Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
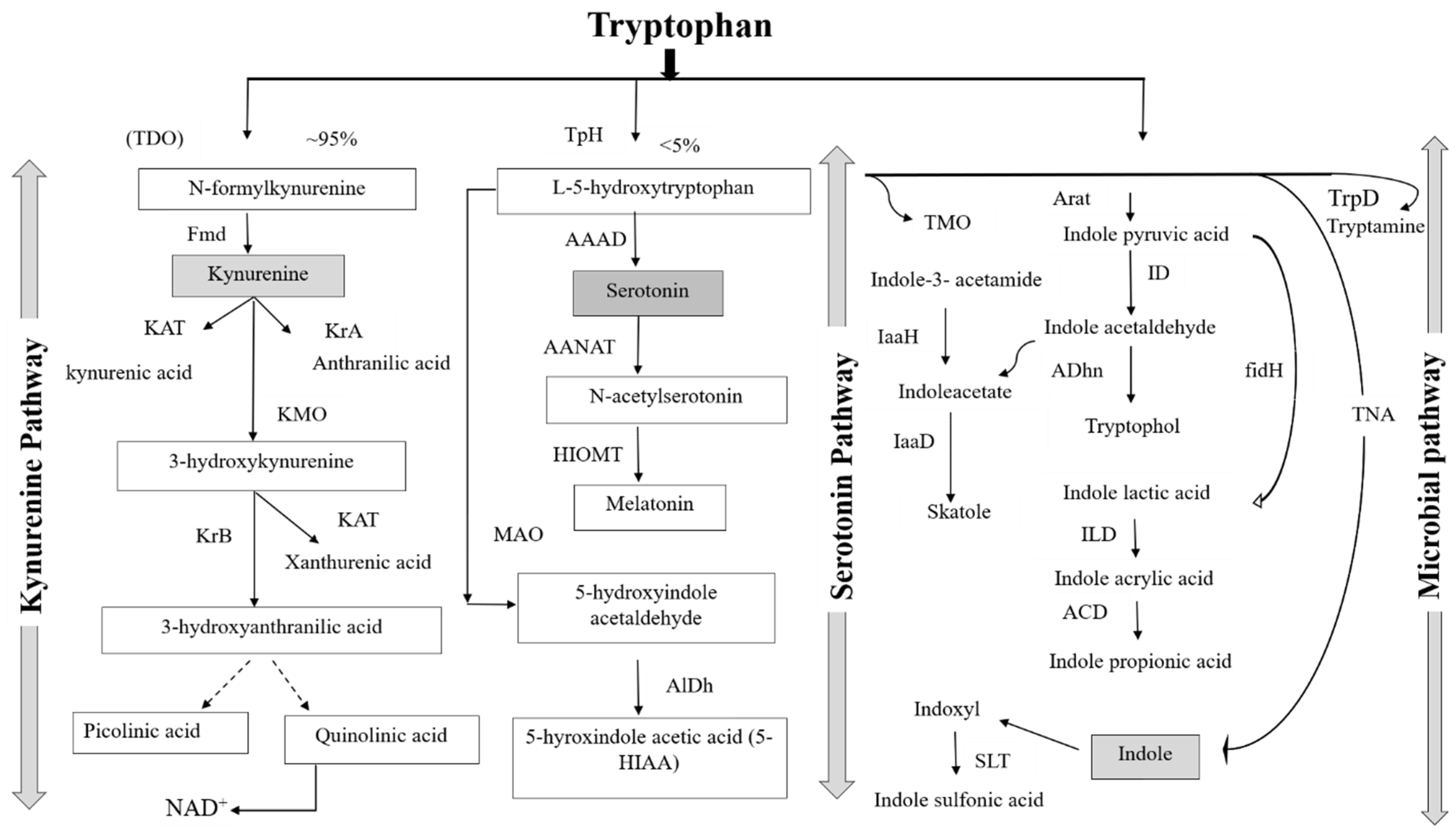
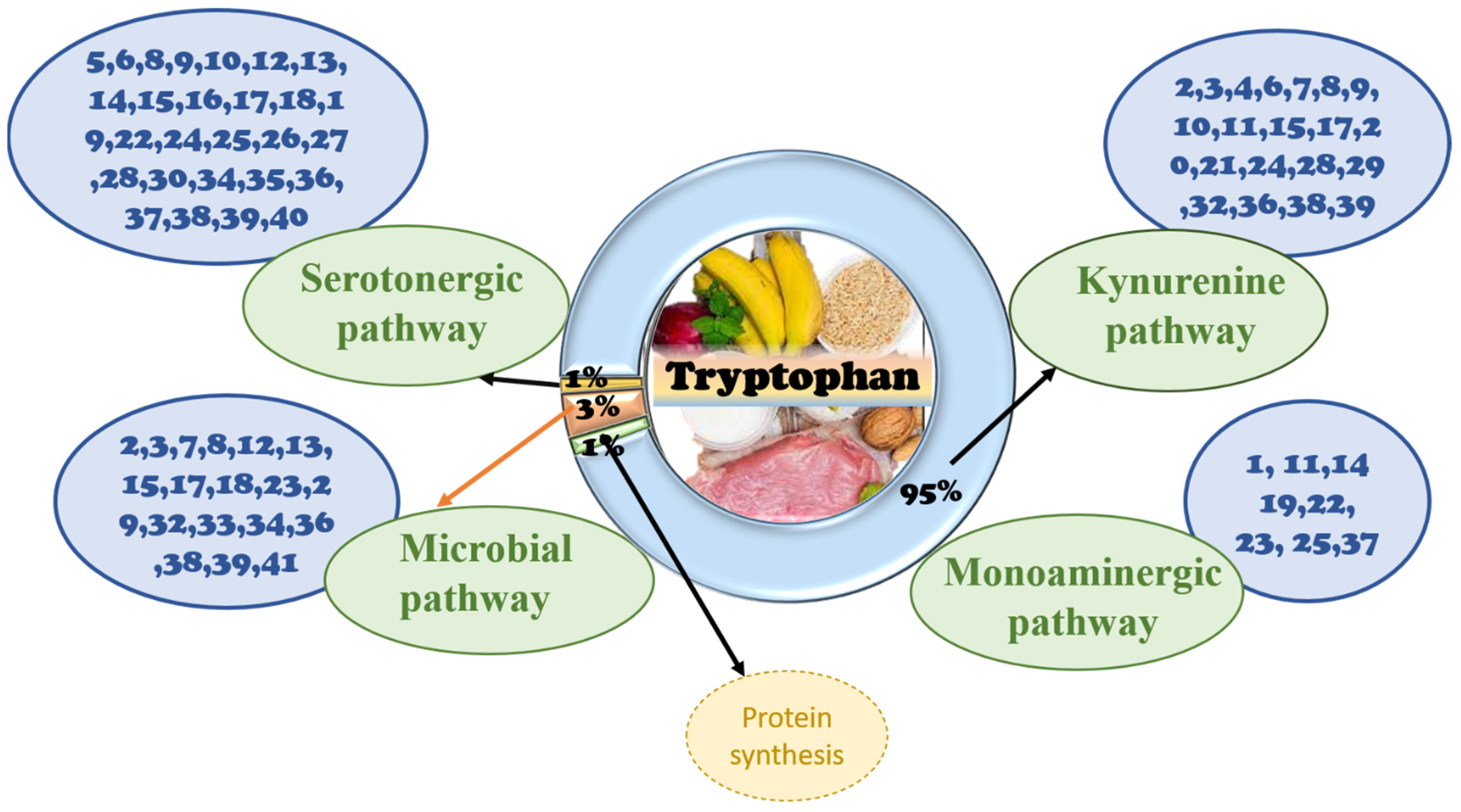
3.1. Tryptophan Analog Structure and Biochemistry
3.2. Signaling Pathway of Tryptophan in the Gut
3.2.1. The Role of Tryptophan Targeting Microorganisms in the Gut
3.2.2. The Role of Tryptophan Targeting the Kynurenine Pathway in the Gut
3.2.3. The Role of the Tryptophan Targeting the Serotonin Pathway in the Gut
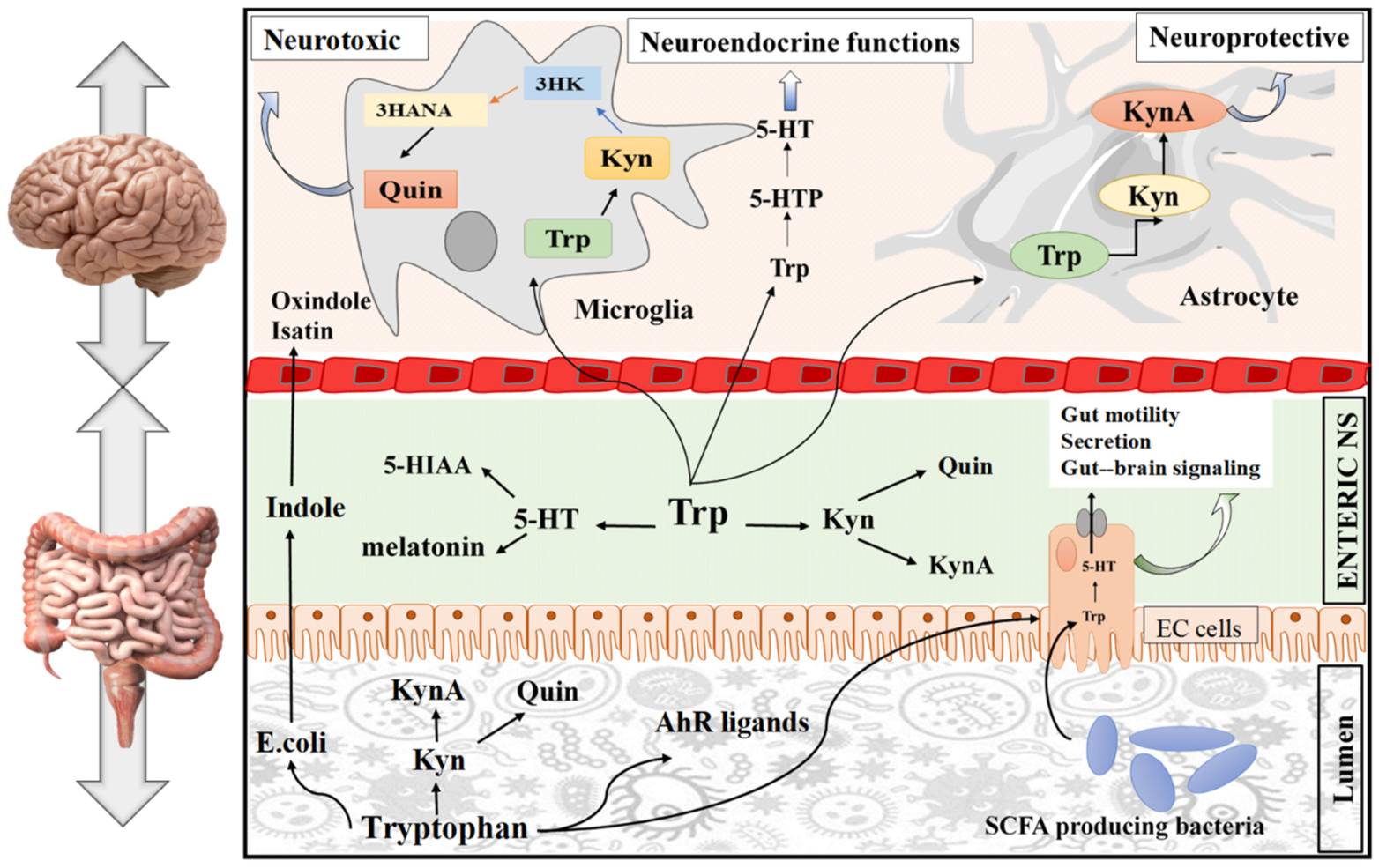
3.3. Signaling Pathway of Tryptophan in the Brain
3.3.1. The Role of Tryptophan Targeting Microbiota in the GBA
3.3.2. The Role of Tryptophan Targeting the Kynurenine Pathway in the Brain
3.3.3. The Role of Tryptophan Targeting the 5-HT Pathway in the Brain
3.4. The Role of Natural Products and Their Metabolites in the GBA Targeting Tryptophan
3.4.1. Natural Products
Human Breast Milk
Moringa oleifera
Nelumbo nucifera
Mimosa pudica
Poria cocos
Salvia officinalis
Tagetes lucida
Theobroma cacao
Tualang Honey
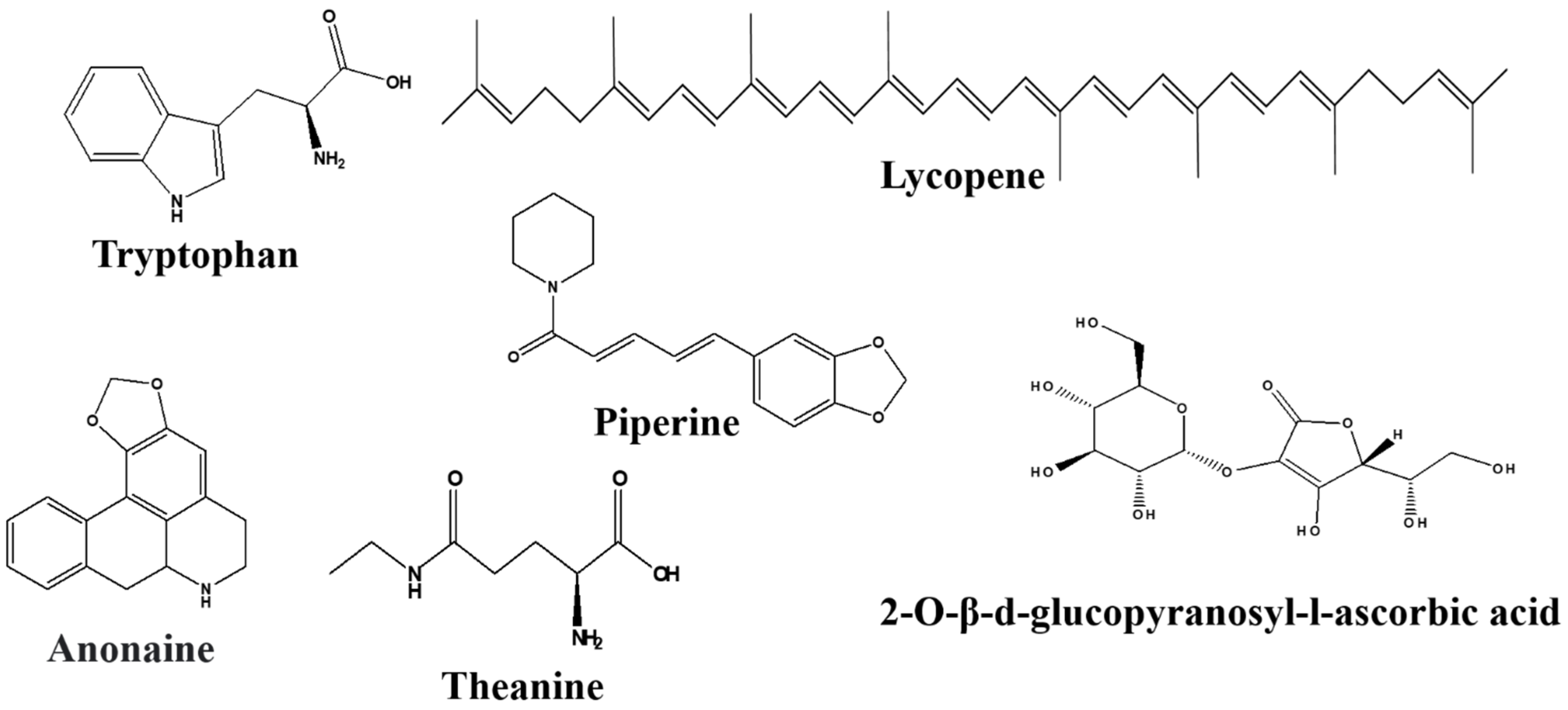
3.4.2. Amino Acids
Theanine
3.4.3. Alkaloids
Anonaine and Asimilobine
Piperine
3.4.4. Carotenoids
Lycopene
2-O-β-d-glucopyranosyl-l-ascorbic Acid
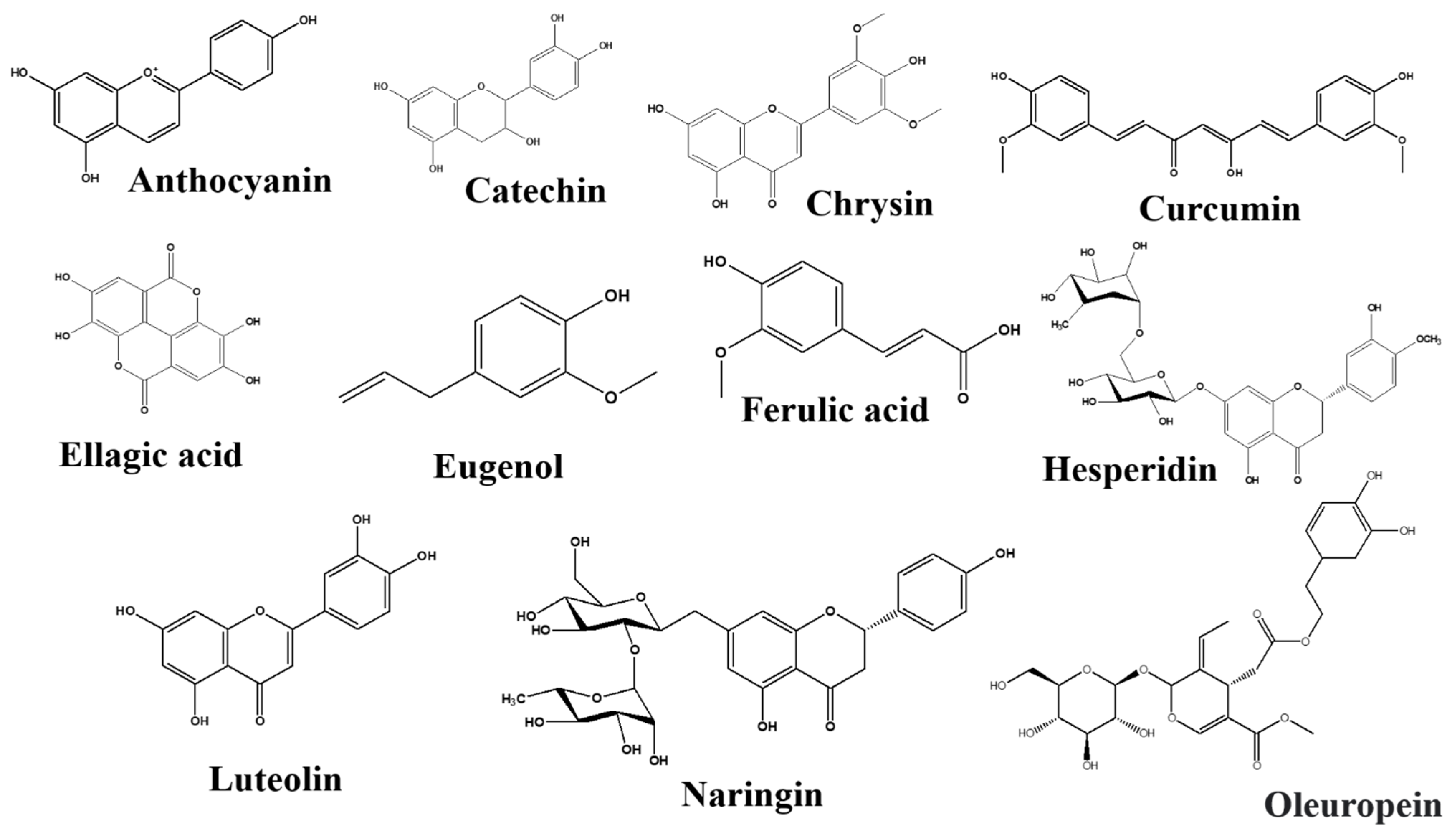
3.4.5. Flavonoids and Phenolics
Anthocyanins
Catechins
Chrysin
Curcumin
Ellagic Acid
Eugenol
Ferulic Acid
Hesperidin
Luteolin
Naringin
Oleuropein
Proanthocyanidins
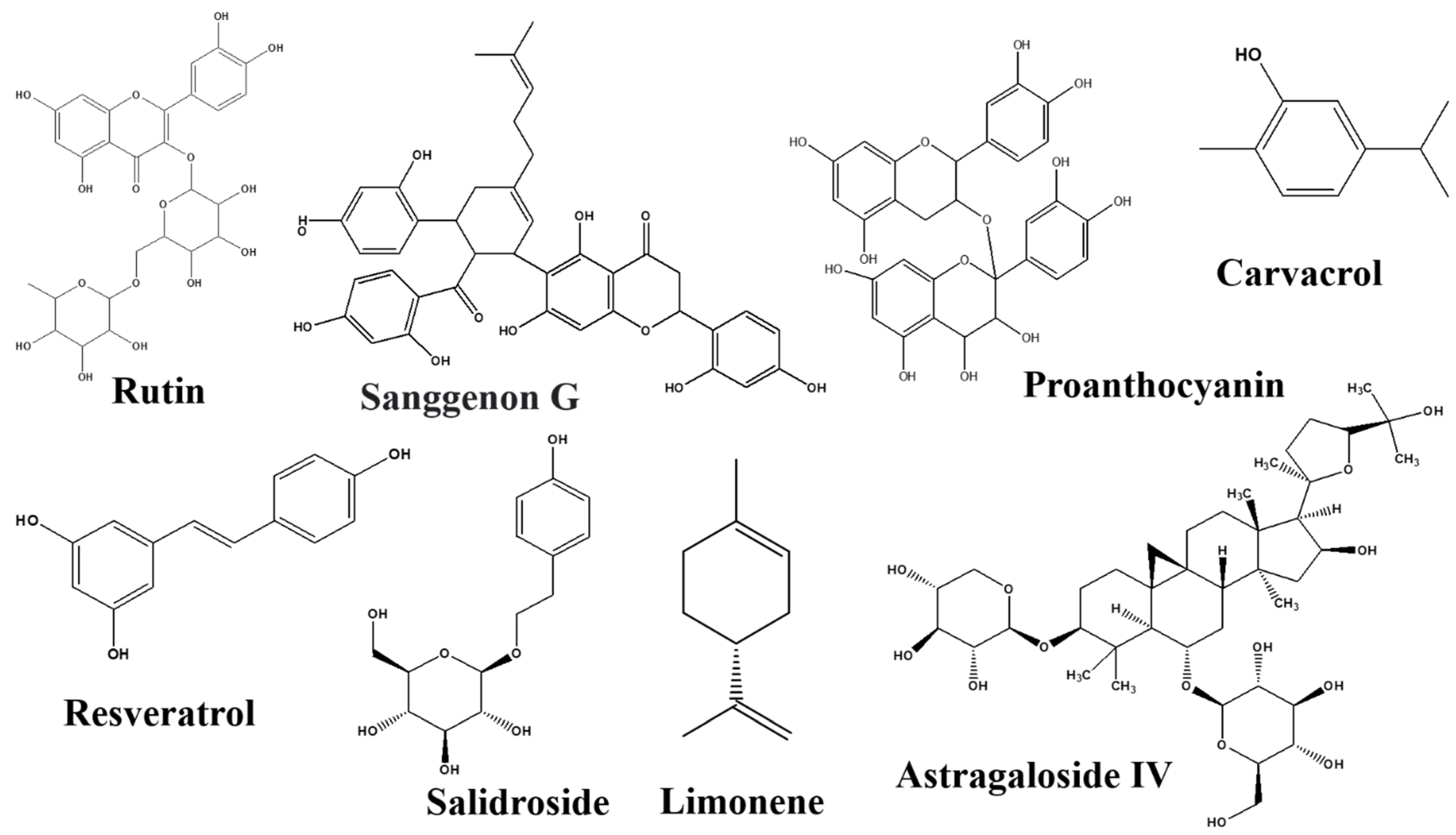
Rutin
Sanggenon G
Salidroside
Resveratrol
3.4.6. Terpenoids
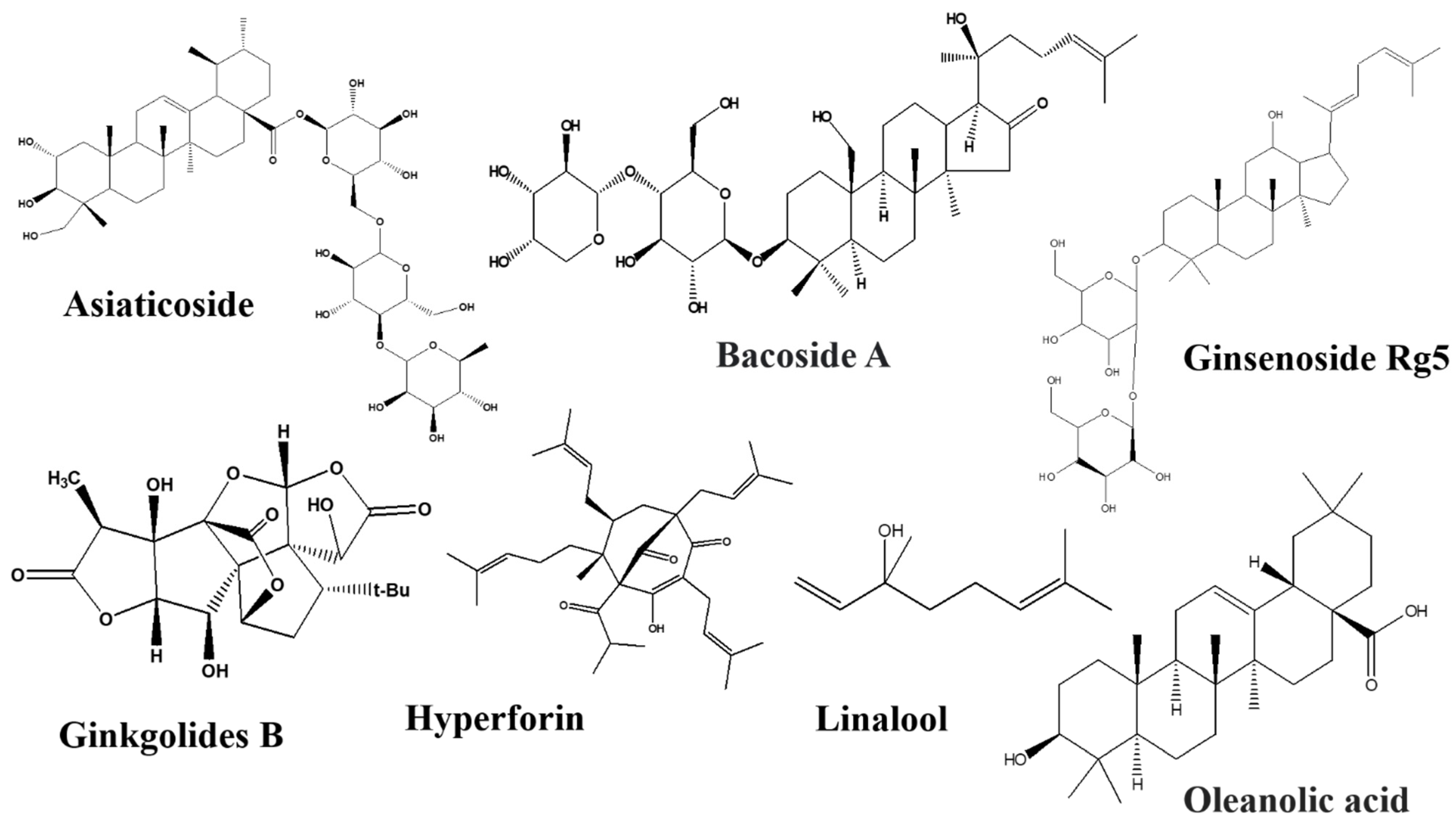
Astragaloside IV
Asiaticoside
Bacosides
Carvacrol
Ginkgolides
Linalool
Ginsenosides Rb1 and Rg5
Limonene
Oleanolic Acid
3.4.7. Fatty Acids
Omega-3 Fatty Acid
3.4.8. Phloroglucinol Derivative
Hyperforin
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.-X.; Wang, Y.-P. Gut microbiota-brain axis. Chin. Med. J. 2016, 129, 2373. [Google Scholar] [CrossRef] [PubMed]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Giaroni, C.; Baj, A. Tryptophan metabolites along the microbiota-gut-brain axis: An interkingdom communication system influencing the gut in health and disease. Int. J. Res. 2020, 13, 1178646920928984. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Pi, Y.; Mu, C.L.; Farzi, A.; Liu, Z.; Zhu, W.Y. Increasing carbohydrate availability in the hindgut promotes hypothalamic neurotransmitter synthesis: Aromatic amino acids linking the microbiota–brain axis. J. Neurochem. 2019, 149, 641–659. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Getselter, D.; Koren, O.; Elliott, E. Role of tryptophan in microbiota-induced depressive-like behavior: Evidence from tryptophan depletion study. Front. Behav. Neurosci. 2019, 13, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Kaur, H.; Bose, C.; Mande, S.S. Tryptophan metabolism by gut microbiome and gut-brain-axis: An in silico analysis. Front. Neurosci. 2019, 13, 1365. [Google Scholar] [CrossRef]
- Gracie, D.J.; Hamlin, P.J.; Ford, A.C. The influence of the brain–gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol. Hepatol. 2019, 4, 632–642. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Waclawiková, B.; El Aidy, S. Role of microbiota and tryptophan metabolites in the remote effect of intestinal inflammation on brain and depression. Pharmaceuticals 2018, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Barik, S. The uniqueness of tryptophan in biology: Properties, metabolism, interactions and localization in proteins. Int. J. Mol. Sci. 2020, 21, 8776. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan biochemistry: Structural, nutritional, metabolic, and medical aspects in humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, L.; Clarke, G.; Nolan, A.; Watkins, C.; Dinan, T.G.; Stanton, C.; Ross, R.P.; Ryan, C.A. Tryptophan metabolic profile in term and preterm breast milk: Implications for health. J. Nutr. Sci. 2018, 7, e13. [Google Scholar] [CrossRef] [Green Version]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [PubMed]
- Zelante, T.; Iannitti, R.G.; Cunha, C.; De Luca, A.; Giovannini, G.; Pieraccini, G.; Zecchi, R.; D’Angelo, C.; Massi-Benedetti, C.; Fallarino, F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013, 39, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breda, C.; Sathyasaikumar, K.V.; Idrissi, S.S.; Notarangelo, F.M.; Estranero, J.G.; Moore, G.G.; Green, E.W.; Kyriacou, C.P.; Schwarcz, R.; Giorgini, F. Tryptophan-2, 3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc. Natl. Acad. Sci. USA 2016, 113, 5435–5440. [Google Scholar] [CrossRef] [Green Version]
- Dong, F.; Hao, F.; Murray, I.A.; Smith, P.B.; Koo, I.; Tindall, A.M.; Kris-Etherton, P.M.; Gowda, K.; Amin, S.G.; Patterson, A.D. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 2020, 12, 1788899. [Google Scholar] [CrossRef]
- Bengmark, S. Gut microbiota, immune development and function. Pharmacol. Res. 2013, 69, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Dehhaghi, M.; Kazemi Shariat Panahi, H.; Guillemin, G.J. Microorganisms’ footprint in neurodegenerative diseases. Front. Cell. Neurosci. 2018, 12, 466. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Alexeev, E.E.; Lanis, J.M.; Kao, D.J.; Campbell, E.L.; Kelly, C.J.; Battista, K.D.; Gerich, M.E.; Jenkins, B.R.; Walk, S.T.; Kominsky, D.J. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 2018, 188, 1183–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardar, P.; Kempken, F. Characterization of indole-3-pyruvic acid pathway-mediated biosynthesis of auxin in Neurospora crassa. PLoS ONE 2018, 13, e0192293. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Daugé, V.; Maguin, E.; Naudon, L.; Rabot, S. Indole, a signaling molecule produced by the gut microbiota, negatively impacts emotional behaviors in rats. Front. Neurosci. 2018, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Mardinoglu, A.; Shoaie, S.; Bergentall, M.; Ghaffari, P.; Zhang, C.; Larsson, E.; Bäckhed, F.; Nielsen, J. The gut microbiota modulates host amino acid and glutathione metabolism in mice. Mol. Syst. Biol. 2015, 11, 834. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Cervantes-Barragan, L.; Chai, J.N.; Tianero, M.D.; Di Luccia, B.; Ahern, P.P.; Merriman, J.; Cortez, V.S.; Caparon, M.G.; Donia, M.S.; Gilfillan, S. Lactobacillus reuteri induces gut intraepithelial CD4+ CD8αα+ T cells. Science 2017, 357, 806–810. [Google Scholar] [CrossRef] [Green Version]
- Wilck, N.; Matus, M.G.; Kearney, S.M.; Olesen, S.W.; Forslund, K.; Bartolomaeus, H.; Haase, S.; Mähler, A.; Balogh, A.; Markó, L. Salt-responsive gut commensal modulates TH 17 axis and disease. Nature 2017, 551, 585–589. [Google Scholar] [CrossRef]
- Dodd, D.; Spitzer, M.H.; Van Treuren, W.; Merrill, B.D.; Hryckowian, A.J.; Higginbottom, S.K.; Le, A.; Cowan, T.M.; Nolan, G.P.; Fischbach, M.A. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 2017, 551, 648–652. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe 2017, 22, 25–37.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, S.; Velagapudi, C.; Redus, L.; Thameem, F.; Kasinath, B.; Hura, C.E.; Lorenzo, C.; Abboud, H.E.; O’Connor, J.C. Tryptophan metabolism in patients with chronic kidney disease secondary to type 2 diabetes: Relationship to inflammatory markers. Int. J. Tryptophan. Res. 2017, 10, 1178646917694600. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Ma, N.; He, T.; Johnston, L.J.; Ma, X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit. Rev. Food Sci. Nutr. 2020, 60, 1760–1768. [Google Scholar] [CrossRef]
- Choi, S.-C.; Brown, J.; Gong, M.; Ge, Y.; Zadeh, M.; Li, W.; Croker, B.P.; Michailidis, G.; Garrett, T.J.; Mohamadzadeh, M. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci. Transl. Med. 2020, 12, eaax2220. [Google Scholar] [CrossRef] [PubMed]
- Galligan, J. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol. Motil. 2018, 30, e13283. [Google Scholar] [CrossRef]
- Gostner, J.M.; Geisler, S.; Stonig, M.; Mair, L.; Sperner-Unterweger, B.; Fuchs, D. Tryptophan metabolism and related pathways in psychoneuroimmunology: The impact of nutrition and lifestyle. Neuropsychobiology 2020, 79, 89–99. [Google Scholar] [CrossRef]
- Yadav, V.K. Serotonin: The central link between bone mass and energy metabolism. In Translational Endocrinology of Bone: Reproduction, Metabolism and the Central Nervous System; Academic Press: Cambridge, MA, USA, 2012; Volume 51. [Google Scholar]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.-E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M. Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [Green Version]
- Brint, E.K.; MacSharry, J.; Fanning, A.; Shanahan, F.; Quigley, E.M. Differential expression of toll-like receptors in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2011, 106, 329–336. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; III, J.F.R.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018, 175, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Lyte, M. Microbial endocrinology: Host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes 2014, 5, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Wu, J.; Zhang, H.; Perry, S.W.; Yin, B.; Tan, X.; Chai, T.; Liang, W.; Huang, Y.; Li, Y. The gut microbiome modulates gut–brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol. Psychiatry 2020, 26, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Ai, Z.; Zeng, S.; Song, Y.; Song, J.; Zeng, Q.; Liao, Z.; Wang, T.; Huang, C.; Su, D. Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway. Psychoneuroendocrinology 2020, 117, 104699. [Google Scholar] [CrossRef] [PubMed]
- Arentsen, T.; Raith, H.; Qian, Y.; Forssberg, H.; Heijtz, R.D. Host microbiota modulates development of social preference in mice. Microb. Ecol. Health Dis. 2015, 26, 29719. [Google Scholar] [CrossRef] [PubMed]
- Du, H.X.; Liu, Y.; Zhang, L.G.; Zhan, C.S.; Chen, J.; Zhang, M.; Chen, X.G.; Zhang, L.; Liang, C.Z. Abnormal gut microbiota composition is associated with experimental autoimmune prostatitis-induced depressive-like behaviors in mice. Prostate 2020, 80, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Malmevik, J.; Petri, R.; Knauff, P.; Brattås, P.L.; Åkerblom, M.; Jakobsson, J. Distinct cognitive effects and underlying transcriptome changes upon inhibition of individual miRNAs in hippocampal neurons. Sci. Rep. 2016, 6, 19879. [Google Scholar] [CrossRef] [Green Version]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.T.; Shanahan, F.; Dinan, T.t.; Cryan, J.t. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Dolšak, A.; Gobec, S.; Sova, M. Indoleamine and tryptophan 2, 3-dioxygenases as important future therapeutic targets. Pharmacol. Ther. 2020, 221, 107746. [Google Scholar] [CrossRef]
- Kindler, J.; Lim, C.; Shannon Weickert, C.; Boerrigter, D.; Galletly, C.; Liu, D.; Jacobs, K.; Balzan, R.; Bruggemann, J.; O’Donnell, M.; et al. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol. Psychiatry 2020, 25, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muneer, A. Kynurenine pathway of tryptophan metabolism in neuropsychiatric disorders: Pathophysiologic and therapeutic considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Morales-Puerto, N.; Giménez-Gómez, P.; Pérez-Hernández, M.; Abuin-Martínez, C.; Leticia, G.d.B.-E.; Vidal, R.; Gutiérrez-López, M.D.; O’Shea, E.; Colado, M.I. Addiction and the kynurenine pathway: A new dancing couple? Pharmacol. Ther. 2021, 223, 107807. [Google Scholar] [CrossRef]
- Savitz, J. Role of Kynurenine Metabolism Pathway Activation in Major Depressive Disorders. Curr. Top. Behav. Neurosci. 2017, 31, 249–267. [Google Scholar]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Escobar Galvis, M.L.; Brundin, P.; Huang, X.; et al. Tryptophan Metabolites Are Associated With Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 2020, 35, 2028–2037. [Google Scholar]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan metabolic pathways and brain serotonergic activity: A comparative review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Wolf, D.; Klasen, M.; Eisner, P.; Zepf, F.D.; Zvyagintsev, M.; Palomero-Gallagher, N.; Weber, R.; Eisert, A.; Mathiak, K. Central serotonin modulates neural responses to virtual violent actions in emotion regulation networks. Brain Struct. Funct. 2018, 223, 3327–3345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyer, D. Targeting the 5-HT system: Potential side effects. Neuropharmacology 2020, 179, 108233. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Type A monoamine oxidase and serotonin are coordinately involved in depressive disorders: From neurotransmitter imbalance to impaired neurogenesis. J. Neural. Transm. 2018, 125, 53–66. [Google Scholar] [CrossRef]
- Bader, M. Inhibition of serotonin synthesis: A novel therapeutic paradigm. Pharmacol. Ther. 2020, 205, 107423. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.; Dinan, T.; Cryan, J. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.L.; Egerod, K.L.; Engelstoft, M.S.; Dmytriyeva, O.; Theodorsson, E.; Patel, B.A.; Schwartz, T.W. Enterochromaffin 5-HT cells–A major target for GLP-1 and gut microbial metabolites. Mol. Metab. 2018, 11, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- Bikomo, E.; Magbagbeola, O.; Ebuehi, O. Antidepressant activity of ethanol leaf extract of Annona muricata L. in Sprague-Dawley rats. Am. J. Biochem. 2017, 7, 1–5. [Google Scholar]
- Diniz, T.C.; de Oliveira Júnior, R.G.; Medeiros, M.A.M.B.; de Silva, M.G.; de Andrade Teles, R.B.; dos Passos Menezes, P.; De Sousa, B.M.H.; Frank, L.A.; de Souza Araújo, A.A.; Serafini, M.R. Anticonvulsant, sedative, anxiolytic and antidepressant activities of the essential oil of Annona vepretorum in mice: Involvement of GABAergic and serotonergic systems. Biomed. Pharmacother. 2019, 111, 1074–1087. [Google Scholar] [CrossRef]
- Monteiro, Á.B.; de Souza Rodrigues, C.K.; do Nascimento, E.P.; dos Santos Sales, V.; de Araújo Delmondes, G.; da Costa, M.H.N.; de Oliveira, V.A.P.; de Morais, L.P.; Boligon, A.A.; Barbosa, R. Anxiolytic and antidepressant-like effects of Annona coriacea (Mart.) and caffeic acid in mice. Food Chem. Toxicol. 2020, 136, 111049. [Google Scholar] [CrossRef] [PubMed]
- Bergland, A.K.; Soennesyn, H.; Dalen, I.; Rodriguez-Mateos, A.; Berge, R.K.; Giil, L.M.; Rajendran, L.; Siow, R.; Tassotti, M.; Larsen, A.I.; et al. Effects of Anthocyanin Supplementation on Serum Lipids, Glucose, Markers of Inflammation and Cognition in Adults With Increased Risk of Dementia—A Pilot Study. Front. Genet. 2019, 10, 536. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J.; Zhou, Z. Anthocyanins from black chokeberry (Aronia melanocarpa Elliot) delayed aging-related degenerative changes of brain. J. Agric. Food Chem. 2017, 65, 5973–5984. [Google Scholar] [CrossRef]
- Fang, J.-L.; Luo, Y.; Jin, S.-H.; Yuan, K.; Guo, Y. Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and up-regulating BDNF expression. J. Funct. Foods 2020, 66, 103757. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, Y.; Tang, J.; Mi, J.; Lu, L.; Li, X.; Ran, L.; Zeng, X.; Cao, Y. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J. Funct. Foods 2018, 48, 533–541. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [Green Version]
- Chanana, P.; Kumar, A. Possible involvement of nitric oxide modulatory mechanisms in the neuroprotective effect of Centella asiatica against sleep deprivation induced anxiety like behaviour, oxidative damage and neuroinflammation. Phytother. Res. 2016, 30, 671–680. [Google Scholar] [CrossRef]
- Marques, N.F.; Stefanello, S.T.; Froeder, A.L.; Busanello, A.; Boligon, A.A.; Athayde, M.L.; Soares, F.A.; Fachinetto, R. Centella asiatica and its fractions reduces lipid peroxidation induced by quinolinic acid and sodium nitroprusside in rat brain regions. Neurochem. Res. 2015, 40, 1197–1210. [Google Scholar] [CrossRef]
- Sbrini, G.; Brivio, P.; Fumagalli, M.; Giavarini, F.; Caruso, D.; Racagni, G.; Dell’Agli, M.; Sangiovanni, E.; Calabrese, F. Centella asiatica L. Phytosome improves cognitive performance by promoting bdnf expression in rat prefrontal cortex. Nutrients 2020, 12, 355. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, X.; Liu, J.; Chen, M.; Huang, M.; Huang, G.; Chen, X.; Du, Q.; Su, J.; Lin, R. Ethanol extract of Centella asiatica alleviated dextran sulfate sodium-induced colitis: Restoration on mucosa barrier and gut microbiota homeostasis. J. Ethnopharmacol. 2021, 267, 113445. [Google Scholar] [CrossRef] [PubMed]
- Abdul Manap, A.S.; Vijayabalan, S.; Madhavan, P.; Chia, Y.Y.; Arya, A.; Wong, E.H.; Rizwan, F.; Bindal, U.; Koshy, S. Bacopa monnieri, a neuroprotective lead in Alzheimer Disease: A review on its properties, mechanisms of action, and preclinical and clinical studies. Drug Target Insights 2019, 13, 1177392819866412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazra, S.; Kumar, S.; Saha, G.K.; Mondal, A.C. Reversion of BDNF, Akt and CREB in hippocampus of chronic unpredictable stress induced rats: Effects of phytochemical, Bacopa monnieri. Psychiatry Investig. 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.J.; Jung, H.Y.; Hahn, K.R.; Kim, W.; Kim, J.W.; Yoo, D.Y.; Yoon, Y.S.; Hwang, I.K.; Kim, D.W. Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus. Lab. Anim. Res. 2018, 34, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivasangari, K.; Rajan, K.E. Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring. Antioxidants 2020, 9, 1229. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Di Stefano, A.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From ancient flavoring to neuromodulatory agent. Molecules 2013, 18, 6161–6172. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, Z.; Salmani, H.; Marefati, N.; Arab, Z.; Gholamnezhad, Z.; Beheshti, F.; Shafei, M.N.; Hosseini, M. Protective effects of carvacrol on brain tissue inflammation and oxidative stress as well as learning and memory in lipopolysaccharide-challenged rats. Neurotox. Res. 2019, 37, 965–976. [Google Scholar] [CrossRef]
- Lee, B.; Yeom, M.; Shim, I.; Lee, H.; Hahm, D.-H. Inhibitory effect of carvacrol on lipopolysaccharide-induced memory impairment in rats. Korean J. Physiol. Pharmacol. 2020, 24, 27. [Google Scholar] [CrossRef] [Green Version]
- Stringer, T.P.; Guerrieri, D.; Vivar, C.; van Praag, H. Plant-derived flavanol (-)epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl. Psychiatry 2015, 5, e493. [Google Scholar] [CrossRef]
- Rothenberg, D.O.N.; Zhang, L. Mechanisms underlying the anti-depressive effects of regular tea consumption. Nutrients 2019, 11, 1361. [Google Scholar] [CrossRef] [Green Version]
- Suganthy, N.; Devi, K.P. Protective effect of catechin rich extract of Rhizophora mucronata against β-amyloid-induced toxicity in PC12 cells. J. Appl. Biom. 2016, 14, 137–146. [Google Scholar] [CrossRef]
- Santamaría-del Ángel, D.; Labra-Ruíz, N.A.; García-Cruz, M.E.; Calderón-Guzmán, D.; Valenzuela-Peraza, A.; Juárez-Olguín, H. Comparative effects of catechin, epicatechin and N-Ω-nitroarginine on quinolinic acid-induced oxidative stress in rat striatum slices. Biomed. Pharmacother. 2016, 78, 210–215. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Salman, M.; Kaushik, P.; Bharadwaj, N.; Aggarwal, N.B.; Tabassum, H.; Parvez, S. Chrysin ameliorates 3 nitropropinoic acid induced neurotoxicity targeting behavioural, biochemical and histological alterations. Int. J. Neurosci. 2020, 132, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, A.Q.; Qamar, W.; Lateef, A.; Ali, F.; Rehman, M.U.; Tahir, M.; Sharma, S.; Sultana, S. Chrysin abrogates cisplatin-induced oxidative stress, p53 expression, goblet cell disintegration and apoptotic responses in the jejunum of Wistar rats. Br. J. Nutr. 2012, 108, 1574–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çelik, H.; Kucukler, S.; Çomaklı, S.; Caglayan, C.; Özdemir, S.; Yardım, A.; Karaman, M.; Kandemir, F.M. Neuroprotective effect of chrysin on isoniazid-induced neurotoxicity via suppression of oxidative stress, inflammation and apoptosis in rats. Neurotoxicology 2020, 81, 197–208. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharm. 2012, 26, 1512–1524. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.-R.; Wang, L.; Li, J.; Wu, D.-S. Analysis of anti-depressant potential of curcumin against depression induced male albino wistar rats. Brain Res. 2016, 1642, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence—A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Li, G.; Chen, X.; Hu, S.; Zheng, L.; Luria, V.; Lv, J.; Sun, Y.; Xu, Y. Sub-acute treatment of curcumin derivative J147 ameliorates depression-like behavior through 5-HT1A-mediated cAMP signaling. Front. Neurosci. 2020, 14, 701. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.Y.; Fatemi, I.; Kalantari, H.; Mombeini, M.A.; Mehrzadi, S.; Goudarzi, M. Ellagic acid prevents oxidative stress, inflammation, and histopathological alterations in acrylamide-induced hepatotoxicity in wistar rats. J. Diet. Suppl. 2020, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Bedel, H.A.; Kencebay Manas, C.; Özbey, G.; Usta, C. The antidepressant-like activity of ellagic acid and its effect on hippocampal brain derived neurotrophic factor levels in mouse depression models. Nat. Prod. Res. 2018, 32, 2932–2935. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, W.; You, B.; Tang, W.; Gan, T.; Feng, C.; Li, C.; Yang, R. Serum Metabonomic Study on the Antidepressant-like Effects of Ellagic Acid in a Chronic Unpredictable Mild Stress-Induced Mouse Model. J. Agric. Food Chem. 2020, 68, 9546–9556. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Lee, R.; Henning, S.M.; Wang, J.; Pan, Y.; Qing, T.; Hsu, M.; Nguyen, A.; Prabha, S. Pomegranate Metabolites Impact Tryptophan Metabolism in Humans and Mice. Curr. Dev. Nutr. 2020, 4, nzaa165. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A.; ur Rahman, U.; Khan, M.R.; Sahar, A.; Mehmood, T.; Khan, M. Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv. 2017, 7, 32669–32681. [Google Scholar] [CrossRef] [Green Version]
- Wie, M.-B.; Won, M.-H.; Lee, K.-H.; Shin, J.-H.; Lee, J.-C.; Suh, H.-W.; Song, D.-K.; Kim, Y.-H. Eugenol protects neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci. Lett. 1997, 225, 93–96. [Google Scholar] [CrossRef]
- Garabadu, D.; Shah, A.; Singh, S.; Krishnamurthy, S. Protective effect of eugenol against restraint stress-induced gastrointestinal dysfunction: Potential use in irritable bowel syndrome. Pharm. Biol. 2015, 53, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Mesole, S.B.; Alfred, O.O.; Yusuf, U.A.; Lukubi, L.; Ndhlovu, D. Apoptotic Inducement of Neuronal Cells by Aluminium Chloride and the Neuroprotective Effect of Eugenol in Wistar Rats. Oxid. Med. Cell. Longev. 2020, 2020, 8425643. [Google Scholar] [CrossRef] [Green Version]
- Erseçkin, V.; Mert, H.; İrak, K.; Yildirim, S.; Mert, N. Nephroprotective effect of ferulic acid on gentamicin-induced nephrotoxicity in female rats. Drug Chem. Toxicol. 2020, 45, 663–669. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, W.; Zhang, Y.; Wang, Q.; Qin, C.; Du, S.; Huang, L.; Ye, F.; Chen, L.; Zheng, T. Pharmacokinetics, bioavailability, and tissue distribution study of angoroside C and its metabolite ferulic acid in rat using UPLC-MS/MS. Front. Pharmacol. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Zaid, O.A.R.; El-Sonbaty, S.M.; Barakat, W. Ameliorative effect of selenium nanoparticles and ferulic acid on acrylamide-induced neurotoxicity in rats. Ann. Med. Biomed. Sci. 2017, 3, 35–45. [Google Scholar]
- Chen, J.; Lin, D.; Zhang, C.; Li, G.; Zhang, N.; Ruan, L.; Yan, Q.; Li, J.; Yu, X.; Xie, X. Antidepressant-like effects of ferulic acid: Involvement of serotonergic and norepinergic systems. Metab. Brain Dis. 2015, 30, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-M.; Shen, J.-D.; Xu, L.-P.; Li, H.-B.; Li, Y.-C.; Yi, L.-T. Ferulic acid inhibits neuro-inflammation in mice exposed to chronic unpredictable mild stress. Int. Immunopharmacol. 2017, 45, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, F.; Razavi, B.M.; Hosseinzadeh, H. The effects of Ginkgo biloba on metabolic syndrome: A review. Phytother. Res. 2020, 34, 1798–1811. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, Q.; Chen, W.; Bai, Y.; Hu, D.; Xie, X. The neuroprotective mechanisms of ginkgolides and bilobalide in cerebral ischemic injury: A literature review. Mol. Med. 2019, 25, 57. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, W.; Zhang, L.; Liu, M. Ginkgolide B attenuates myocardial infarction-induced depression-like behaviors via repressing IL-1β in central nervous system. Int. Immunopharmacol. 2020, 85, 106652. [Google Scholar] [CrossRef]
- Chen, P.; Hei, M.; Kong, L.; Liu, Y.; Yang, Y.; Mu, H.; Zhang, X.; Zhao, S.; Duan, J. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019, 10, 8161–8171. [Google Scholar] [CrossRef]
- Wang, G.-L.; He, Z.-M.; Zhu, H.-Y.; Gao, Y.-G.; Zhao, Y.; Yang, H.; Zhang, L.-X. Involvement of serotonergic, noradrenergic and dopaminergic systems in the antidepressant-like effect of ginsenoside Rb1, a major active ingredient of Panax ginseng C.A. Meyer. J. Ethnopharmacol. 2017, 204, 118–124. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Zhao, W.; Gao, S.; Cui, Z. Antidepressant-like effects of ginsenoside Rg5 in mice: Involving of hippocampus BDNF signaling pathway. Neurosci. Lett. 2017, 645, 97–105. [Google Scholar] [CrossRef]
- Wang, G.; Lei, C.; Tian, Y.; Wang, Y.; Zhang, L.; Zhang, R. Rb1, the Primary Active Ingredient in Panax ginseng C.A. Meyer, Exerts Antidepressant-Like Effects via the BDNF–Trkb–CREB Pathway. Front. Pharmacol. 2019, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Abbasi, M.M.; Salari, A.-A. Hesperidin attenuates depression-related symptoms in mice with mild traumatic brain injury. Life Sci. 2018, 213, 198–205. [Google Scholar] [CrossRef]
- Noshy, P.A.; Azouz, R.A. Neuroprotective effect of hesperidin against emamectin benzoate-induced neurobehavioral toxicity in rats. Neurotoxicol. Teratol. 2021, 106981. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.; Kim, J.N.; Kim, B.J. Hesperidin depolarizes the pacemaker potentials through 5-HT4 receptor in murine small intestinal interstitial cells of Cajal. Anim. Cells Syst. 2020, 24, 84–90. [Google Scholar] [CrossRef] [Green Version]
- El-Bakly, W.M.; Hasanin, A.H. Hypericum perforatum decreased hippocampus TNF-α and corticosterone levels with no effect on kynurenine/tryptophan ratio in bilateral ovariectomized rats. Korean J. Physiol. Pharmacol. 2014, 18, 233. [Google Scholar] [CrossRef] [Green Version]
- Eatemadnia, A.; Ansari, S.; Abedi, P.; Najar, S. The effect of Hypericum perforatum on postmenopausal symptoms and depression: A randomized controlled trial. Complementary Ther. Med. 2019, 45, 109–113. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C. Neuroprotective activity of Hypericum perforatum and its major components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Zhang, F.; Cheng, J.; Guo, S.; Liu, P.; Wang, H. Antidepressant-like activity of adhyperforin, a novel constituent of Hypericum perforatum L. Sci. Rep. 2014, 4, 5632. [Google Scholar] [CrossRef] [Green Version]
- Yun, J. Limonene inhibits methamphetamine-induced locomotor activity via regulation of 5-HT neuronal function and dopamine release. Phytomedicine 2014, 21, 883–887. [Google Scholar] [CrossRef]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Funct. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Piccialli, I.; Ciccone, R.; de Caprariis, P.; Massa, A.; De Feo, V.; Pannaccione, A. Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phytother. Res. 2021, 35, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Khaleghi Ghadiri, M.; Gorji, A. Lavender and the nervous system. Evid. Based Complementary Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef] [Green Version]
- Fißler, M.; Quante, A. A case series on the use of lavendula oil capsules in patients suffering from major depressive disorder and symptoms of psychomotor agitation, insomnia and anxiety. Complementary Ther. Med. 2014, 22, 63–69. [Google Scholar] [CrossRef]
- Chioca, L.R.; Ferro, M.M.; Baretta, I.P.; Oliveira, S.M.; Silva, C.R.; Ferreira, J.; Losso, E.M.; Andreatini, R. Anxiolytic-like effect of lavender essential oil inhalation in mice: Participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J. Ethnopharmacol. 2013, 147, 412–418. [Google Scholar] [CrossRef] [Green Version]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Tambe, R.; Patil, A.; Jain, P.; Sancheti, J.; Somani, G.; Sathaye, S. Assessment of luteolin isolated from Eclipta alba leaves in animal models of epilepsy. Pharm. Biol. 2017, 55, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Lei, S.; Zhou, S.; Jin, L.; Zeng, S.; Jiang, H.; Zhou, H. Luteolin shows antidepressant-like effect by inhibiting and downregulating plasma membrane monoamine transporter (PMAT, Slc29a4). J. Funct. Foods 2019, 54, 440–448. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, N.; Bao, B.; Wang, L.; Chen, J.; Liu, J. Luteolin reduces fat storage in Caenorhabditis elegans by promoting the central serotonin pathway. Food Funct. 2020, 11, 730–740. [Google Scholar] [CrossRef]
- Li, F.; Xiang, H.; Lu, J.; Chen, Z.; Huang, C.; Yuan, X. Lycopene ameliorates PTSD-like behaviors in mice and rebalances the neuroinflammatory response and oxidative stress in the brain. Physiol. Behav. 2020, 224, 113026. [Google Scholar] [CrossRef] [PubMed]
- Farouk, S.M.; Gad, F.A.; Almeer, R.; Abdel-Daim, M.M.; Emam, M.A. Exploring the possible neuroprotective and antioxidant potency of lycopene against acrylamide-induced neurotoxicity in rats’ brain. Biomed. Pharmacother. 2021, 138, 111458. [Google Scholar] [CrossRef] [PubMed]
- El Morsy, E.; Ahmed, M. Protective effects of lycopene on hippocampal neurotoxicity and memory impairment induced by bisphenol A in rats. Hum. Exp. Toxicol. 2020, 39, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, H.; Aschner, M.; Hasan, M.M.; Hassan, S.T. Therapeutic potential of naringin in neurological disorders. Food Chem. Toxicol. 2019, 132, 110646. [Google Scholar] [CrossRef]
- Cui, J.; Wang, G.; Kandhare, A.D.; Mukherjee-Kandhare, A.A.; Bodhankar, S.L. Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: Possible role of PPAR-γ, Bax/Bcl-2, and caspase-3. Food Chem. Toxicol. 2018, 121, 95–108. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; Polepally, P.R.; Chaurasiya, N.D.; Tekwani, B.L.; Zjawiony, J.K.; Costa, E.A. Oleanolic acid acrylate elicits antidepressant-like effect mediated by 5-HT 1A receptor. Sci. Rep. 2015, 5, 11582. [Google Scholar] [CrossRef] [Green Version]
- Niculau, E.D.S.; Ribeiro, L.d.P.; Ansante, T.F.; Fernandes, J.B.; Forim, M.R.; Vieira, P.C.; Vendramim, J.D.; Da Silva, M.F.d.G.F. Isolation of Chavibetol and Methyleugenol from Essential Oil of Pimenta pseudocaryophyllus by High Performance Liquid Chromatography. Molecules 2018, 23, 2909. [Google Scholar] [CrossRef] [Green Version]
- Fajemiroye, J.O.; Martins, J.L.R.; Ghedini, P.C.; Galdino, P.M.; Paula, J.A.M.D.; Realino de Paula, J.; Da Rocha, F.F.; Costa, E.A. Antidepressive-Like Property of Dichloromethane Fraction of Pimenta pseudocaryophyllus and Relevance of Monoamine Metabolic Enzymes. Evid. Based Complementary Altern. Med. 2013, 2013, 659391. [Google Scholar] [CrossRef] [Green Version]
- Fajemiroye, J.O.; Galdino, P.M.; De Paula, J.A.M.; Rocha, F.F.; Akanmu, M.A.; Vanderlinde, F.A.; Zjawiony, J.K.; Costa, E.A. Anxiolytic and antidepressant like effects of natural food flavour (E)-methyl isoeugenol. Food Funct. 2014, 5, 1819–1828. [Google Scholar] [CrossRef]
- Achour, I.; Arel-Dubeau, A.-M.; Renaud, J.; Legrand, M.; Attard, E.; Germain, M.; Martinoli, M.-G. Oleuropein prevents neuronal death, mitigates mitochondrial superoxide production and modulates autophagy in a dopaminergic cellular model. Int. J. Mol. Sci. 2016, 17, 1293. [Google Scholar] [CrossRef] [Green Version]
- Millman, J.F.; Okamoto, S.; Teruya, T.; Uema, T.; Ikematsu, S.; Shimabukuro, M.; Masuzaki, H. Extra-virgin olive oil and the gut-brain axis: Influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutr. Rev. 2021, 79, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- De Gomes, M.G.; Souza, L.C.; Goes, A.R.; Del Fabbro, L.; Borges Filho, C.; Donato, F.; Prigol, M.; Luchese, C.; Roman, S.S.; Puntel, R.L. Fish oil ameliorates sickness behavior induced by lipopolysaccharide in aged mice through the modulation of kynurenine pathway. J. Nutr. Biochem. 2018, 58, 37–48. [Google Scholar] [CrossRef]
- Correa, C.R.; Schena, C.; Lopes, S.C.; Prediger, R.D.; Silva, E.; Venske, D.K.; Ribeiro, L.; Moreira, J. Combined effects of caloric restriction and fish oil attenuated anti-depressant and anxiolytic-like effects of fish oil: Association with hippocampal BDNF concentrations. Behav. Brain Res. 2020, 393, 112770. [Google Scholar] [CrossRef]
- Carabelli, B.; Delattre, A.M.; Waltrick, A.P.F.; Araújo, G.; Suchecki, D.; Machado, R.B.; de Souza, L.E.R.; Zanata, S.M.; Zanoveli, J.M.; Ferraz, A.C. Fish-oil supplementation decreases Indoleamine-2, 3-Dioxygenase expression and increases hippocampal serotonin levels in the LPS depression model. Behav. Brain Res. 2020, 390, 112675. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-E.; Kim, E.-Y.; Park, Y. N-3 PUFA improved pup separation-induced postpartum depression via serotonergic pathway regulated by miRNA. J. Nutr. Biochem. 2020, 84, 108417. [Google Scholar] [CrossRef]
- Salman, M.; Tabassum, H.; Parvez, S. Piperine mitigates behavioral impairments and provides neuroprotection against 3-nitropropinoic acid-induced Huntington disease-like symptoms. Nutr. Neurosci. 2020, 25, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Z.; Wang, W.; Wei, M.; Kou, D.; Li, T.; Yang, Z.; Guo, H.; Le, W.; Li, S. Piperine attenuates cognitive impairment in an experimental mouse model of sporadic Alzheimer’s disease. J. Nutr. Biochem. 2019, 70, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, X.; Shi, M.; Xue, L.; Kuang, S.; Xu, R.; Qi, W.; Li, Y.; Ma, X.; Zhang, R.; et al. Identification and optimization of piperine analogues as neuroprotective agents for the treatment of Parkinson’s disease via the activation of Nrf2/keap1 pathway. Eur. J. Med. Chem. 2020, 199, 112385. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, C.; Wu, F.; Xu, X.; Wang, G.; Lin, M.; Yu, Y.; An, Y.; Pan, J. Piperine potentiates the effects of trans-resveratrol on stress-induced depressive-like behavior: Involvement of monoaminergic system and cAMP-dependent pathway. Metab. Brain Dis. 2016, 31, 837–848. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Chen, R.; Li, G.; Barish, P.A.; You, W.; Chen, L.; Lin, M.; Ku, B.; Pan, J. Antidepressant-like effect of low molecular proanthocyanidin in mice: Involvement of monoaminergic system. Pharmacol. Biochem. Behav. 2010, 94, 447–453. [Google Scholar] [CrossRef]
- Sun, Q.; Jia, N.; Ren, F.; Li, X. Grape seed proanthocyanidins improves depression-like behavior by alleviating oxidative stress and NLRP3 activation in the hippocampus of prenatally-stressed female offspring rats. J. Histotechnol. 2021, 44, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and depression in animal models: A systematic review of the biological mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, I.; Shih, J.-H.; Jhao, Y.-T.; Chen, H.-C.; Chiu, C.-H.; Chen, C.-F.F.; Huang, Y.-S.; Shiue, C.-Y.; Ma, K.-H. Regulation of noise-induced loss of serotonin transporters with resveratrol in a rat model using 4-[18f]-ADAM/small-animal positron emission tomography. Molecules 2019, 24, 1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.C.; Li, J.; Zhang, M.; Pan, J.C.; Yu, Y.; Zhang, J.B.; Zheng, L.; Si, J.M.; Xu, Y. Resveratrol Improves Brain-Gut Axis by Regulation of 5-HT-Dependent Signaling in the Rat Model of Irritable Bowel Syndrome. Front. Cell. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef] [Green Version]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef]
- Çelik, H.; Kandemir, F.M.; Caglayan, C.; Özdemir, S.; Çomaklı, S.; Kucukler, S.; Yardım, A. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol. Biol. Rep. 2020, 47, 2023–2034. [Google Scholar] [CrossRef]
- Xie, Z.; Lu, H.; Yang, S.; Zeng, Y.; Wei, L.; Wang, L.; Luo, G.; Fang, F.; Zeng, T.; Cheng, W. Salidroside Attenuates Cognitive Dysfunction in Senescence-Accelerated Mouse Prone (SAMP8) Mice and Modulates Inflammation of the Gut-brain Axis. Front. Pharmacol. 2020, 11, 1905. [Google Scholar] [CrossRef] [PubMed]
- Jówko, E.; Sadowski, J.; Długołęcka, B.; Gierczuk, D.; Opaszowski, B.; Cieśliński, I. Effects of Rhodiola rosea supplementation on mental performance, physical capacity, and oxidative stress biomarkers in healthy men. J. Sport Health Sci. 2018, 7, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zeng, Y.; Qu, Z.; Tang, J.; Qin, Y.; Chung, P.; Wong, R.; Hägg, U. The effects of Rhodiola rosea extract on 5-HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine 2009, 16, 830–838. [Google Scholar] [CrossRef]
- Nade, V.S.; Kawale, L.A.; Naik, R.A.; Yadav, A.V. Adaptogenic effect of Morus alba on chronic footshock-induced stress in rats. Indian J. Pharmacol. 2009, 41, 246. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.W.; Jung, J.-W.; Park, J.-H.; Baek, N.-I.; Kim, Y.T.; Kim, I.-H.; Han, D. Antidepressant-like effects of sanggenon G, isolated from the root bark of Morus alba, in rats: Involvement of the serotonergic system. Biol. Pharm. Bull. 2015, b15-00471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawvised, S.; Wattanathorn, J.; Thukham-mee, W. Neuroprotective and Cognitive-Enhancing Effects of Microencapsulation of Mulberry Fruit Extract in Animal Model of Menopausal Women with Metabolic Syndrome. Oxid. Med. Cell. Longev. 2017, 2017, 2962316. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Gong, Z.; Lin, L.; Xu, W.; Zhang, T.; Zhang, S.; Li, Y.; Chen, J.; Xiao, W. Effects of l-theanine on glutamine metabolism in enterotoxigenic Escherichia coli (E44813)-stressed and non-stressed rats. J. Funct. Foods 2020, 64, 103670. [Google Scholar] [CrossRef]
- Tamano, H.; Fukura, K.; Suzuki, M.; Sakamoto, K.; Yokogoshi, H.; Takeda, A. Advantageous effect of theanine intake on cognition. Nutr. Neurosci. 2014, 17, 279–283. [Google Scholar] [CrossRef] [PubMed]
- White, D.J.; De Klerk, S.; Woods, W.; Gondalia, S.; Noonan, C.; Scholey, A.B. Anti-Stress, Behavioural and Magnetoencephalography Effects of an l-Theanine-Based Nutrient Drink: A Randomised, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2016, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Yang, Y.; Wu, Y.; Zhang, B.; Wu, H.; Wang, L.; Tang, H.; Chen, J. L-theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic–cortical–striatal–pallidal–thalamic-circuit related brain regions. Phytother. Res. 2019, 33, 412–421. [Google Scholar] [CrossRef]
- Singh, R. Role of tryptophan in health and disease: Systematic review of the anti-oxidant, anti-inflammation, and nutritional aspects of tryptophan and its metabolites. World Heart J. 2019, 11, 161–178. [Google Scholar]
- Molad, M.; Ashkenazi, L.; Gover, A.; Lavie-Nevo, K.; Zaltsberg-Barak, T.; Shaked-Mishan, P.; Soloveichik, M.; Kessel, I.; Rotschild, A.; Etzioni, T. Melatonin stability in human milk. Breastfeed. Med. 2019, 14, 680–682. [Google Scholar] [CrossRef]
- Engler, A.C.; Hadash, A.; Shehadeh, N.; Pillar, G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. Eur. J. Pediatr. 2012, 171, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Camps-Bossacoma, M.; Garcia-Aloy, M.; Saldaña-Ruiz, S.; Cambras, T.; González-Domínguez, R.l.; Franch, A.n.; Pérez-Cano, F.J.; Andres-Lacueva, C.; Castell, M. Role of theobromine in cocoa’s metabolic properties in healthy rats. J. Agric. Food Chem. 2019, 67, 3605–3614. [Google Scholar] [CrossRef]
- Bertazzo, A.; Comai, S.; Brunato, I.; Zancato, M.; Costa, C.V. The content of protein and non-protein (free and protein-bound) tryptophan in Theobroma cacao beans. Food Chem. 2011, 124, 93–96. [Google Scholar] [CrossRef]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect, and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.S.; Chintalapati, M.; Mohan, G.K. A Comparative Study of Methanolic and Hydro-Alcoholic Extracts of Moringa oleifera Pods on Memory Enhancing Activity. J. Sci. Res. 2020, 64, 27–33. [Google Scholar] [CrossRef]
- Yunusa, S.; Musa, A. Ethyl-acetate and aqueous fractions of Moringa oleifera Lam (Moringaceae) leaf extract possess antidepressant activity in mice. Afr. J. Pharmacol. Ther. 2018, 7, 1–6. [Google Scholar]
- Bhattacharya, A.; Santra, S.; Mahapatra, S.; Sahu, P.K.; Agrawal, D.; Kumar, S. Study of anxiolytic effect of ethanolic extract of drumstick tree leaves on albino mice in a basic neuropharmacology laboratory of a postgraduate teaching institute. J. Health Res. Rev. 2016, 3, 41. [Google Scholar] [CrossRef]
- Debnath, S.; Guha, D. Role of Moringa oleifera on enterochromaffin cell count and serotonin content of experimental ulcer model. Indian J. Exp. Biol. 2007, 45, 726–731. [Google Scholar]
- Kaur, G.; Invally, M.; Sanzagiri, R.; Buttar, H.S. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J. Ayurveda Integr. Med. 2015, 6, 273. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)—Phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ng, T.; Wang, C.; Li, N.; Wen, T.; Qiao, W.; Zhang, D.; Cheng, Z.; Liu, F. First isolation of tryptophan from edible lotus (Nelumbo nucifera Gaertn) rhizomes and demonstration of its antioxidant effects. Int. J. Food Sci. Nutr. 2010, 61, 346–356. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Nishimura, K.; Itoh, A.; Tanahashi, T.; Nakajima, H.; Oshiro, H.; Sun, S.; Toda, T.; Yamada, J. Serotonergic mechanisms are involved in antidepressant-like effects of bisbenzylisoquinolines liensinine and its analogs isolated from the embryo of Nelumbo nucifera Gaertner seeds in mice. J. Pharm. Pharmacol. 2015, 67, 1716–1722. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Furutani, S.; Nishimura, K.; Itoh, A.; Tanahashi, T.; Nakajima, H.; Oshiro, H.; Sun, S.; Yamada, J. Antidepressant-like effects of neferine in the forced swimming test involve the serotonin1A (5-HT1A) receptor in mice. Eur. J. Pharmacol. 2010, 634, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Huang, K.; Yan, Y.; Wan, P.; Peng, Y.; Zeng, X.; Cao, Y. Long-term consumption of 2-O-β-D-glucopyranosyl-L-ascorbic acid from the fruits of Lycium barbarum modulates gut microbiota in c57bl/6 mice. J. Agric. Food Chem. 2020, 68, 8863–8874. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, F.P.; Coşkun, H.; Sağlam, K.; Bozat, B.G. Lycium barbarum L.(goji berry) fruits improve anxiety, depression-like behaviors, and learning performance: The moderating role of sex. Turk. J. Biol. 2016, 40, 762–771. [Google Scholar] [CrossRef]
- Zhao, R.; Master, B.Q.; Master, B.M.; Cai, Y. Improving Activity of Lycium barbarum. Polysaccharide on Depressive Mice Induced by Reserpine. Iran. J. Pharm. Res. 2019, 18, 1556. [Google Scholar]
- Zhao, X.-Q.; Guo, S.; Lu, Y.-Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.-X.; Wang, H.-Q.; Zhang, W.-H.; Duan, J.-A. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; George, J.; Mohan, J. Pharmacology and Traditional Uses of Mimosa pudica. Int. J. Pharm. Sci. Drug Res. 2013, 5, 41–44. [Google Scholar]
- Patro, G.; Kumar Bhattamisra, S.; Kumar Mohanty, B. Effects of Mimosa pudica L. leaves extract on anxiety, depression and memory. Avicenna J. Phytomed. 2016, 6, 696–710. [Google Scholar] [PubMed]
- Duyu, T.; Khanal, P.; Khatib, N.A.; Patil, B.M. Mimosa pudica modulates neuroactive ligand-receptor interaction in Parkinson’s disease. Indian J. Pharm. Educ. 2020, 54, 732–739. [Google Scholar] [CrossRef]
- Gao, X.; Feng, Y.; Xue, H.; Meng, M.; Qin, X. Antidepressant-like effect of triterpenoids extracts from Poria cocos on the CUMS rats by 16S rRNA gene sequencing and LC–MS metabolomics. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 494–507. [Google Scholar] [CrossRef]
- Huang, H.-S.; Wu, H.-Y.; Chang, W.-T.; Lin, Y.-E.; Huang, Y.-J.; Lu, K.-H.; Lu, Y.-S.; Chen, M.-H.; Sheen, L.-Y. The Antidepressive and Anxiolytic Effects of Formula Consisted of Poria cocos and Cordyceps militaris Waster Medium Extract in Unpredictable Chronic Mild Stress Animal Model. Curr. Dev. Nutr. 2020, 4, 1212. [Google Scholar] [CrossRef]
- Sheihk, N.A.M.E.; Khalil, N.A. The effects of sage (Salvia officinalis) supplementations on the health stats of diabetic animal models: Gut microbiota in focus. Sci. J.Specif. Educ. Appl. Sci. 2018, 1, 254–266. [Google Scholar]
- Sarkoohi, P.; Fathalipour, M.; Ghasemi, F.; Javidnia, K.; Emamghoreishi, M. Antidepressant effects of the aqueous and hydroalcoholic extracts of Salvia mirzayanii and Salvia macrosiphon in male mice. Shiraz E Med. J. 2020, 21, e91276. [Google Scholar] [CrossRef] [Green Version]
- Tober, C.; Schoop, R. Modulation of neurological pathways by Salvia officinalis and its dependence on manufacturing process and plant parts used. BMC Complementary Altern. Med. 2019, 19, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Ortega, G.; González-Trujano, M.; Ángeles-López, G.; Brindis, F.; Vibrans, H.; Reyes-Chilpa, R. Tagetes lucida Cav.: Ethnobotany, phytochemistry and pharmacology of its tranquilizing properties. J. Ethnopharmacol. 2016, 181, 221–228. [Google Scholar] [CrossRef]
- Gabriela, G.-C.; Javier, A.-A.F.; Elisa, V.-A.; Gonzalo, V.-P.; Herlinda, B.-J. Antidepressant-like effect of Tagetes lucida Cav. extract in rats: Involvement of the serotonergic system. Am. J. Chin. Med. 2012, 40, 753–768. [Google Scholar] [CrossRef]
- Bonilla-Jaime, H.; Guadarrama-Cruz, G.; Alarcon-Aguilar, F.; Limón-Morales, O.; Vazquez-Palacios, G. Antidepressant-like activity of Tagetes lucida Cav. is mediated by 5-HT 1A and 5-HT 2A receptors. J. Nat. Med. 2015, 69, 463–470. [Google Scholar] [CrossRef]
- Al-Rahbi, B.; Zakaria, R.; Othman, Z.; Hassan, A.; Ahmad, A.H. Enhancement of BDNF concentration and restoration of the hypothalamic-pituitary-adrenal axis accompany reduced depressive-like behaviour in stressed ovariectomised rats treated with either Tualang honey or estrogen. Sci. World J. 2014, 2014, 310821. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R.; Abdul Aziz, C.B.; Othman, Z. Tualang honey attenuates noise stress-induced memory deficits in aged rats. Oxidative Med. Cell. Longev. 2016, 2016, 1549158. [Google Scholar] [CrossRef] [Green Version]
- Azman, K.F.; Zakaria, R. Honey as an antioxidant therapy to reduce cognitive ageing. Iran. J. Basic Med. Sci. 2019, 22, 1368–1377. [Google Scholar]
- Pannaraj, P.S.; Li, F.; Cerini, C.; Bender, J.M.; Yang, S.; Rollie, A.; Adisetiyo, H.; Zabih, S.; Lincez, P.J.; Bittinger, K. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatrics 2017, 171, 647–654. [Google Scholar] [CrossRef]
- Pogliani, L.; Baldelli, S.; Cattaneo, D.; Pileri, P.; Clementi, E.; Cetin, I.; Zuccotti, G. Selective serotonin reuptake inhibitors’ passage into human milk of lactating women. J. Matern. Fetal Neonatal Med. 2019, 32, 3020–3025. [Google Scholar] [CrossRef]
- Walker, W.A.; Meng, D. Breast Milk and Microbiota in the Premature Gut: A Method of Preventing Necrotizing Enterocolitis. Nestle Nutr. Inst. Workshop Ser. 2020, 94, 103–112. [Google Scholar] [PubMed]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Chen, X. Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 2020, 7, 53. [Google Scholar] [CrossRef]
- Ijaz, S.; Shoaib Khan, H.M.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef]
- Mahadevan, M.; Ramaswamy, R.; Banumathi, V. Mimosa pudica exerts Neuroprotection against mpp+ induced neurotoxicity in shsy5y cell lines-an in vitro model of Anti-parkinsonism. Int. J. Pharm. Pharm. Sci. 2017, 9, 21–26. [Google Scholar]
- Lopresti, A.L. Salvia (sage): A review of its potential cognitive-enhancing and protective effects. Drugs R&D 2017, 17, 53–64. [Google Scholar]
- Boussadia, A.; Kharoubi, O.; Lahouel, Z.; Benglia, A.; Aoues, A. Effect of aqueous Salvia officinalis extract on Aluminum chloride-induced neurotoxicity in female rats. Int. J. Pharm. Res. Allied Sci. 2020, 9, 139–150. [Google Scholar]
- Mohseni, I.; Peeri, M.; Azarbayjani, M.A. Dietary supplementation with Salvia officinalis L. and aerobic training attenuates memory deficits via the CREB-BDNF pathway in amyloid beta-injected rats. J. Med. Plants 2020, 1, 119–132. [Google Scholar] [CrossRef]
- Yahaya, R.; Zahary, M.N.; Othman, Z.; Ismail, R.; Him, N.A.S.N.; Abd Aziz, A.; Dahlan, R.; Jusoh, A.F. Tualang honey supplementation as cognitive enhancer in patients with schizophrenia. Heliyon 2020, 6, e03948. [Google Scholar] [CrossRef]
- Jiang, L.; Xie, M.; Chen, G.; Qiao, J.; Zhang, H.; Zeng, X. Phenolics and carbohydrates in buckwheat honey regulate the human intestinal microbiota. Evid. Based Complementary Altern. Med. 2020, 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Zahir, F.; Alhewairini, S.S.; Mahamood, M. The Gut–Brain Axis, Cognition and Honey. In Therapeutic Applications of Honey and its Phytochemicals: Vol. 1, Rehman, M.U., Majid, S., Eds.; Springer: Singapore, 2020; pp. 331–343. [Google Scholar]
- Martínez-Vázquez, M.; Estrada-Reyes, R.; Escalona, A.A.; Velázquez, I.L.; Martínez-Mota, L.; Moreno, J.; Heinze, G. Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J. Ethnopharmacol. 2012, 139, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Huang, Z.; Zhong, X.-M.; Xian, Y.-F.; Ip, S.-P. Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 2014, 261, 140–145. [Google Scholar] [CrossRef]
- Dong, J.; Gao, W.; Lu, D.; Wang, Y. Simultaneous extraction and analysis of four polyphenols from leaves of Lycium barbarum L. J. Food Biochem. 2011, 35, 914–931. [Google Scholar] [CrossRef]
- Jurado, S.R. Ingestion of goji berry lyciumbarbarum evaluation on plasma levels of total cholesterol lipid fractions glycaemia serotonin and arterial pressure. Cardiol. Res. Cardiovasc. Med. 2017, 2, 116. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; Abdel-Motal, S.M.; Malhat, S.M.; Mostafa, H.I.; Moselhy, A.A.; Beheiry, R.R.; Said, E.N. Curcumin mitigates neurotoxic and neurobehavioral changes of gentamicin and sodium salicylate in rats by adjusting oxidative stress and apoptosis. Life Sci. 2021, 265, 118824. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mombeini, M.A.; Fatemi, I.; Aminzadeh, A.; Kalantari, H.; Nesari, A.; Najafzadehvarzi, H.; Mehrzadi, S. Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol. Res. 2019, 41, 419–428. [Google Scholar] [CrossRef]
- Prasad, S.N.; Bharath, M.S. Neurorestorative effects of eugenol, a spice bioactive: Evidence in cell model and its efficacy as an intervention molecule to abrogate brain oxidative dysfunctions in the streptozotocin diabetic rat. Neurochem. Int. 2016, 95, 24–36. [Google Scholar] [CrossRef]
- Westfall, S.; Pasinetti, G.M. The gut microbiota links dietary polyphenols with management of psychiatric mood disorders. Front. Neurosci. 2019, 13, 1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Gu, Z.; Liu, H.; Jia, B.; Wang, Y.; Cao, M.; Song, R.; Zhang, Z.; Bian, Y. The Anti-Depressive Effects of Hesperidin and the Relative Mechanisms Based on the NLRP3 Inflammatory Signaling Pathway. Front. Pharmacol. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New Insights into the Metabolism of the Flavanones Eriocitrin and Hesperidin: A Comparative Human Pharmacokinetic Study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Anjomshoa, M.; Boroujeni, S.N.; Ghasemi, S.; Lorigooini, Z.; Amiri, A.; Balali-Dehkordi, S.; Amini-Khoei, H. Rutin via increase in the CA3 diameter of the Hippocampus exerted antidepressant-like effect in mouse model of maternal separation stress: Possible involvement of NMDA receptors. Behav. Neurol. 2020, 2020, 4813616. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.-D.; Zhang, Y.-W.; Wang, B.-Y.; Bai, L.; Lu, S.-F.; Zhu, L.-L.; Bai, M.; Li, Y.-C.; Xu, E.-P. Effects of resveratrol on the levels of ATP, 5-HT and GAP-43 in the hippocampus of mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 2020, 735, 135232. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y. Resveratrol suppresses rotenone-induced neurotoxicity through activation of SIRT1/Akt1 signaling pathway. Anat. Rec. 2018, 301, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Zhao, Y.; Zhou, Z.; Yan, J.; Zhou, B.; Chi, X.; Luo, A.; Li, S. Resveratrol mitigates sevoflurane-induced neurotoxicity by the SIRT1-dependent regulation of BDNF expression in developing mice. Oxid. Med. Cell. Longev. 2020, 2020, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-C.; Tsay, H.-J.; Lu, M.-K.; Lin, C.-H.; Yeh, C.-W.; Liu, H.-K.; Shiao, Y.-J. Astragalus membranaceus-polysaccharides ameliorates obesity, hepatic steatosis, neuroinflammation and cognition impairment without affecting amyloid deposition in metabolically stressed APPswe/PS1dE9 mice. Int. J. Mol. Sci. 2017, 18, 2746. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, W.; Chen, H.; Li, W.; Li, W.; Zhu, G. Astragaloside IV prevents Aβ1-42 oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020, 1747, 147041. [Google Scholar] [CrossRef]
- Abd Elkader, H.-T.A.E.; Abdou, H.M.; Khamiss, O.A.; Essawy, A.E. Anti-anxiety and antidepressant-like effects of astragaloside IV and saponins extracted from Astragalus spinosus against the bisphenol A-induced motor and cognitive impairments in a postnatal rat model of schizophrenia. Environ. Sci. Pollut. Res. 2021, 28, 35171–35187. [Google Scholar] [CrossRef]
- Xu, J.; Guan, Z.; Wang, X.; Sun, D.; Li, Y.; Pei, B.; Lu, Y.; Yuan, L.; Zhang, X. Network Pharmacology and Experimental Evidence Identify the Mechanism of Astragaloside IV in Oxaliplatin Neurotoxicity. Drug Des. Devel. Ther. 2021, 15, 99. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Shou, S.S.; Lin, Y.X.; Chen, C.C.; Chiang, C.Y.; Yeh, C.Y. Effect of Ginkgo biloba extract on lipopolysaccharide-induced anhedonic depressive-like behavior in male rats. Phytother. Res. 2015, 29, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, S.; E Barreto, G.; Aliev, G.; Echeverria, V. Ginkgo biloba as an alternative medicine in the treatment of anxiety in dementia and other psychiatric disorders. Curr. Drug Metab. 2017, 18, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, S.; Wang, J.; Jiao, Y.; Zhang, X.; Zhang, Y.; Wang, Z.; Zhang, Q.; Li, S. Effect of Ginkgo biloba extract on cognitive function and neurotransmitter levels in rats with vascular dementia. Indian J. Anim. Res. 2018, 52, 1141–1145. [Google Scholar] [CrossRef]
- Dai, C.-X.; Hu, C.-C.; Shang, Y.-S.; Xie, J. Role of Ginkgo biloba extract as an adjunctive treatment of elderly patients with depression and on the expression of serum S100B. Medicine 2018, 97, e12421. [Google Scholar] [CrossRef]
- Ayatollahi, S.A.; Khoshsirat, S.; Peyvandi, A.A.; Rezaei, O.; Mehrjardi, F.Z.; Nahavandi, A.; Niknazar, S. Ginkgo biloba modulates hippocampal BDNF expression in a rat model of chronic restraint stress-induced depression. Physiol. Pharmacol. 2020, 24, 285–297. [Google Scholar] [CrossRef]
- Machado, M.M.F.; Banin, R.M.; Thomaz, F.M.; de Andrade, I.S.; Boldarine, V.T.; de Souza Figueiredo, J.; Hirata, B.K.S.; Oyama, L.M.; Lago, J.H.G.; Ribeiro, E.B. Ginkgo biloba extract (GbE) restores serotonin and leptin receptor levels and plays an antioxidative role in the hippocampus of ovariectomized rats. Mol. Neurobiol. 2021, 58, 2692–2703. [Google Scholar] [CrossRef]
- Jang, D.; Lee, H.-J.; Lee, K.; Kim, K.-R.; Won, R.; Lee, S.E.; Shim, I. White Ginseng Ameliorates Depressive Behavior and Increases Hippocampal 5-HT Level in the Stressed Ovariectomized Rats. Biomed. Res. Int. 2019, 2019, 5705232. [Google Scholar] [CrossRef]
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax ginseng action as an antidepressant. Cell Prolif. 2019, 52, e12696. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.; Zheng, X.; Qu, L.; Zhang, H.; Yuan, H.; Hui, J.; Mi, Y.; Ma, P.; Fan, D. Ginsenoside Rg5/Rk1 ameliorated sleep via regulating the GABAergic/serotoninergic signaling pathway in a rodent model. Food Funct. 2020, 11, 1245–1257. [Google Scholar] [CrossRef]
- Salomon, R.M.; Miller, H.; Delgado, P.L.; Charney, D.S. The use of tryptophan depletion to evaluate central serotonin function in depression and other neuropsychiatric disorders. Int. Clin. Psychopharmacol. 1993, 8, 41–46. [Google Scholar] [CrossRef]
- Abdul Aziz, N.U.; Chiroma, S.M.; Mohd Moklas, M.A.; Adenan, M.I.; Ismail, A.; Hidayat Baharuldin, M.T. Antidepressant-like properties of fish oil on postpartum depression-like rats model: Involvement of serotonergic system. Brain Sci. 2020, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-H.; Lee, Y.-K.; Shon, W.-J.; Kim, B.; Jeon, C.O.; Cho, J.-Y.; Morse, H.C.; Choi, E.Y.; Shin, D.-M. Gut microorganisms and their metabolites modulate the severity of acute colitis in a tryptophan metabolism-dependent manner. Eur. J. Nutr. 2020, 59, 3591–3601. [Google Scholar] [CrossRef] [PubMed]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic efficacy of palmitoylethanolamide and its new formulations in synergy with different antioxidant molecules present in diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.M. Factors associated with the suicide rates in Korea. Psychiatry Res. 2020, 284, 112745. [Google Scholar] [CrossRef]
- Lee, H.; Park, C.H.K.; Rhee, S.J.; Kim, J.; Kim, B.; Lee, S.S.; Ha, K.; Baik, C.J.; Ahn, Y.M. An integrated model for the relationship between socio-cultural factors, Attitudes Toward Suicide, and intensity of suicidal ideation in Korean, Japanese, and American populations. J. Affect. Disord. 2021, 280, 203–210. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-López, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef]

| Compound | Origin | Targeted Pathway | Family | Class | Pharmacological Target | Pharmacological Action | References |
|---|---|---|---|---|---|---|---|
| Anonaine | Annona muricata | Monoaminergic | Annonaceae | Alkaloid | 5-HT | Antidepressant, sedative, and anxiolytic | [69,70,71,72] |
| Anthocyanins | Rubus fruticosus | Microbial, kynurenine, MAO | Rosaceae | Flavonoid | BNDF, 5-HT, gut | Anti-neuroinflammatory, antidepressant, prevents brain aging, and regulates the gut microbiota | [73,74,75,76,77] |
| Asiaticoside | Centella asiatica | Kynurenine, microbial | Apiaceae | Terpenoid | BDNF, QnA, inflammatory markers, microbiota homeostasis, and mucosal barrier | Prevents neurotoxicity, lipid peroxidation, neuroinflammation; improves colitis, GI motility, and homeostasis | [78,79,80,81] |
| Astragaloside IV | Astragalus membranaceus | BDNF, kynurenine | Fabaceae | Polyphenol | 5-HT, dopamine, and MAO levels; oxidative, apoptotic, and inflammatory parameters | Neuroprotection against toxicity, inflammation, oxidative stress, apoptosis, and depression | [78,79,80,81] |
| Bacoside A | Bacopa monnieri | Serotonergic system | Plantaginaceae | Terpenoid | BDNF, 5-HT receptors, and synaptic proteins | Antidepressant and anti-anxiolytic | [82,83,84,85] |
| Carvacrol | Origanum vulgare | Serotoninergic, kynurenine | Lamiaceae | Phenol | 5-HT and BDNF | Gastroprotective and provides neuroprotection against memory degeneration, inflammation, oxidative stress, and depression | [86,87,88,89] |
| Catechins | Rhizophora mucronata | Microbial, kynurenine | Rhizophoraceae | Flavonoid | QnA, BDNF, microbes, and MAO | Neuroprotection against anxiety, oxidative stress, and neurotoxicity | [90,91,92,93,94] |
| Chrysin | Matricaria chamomilla | Kynurenine, serotoninergic, and microbial | Asteraceae | Flavonoid | MAO and 5-HT | Gut and neuroprotection against oxidative stress, apoptosis, and inflammation | [95,96,97,98] |
| Curcumin | Curcuma longa | Kynurenic, serotoninergic | Zingiberaceae | Phenol | 5-HT, kynurenine, QnA, BDNF | Neuroprotection against oxidative stress, apoptosis, and depression | [99,100,101,102] |
| Ellagic acid | Punica granatum | Kynurenine, serotoninergic | Lythraceae | Phenol | 5-HT and BDNF | Neuroprotection against inflammation, oxidative stress, and depression; improves memory | [103,104,105,106] |
| Eugenol | Syzygium aromaticum | Monoaminergic, kynurenine | Myrtaceae | Phenol | Neuroprotection against toxicity, oxidative stress, and IBS-induced stress | [107,108,109,110] | |
| Ferulic acid | Ferula foetida | Serotoninergic, microbial | Poaceae | Phenol | 5-HT, MAOA, and BDNF | Elevates 5-HT levels and has antidepressant and anti-neurotoxic effects | [111,112,113,114,115] |
| Ginkgolides B | Ginkgo biloba | Serotonergic, microbial | Ginkgoaceae | Terpenoid | BNDF and 5-HT | Reduces depression and anxiety and improves cognitive abilities | [116,117,118,119] |
| Ginsenoside Rg5 | Panax ginseng | Serotonergic, dopaminergic, GABAergic system | Araliaceae | Terpenoid | BNDF and 5-HT | Antidepressant | [120,121,122] |
| Hesperidin | Citrus limon | Serotonergic, kynurenine, microbial | Rutaceae | Flavonoid | BDNF | Regulates GI motility and provides neuroprotection against toxicity, inflammation, and depression | [123,124,125,126] |
| Hyperforin | Hypericum perforatum | MAOA, serotonergic system | Hypericaceae | Terpenoid | 5-HT, MAOA, and the kynurenine/Trp ratio | Antidepressant | [127,128,129,130] |
| Limonene | Citrus sinensis | Kynurenine, serotonergic, microbial | Rutaceae | Terpene | Melatonin, BDNF, and gut microbiome | Neuroprotection against inflammation and oxidative stress (IBD) | [131,132,133] |
| Linalool | Lavandula angustifolia | Serotonergic system, microbial | Lamiaceae | Terpenoid | 5-HT, gut microbiota, inflammatory markers, and mucosal immunity | Neuroprotection against inflammation, oxidative stress, and neural death; improves gut microbiota homeostasis and cognitive health | [134,135,136,137] |
| Luteolin | Eclipta prostrata | Monoaminergic, serotonergic | Asteraceae | Flavonoid | MAO neurotransmitters, 5-HT-related receptors, BDNF, 5-HT, and monoamine transporter | Antidepressant-like effect, inhibits serotonin reuptake, and promotes lipolysis and fatty acid β-oxidation | [138,139,140,141] |
| Lycopene | Citrullus lanatus | BDNF, kynurenine | Cucurbitaceae | Carotenoid | BDNF, serotonin, dopamine, inflammatory, and oxidative markers | Neuroprotection against inflammation, oxidative stress, toxicity, and stress | [142,143,144] |
| Naringin | Citrus paradisi | Kynurenine | Rutaceae | Flavonoid | Neuroinflammatory, apoptotic, and oxidative markers | Neuroprotection against inflammation, oxidative stress, toxicity, and stress | [145,146] |
| Oleanolic acid | Pimenta pseudocaryophyllus | Monoaminergic, serotonergic system | Myrtaceae | Terpenoid | 5-HT and MAOA | Anxiolytic, antidepressant | [147,148,149,150] |
| Oleuropein | Olea europaea | Dopaminergic, microbial system | Oleaceae | Phenol | Inflammatory markers, gut microbiota, and 5-HT | Neuroprotection against inflammation, improvement of the gut microbiota homeostasis and cognitive health | [151,152] |
| Omega-3 fatty acids | Fish oil | Kynurenine or serotonergic | Fatty acid | BDNF, serotonin, and IDO | Antidepressant and anti-anxiolytic; improves memory | [153,154,155,156] | |
| Piperine | Piper nigrum | Monoaminergic, serotonergic, BDNF | Piperaceae | Alkaloid | 5-HT and MAOA | Neuroprotection against inflammation, oxidative stress, toxicity, and stress | [157,158,159,160] |
| Proanthocyanidins | Cinnamomum zeylanicum | Serotonergic | Lauraceae | Polyphenol | 5-HT | Antidepressant | [161,162] |
| Resveratrol | Polygonum cuspidatum | Serotoninergic | Polygonaceae | Polyphenol | 5-HT, SERT, and BDNF | Enhances 5-HT levels, inhibits 5-HT reuptake, provides neuroprotection against toxicity, oxidative damage, and IBS-like effect | [163,164,165] |
| Rutin | Fagopyrum esculentum | Kynurenine, serotonergic | Polygonaceae | Flavonoid | NMDA, BDNF, QnA, oxidative, apoptotic, and inflammatory parameters | Neuroprotection against inflammation, oxidative stress, apoptosis, toxicity, and depression | [166,167] |
| Salidroside | Rhodiola rosea | Microbial, kynurenine | Crassulaceae | Glycoside | Inflammatory markers, gut microbiota, and 5-HT | Antidepressant, regulates gut–brain axis by modulation of gut microbiota and inflammation | [168,169,170] |
| Sanggenon G | Morus alba | Serotonergic | Moraceae | Flavonoid | BNDF | Antidepressant, antistress agent, memory-enhancing agent | [171,172,173] |
| Theanine | Camellia sinensis | BDNF | Theaceae | Amino acid | 5-HT, BDNF, and dopamine | Antidepressant | [174,175,176,177] |
| Tryptophan | Human breast milk | Kynurenine, microbial | Amino acid | AhR | Improves the sleep cycle and reduces infantile colic | [178,179,180] | |
| Tryptophan | Theobroma cacao | Microbial | Sterculiaceae | Amino acid | 5-HT and gut microbiota | Neuroprotective and improves cognition | [181,182,183] |
| Tryptophan | Moringa oleifera | Microbial, serotonergic | Moringaceae | Amino acid | SSRI, 5-HT, and EC cell count | Anxiolytic, antidepressant, and protects against ulcers | [184,185,186,187,188] |
| Tryptophan | Nelumbo nucifera | Serotonergic system | Nymphaeaceae | Amino acid | 5-HT | Antidepressant, 5-HT reuptake inhibitor, and 5-HT metabolism activator | [189,190,191,192] |
| 2-O-β-D-Glucopyranosyl-l-ascorbic acid | Lycium barbarum | Serotonergic, microbial, kynurenine | Solanaceae | Vitamin | Proinflammatory cytokines, 5-HT, and antioxidative markers | Reduces depression and anxiety and stabilizes the gut microbiota | [193,194,195,196] |
| Mimosa pudica | Serotonergic, dopaminergic | Fabaceae | 5-HT receptor | Antidepressant, memory enhancer, and regulates neuroactive ligand–receptor interaction | [197,198,199] | ||
| Poria cocos | Kynurenine, serotonergic, microbial | Polyporaceae | BDNF, gut microbiome, and serotonin | Antidepressant, anxiolytic, and mediates the gut microbiota | [200,201] | ||
| Salvia officinalis | Kynurenine, serotonergic, microbial system | Lamiaceae | BNDF, 5-HT, oxidative markers, and gut microbiota | Protects against neurotoxicity, improves depression, memory, behavioral activities, and the gut microbiota | [202,203,204] | ||
| Tagetes lucida | Serotonergic system | Asteraceae | 5-HT | Antidepressant, modulates 5-HT reuptake/release | [205,206,207] | ||
| Tualang honey | Microbial, BDNF | Memory restoration and improvement in depression and cognitive and neural stresses | [208,209,210] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liaqat, H.; Parveen, A.; Kim, S.Y. Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan. Nutrients 2022, 14, 3270. https://doi.org/10.3390/nu14163270
Liaqat H, Parveen A, Kim SY. Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan. Nutrients. 2022; 14(16):3270. https://doi.org/10.3390/nu14163270
Chicago/Turabian StyleLiaqat, Humna, Amna Parveen, and Sun Yeou Kim. 2022. "Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan" Nutrients 14, no. 16: 3270. https://doi.org/10.3390/nu14163270
APA StyleLiaqat, H., Parveen, A., & Kim, S. Y. (2022). Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut–Brain Axis Targeting Tryptophan. Nutrients, 14(16), 3270. https://doi.org/10.3390/nu14163270






