| Fraga et al. [122] | Vitamin C (250 mg) Depletion to 5 mg/day, Repletion to 250 mg/day | 15 weeks | Prospective, observational study; 24 normal volunteers | 8-OHdG | DNA damage increased by 91% upon depletion due to reduced seminal ascorbic acid, 36% could be restored by repletion |
| Kodama et al. [123] | GSH (400 mg) Vitamin C (200 mg) Vitamin E (200 mg) | 2 months | Prospective, observational study; 14 infertile men | 8-OHdG | Modest decrease in 8-OHdG levels from 1.5 ± 0.2 to 1.1 ± 0.1/105 deoxyguanosine (p < 0.05) |
| Greco et al. [124] | Vitamin C (1000 mg) Vitamin E (1000 mg) | 2 months | Prospective, observational; 38 infertile males with DFI > 15% | TUNEL | 29/38 responded with a decrease in SDF from 24.0 ± 7.9 to 8.2 ± 4.3 (p ≤ 0.001) while 9/38 showed no difference in SDF values from 25.1 ± 8.5 to 23.8 ± 9.2% |
| Greco et al. [125] | Vitamin C (1000 mg) Vitamin E (1000 mg) | 2 months | Randomized placebo-controlled study; 64 infertile males with DFI > 15% | TUNEL | Decrease in SDF from 22.1 ± 7.7% to 9.1 ± 7.2 (p < 0.001) in the treatment group, but not in the placebo group (from 24.4 ± 7.8 to 22.9 ± 7.9) |
| Menezo et al. [118] | Vitamin C (400 mg) Vitamin E (400 mg) Zinc (33 mg) Selenium (80 µg) β-carotene (18 mg) | 90 days | Double-centered, observational study; 58 males with DFI >15% | SCSA | DFI decreased from 32.4% to 26.2% (p < 0.001) but, sperm decondensation increased from 17.5% to 21.5% (p < 0.001) |
| Tremellen et al. [126] | MenevitR: zinc (25 mg) Vitamin C (100 mg) Vitamin E (400 IU) Lycopene (6 mg) Garlic oil (33 µg) Selenium (26 µg) Folic acid (500 µg) | 3 months | Double-blind randomized, controlled study; 60 with severe male factor infertility | TUNEL | DNA damage reduced from 37.9% to 33.3%; but from 40.03% to 32.0% in controls |
| Piomboni et al. [127] | Beta-glucan (20 mg) fermented Papaya (50 mg) Lactoferrin (97 mg) Vitamin C (30 mg) Vitamin E (5 mg) | 3 months | Prospective study; 36 men with leukocytospermia and 15 controls | SCSA | No significant decrease in SDF in the control (15.8 ± 6.7 to 16.1 ± 5.4) and treatment (16.7 ± 8.0 to 14.4 ± 6.0) groups |
| Omu et al. [128] | Group1: zinc (400 mg) Group 2: zinc (400 mg) + vitamin E (20 mg) Group 3: zinc (400 mg) + vitamin E (20 mg) + vitamin C (10 mg) | 3 months | Randomized placebo-controlled study; 45 men with asthenozoospermia, 37 treatment group (group 1 = 11; group 2 = 12; group 3 = 14), 8 placebo group | SCSA | Zinc supplementation resulted in significantly lower DFI (14–29%, p< 0.05) compared to zinc deficiency. |
| Tunc et al. [129] | MenevitR: as above | 3 months | Prospective, observational study; 50 males with oxidative stress | TUNEL | SDF levels dropped from 22.2% to 18.2% (p = 0.002) and sperm DNA protamination improved from 69.0% to 73.6% (p< 0.001) |
| Vani et al. [130] | Vitamin C (1000 mg) 5 consecutive days in a week | 3 months | Prospective, comparative study; 120 men exposed to lead, and 120 healthy human subjects | Comet | Decrease in alkaline-labile sites and mean tail length of the comet when compared to the control group (p < 0.01) |
| Abad et al. [131] | L-carnitine (1500 mg) Coenzyme Q10 (20 mg) Vitamin C (60 mg) Vitamin E (10 mg) Vitamin B9 (200 µg) Vitamin B12 (1 µg) Zinc (10 mg) Selenium (50 µg) | 3 months | Prospective, observational study; 20 asthenoterato-zoospermic infertile males | SCD | DNA damage reduced from 28.5% ± 14.97% to 20.12% ± 8.26% (p = 0.004) DNA degraded sperm also reduced from 7.32% ± 4.12% to 5.66% ± 3.21% (p = 0.04) |
| Dattilo et al. [119] | CondensylR: Opuntia fig fruit (100 mg) Quercetin (0.05 mg) Betalain (0.001 mg) Vitamin B2 (1.4 mg) Vitamin B3 (16 mg) Vitamin B6 (1.4 mg) Vitamin B9 (400 µg) Vitamin B12 (2.5 µg) Vitamin E (12 mg) n-acetyl-cysteine (250 mg) | 4 months | Prospective, observational study; 84 infertile men | TUNEL | DFI decreased from 29.7% to 23.1% (p < 0.001); sperm nuclear decondensation index decreased from 40.1% to 36.3% (p < 0.001) |
| Gual-Frau et al. [132] | L-carnitine (1500 mg) Vitamin C (60 mg) Coenzyme Q10 (20 mg) Vitamin E (10 mg) Zinc (10 mg) Vitamin B9 (200 μg) Selenium (50 μg) Vitamin B12 (1 µg) | 3 months | Prospective, observational study; 20 infertile men with grade I varicocele | SCD | After treatment, an average relative reduction of 22.1% in SDF (p = 0.02) and 31.3% fewer highly degraded sperm cells (p = 0.07) were observed |
| Martínez-Soto et al. [133] | Docosahexaenoic acid (1500 mg) | 10 weeks | Randomized, double-blind, placebo-controlled, parallel-group study | TUNEL | Decrease in SDF values (−17.2 ± 2.8%, p < 0.001) in treatment group vs. (+11.2 ± 1.9%, p > 0.05) in the placebo group |
| Barekat et al. [134] | N-acetyl-L-cysteine (NAC; 200 mg) three times daily | 3 months | Randomized controlled trial; 35 infertile men with varicocele, subjected to varicocele repair; 20 control group; 15 treatment group | TUNEL | Improvement in sperm chromatin integrity in men subjected to varicocelectomy receiving NAC post-surgery compared to those who did not (11.8% ± 2.01 vs. 4.7% ± 1.3, p < 0.01) |
| Stenqvist et al. [135] | Vitamin C (30 mg) Vitamin E (5 mg) Vitamin B12 (0.5μg) l-carnitine (750 mg) coenzyme Q10 (10 mg) Folic acid (100 μg) Zinc (5 mg) Selenium (25 μg) | 3 and 6 months | Randomized, double-blind, placebo-controlled study; 37 treatment group; 40 placebo group | SCSA | No significant decrease in DFI both in the placebo and treatment groups, after 3 and 6 months of supplementation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punjabi, U.; Goovaerts, I.; Peeters, K.; Van Mulders, H.; De Neubourg, D. Sperm as a Carrier of Genome Instability in Relation to Paternal Lifestyle and Nutritional Conditions. Nutrients 2022, 14, 3155. https://doi.org/10.3390/nu14153155
Punjabi U, Goovaerts I, Peeters K, Van Mulders H, De Neubourg D. Sperm as a Carrier of Genome Instability in Relation to Paternal Lifestyle and Nutritional Conditions. Nutrients. 2022; 14(15):3155. https://doi.org/10.3390/nu14153155
Chicago/Turabian StylePunjabi, Usha, Ilse Goovaerts, Kris Peeters, Helga Van Mulders, and Diane De Neubourg. 2022. "Sperm as a Carrier of Genome Instability in Relation to Paternal Lifestyle and Nutritional Conditions" Nutrients 14, no. 15: 3155. https://doi.org/10.3390/nu14153155






