Trans Fatty Acids Content in Whole-Day Diets Intended for Pregnant and Breastfeeding Women in Gynaecological and Obstetric Wards: Findings from the Study under the “Mum’s Diet” Pilot Program in Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection and Preparation
2.2. Determination of Fat and Fatty Acids Content, including Trans Isomers
2.2.1. Fat Extraction
2.2.2. Determination of Fatty Acids by Gas Chromatography-Mass Spectrometry
2.3. Statistical Analysis
3. Results
3.1. Samples Characteristics
3.2. Fat and Fatty Acid Content of Individual Hospital Meals and Whole-Day Hospital Diet
3.3. Fat and Trans Fatty Acids Content of Individual Meals and Whole-Day Hospital Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Regulation (EU) No 1169/2011 of The European Parliament and of The Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004. Off. J. Eur. Union 2018, 304, 1–59. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02011R1169-20180101&from=EN (accessed on 20 April 2022).
- European Food Safety Authority (EFSA). Scientific and technical assistance on trans fatty acids. EFSA Supporting Publ. 2018, EN-1433, 1–16. [Google Scholar] [CrossRef]
- Wanders, A.J.; Zock, P.L.; Brouwer, I.A. Trans fat intake and its dietary sources in general populations worldwide: A systematic review. Nutrients 2017, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Precht, D.; Molkentin, J. Rapid analysis of the isomers of trans-octadecenoic acid in milk fat. Int. Dairy J. 1996, 6, 791–809. [Google Scholar] [CrossRef]
- European Commission (EC); Directorate-General Join Research Centre (JRC). Analytical Approach for Checking the Compliance of Fats and Oils against a Possible Regulated Limit for Industrial Trans Fatty Acids. Ref. Ares(2018)3313247-22/06/2018. Available online: File:///C:/Users/Karol/Downloads/EU602_EN_5%20(2).pdf (accessed on 20 April 2022).
- European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the presence of trans fatty acids in foods and the effect on human health of the consumption of trans fatty acids. EFSA J. 2004, 81, 1–49. [Google Scholar] [CrossRef]
- Aro, A.; Antoine, J.M.; Pizzoferrato, L.; Reykdal, O.; van Poppel, G. Trans Fatty Acids in Dairy and Meat Products from 14 European Countries: The TRANSFAIR Study. J. Food Compos. Anal. 1998, 11, 150–160. [Google Scholar] [CrossRef]
- Aro, A.; Van Amelsvoort, J.; Becker, W.; van Erp-Baart, M.A.; Kafatos, A.; Leth, T.; van Poppel, G. Trans Fatty Acids in Dietary Fats and Oils from 14 European Countries: The TRANSFAIR Study. J. Food Compos. Anal. 1998, 11, 137–149. [Google Scholar] [CrossRef]
- Aro, A.; Amaral, E.; Kesteloot, H.; Rimestad, A.; Thamm, M.; van Poppel, G. Trans Fatty Acids in French Fries, Soups, and Snacks from 14 European Countries: The TRANSFAIR Study. J. Food Compos. Anal. 1998, 11, 170–177. [Google Scholar] [CrossRef]
- National Institute of Public Health NIH–National Research Institute (NIPH NIH-NRI). Database of Trans Fatty Acids Content in Polish Food (Baza Zawartości Izomerów Trans Kwasów Tłuszczowych w Środkach Spożywczych). Available online: https://izomery.pzh.gov.pl (accessed on 20 April 2022).
- European Commission (EC). Report from the Commission to the European Parliament and the Council Regarding Trans Fats in Foods and in the Overall Diet of the Union Population. Available online: https://ec.europa.eu/food/system/files/2016-10/fs_labelling-nutrition_trans-fats-report_en.pdf (accessed on 20 April 2022).
- Okręglicka, K.; Mojska, H.; Jarosz, A.; Jarosz, M. Fatty acid composition, including trans isoforms in selected food fats available on Polish market. Pol. J. Hum. Nutr. 2017, XLIV, 5–17. [Google Scholar]
- European Commission (EC). Commission Staff Working Document Impact Assessment Accompanying the Document Commission Regulation (EU) Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Animal Fat, in Foods Intended for the Final Consumer; 162 Final, Part 1/6; SWD(2019): Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:e259bb15-667c-11e9-b6eb-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 20 April 2022).
- Zuchowska-Grzywacz, M.; Kowalska, M. Trans fatty acids in food–current legal regulations as protections for consumers and food manufacturers. Acta Aliment. 2019, 48, 105–114. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Countdown to 2023: WHO Report on Global Trans-Fat Elimination 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: file:///C:/Users/Karol/Downloads/9789240010178-eng%20(1).pdf (accessed on 20 April 2022).
- European Union (EU). Commission Regulation (EU) 2019/649 of 24 April 2019 amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as regards trans fat, other than trans fat naturally occurring in fat of animal origin. Off. J. Eur. Union 2019, 110, 17–20. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0649&from=EN (accessed on 20 April 2022).
- Bousset-Alféres, C.M.; Chávez-Servín, J.L.; Vázquez-Landaverde, P.A.; Betancourt-López, C.A.; Caamaño, M.D.C.; Ferriz-Martínez, R.A.; Chávez-Alabat, E.F.; Lovatón-Cabrera, M.G.; de la Torre-Carbot, K. Content of industrially produced trans fatty acids in breast milk: An observational study. Food Sci. Nutr. 2022, 10, 2568–2581. [Google Scholar] [CrossRef]
- Verneque, B.J.F.; Machado, A.M.; de Abreu Silva, L.; Lopes, A.C.S.; Duarte, C.K. Ruminant and industrial trans-fatty acids consumption and cardiometabolic risk markers: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 62, 2050–2060. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Brouwer, I. A. Effect of Trans-Fatty Acid Intake on Blood Lipids and Lipoproteins: A Systematic Review and Meta-Regression Analysis; World Health Organization: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/bitstream/handle/10665/246109/9789241510608-eng.pdf?sequence=1&isAllowed=y (accessed on 15 April 2022).
- Bendsen, N.; Christensen, R.; Bartels, E.; Astrup, A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: A systematic review and meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 773–783. [Google Scholar] [CrossRef]
- Mozaffarain, D.; Aro, A.; Willet, W.C. Health effects of trans-fatty acids: Experimental and observational evidence. Eur. J. Clin. Nutri. 2009, 63 (Suppl. 2), 5–21. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all-cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 11, h3978. [Google Scholar] [CrossRef]
- Wang, Q.; Afshin, A.; Yakoob, M.Y.; Singh, G.M.; Rehm, C.D.; Khatibzadeh, S.; Micha, R.; Shi, P.; Mozaffarian, D.; Ezzati, M.; et al. Impact of on optimal intakes of saturated, polyunsaturated, and trans fat on global burdens of coronary heart disease. JAHA 2016, 5, e002891. [Google Scholar] [CrossRef]
- Golomb, B.A.; Evans, M.A.; White, H.L.; Dimsdale, J.E. Trans Fat Consumption and Aggression. PLoS ONE 2012, 7, e32175. [Google Scholar] [CrossRef]
- Li, D.; Tong, Y.; Li, Y. Association of dietary trans fatty acid intake with depressive symptoms in midlife women. J. Affect. Disord. 2020, 260, 194–199. [Google Scholar] [CrossRef]
- Ren, X.; Vilhjálmsdóttir, B.L.; Rohde, J.F.; Walker, K.C.; Runstedt, S.E.; Lauritzen, L.; Heitmann, B.L.; Specht, I.O. Systematic Literature Review and Meta-Analysis of the Relationship Between Polyunsaturated and Trans Fatty Acids During Pregnancy and Offspring Weight Development. Front. Nutr. 2021, 8, 1–18. [Google Scholar] [CrossRef]
- Decsi, T.; Boehm, G. trans Isomeric fatty acids are inversely related to the availability of long-chain PUFAs in the perinatal period. Am. J. Clin. Nutr. 2013, 98, 543–548. [Google Scholar] [CrossRef]
- Decsi, T.; Campoy, C.; Demmelmair, H.; Szabó, É.; Marosvölgyi, T.; Escolano, M.; Marchal, G.; Krauss-Etschmann, S.; Cruz, M.; Koletzko, B. Inverse Association between trans Isomeric and Long-Chain Polyunsaturated Fatty Acids in Pregnant Women and Their Newborns. Ann. Nutr. Metab. 2011, 59, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.L.; Innis, S.M. Infant plasma trans, n-6, and n-3 fatty acids and conjugated linoleic acids are related to maternal plasma fatty acids, length of gestation, and birth weight and length. Am. J. Clin. Nutr. 2001, 73, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Decsi, T.; Burus, I.; Molnár, S.; Minda, H.; Veitl, V. Inverse association between trans isomeric and long-chain polyunsaturated fatty acids in cord blood lipids of full-term infants. Am. J. Clin. Nutr. 2001, 74, 364–368. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion. DHA and ARA and visual development. Scientific substantiation of a health claim related to docosahexaenoic acid (DHA) and arachidonic acid (ARA) and visual development pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2009, 941, 1–14. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion DHA and ARA and development of brain and eyes. Scientific substantiation of a health claim related to Docosahexaenoic Acid (DHA) and Arachidonic Acid (ARA) and support of the neural development of the brain and eyes pursuant to Article 14 of Regulation (EC). EFSA J. 2008, 794, 1–11. [Google Scholar]
- Sarnecki, J. Wpływ zwiększenia spożycia omega-3 LC-PUFA na przebieg ciąży–wyniki przeglądu Cochrane. Med. Stand.-Pediatr. 2019, 16, 589–590. [Google Scholar]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 fatty acid addition during pregnancy (Review). Cochrane Database Syst. Rev. 2018, 11, 1–421. [Google Scholar] [CrossRef]
- Newberry, S.J.; Chung, M.; Booth, M.; Maglione, M.A.; Tang, A.M.; O’Hanlon, C.E.; Wang, D.D.; Okunogbe, A.; Huang, C.; Motala, A.; et al. Omega-3 Fatty Acids and Maternal and Child Health: An Updated Systematic Review, Evidence Report/Technology Assessment; Southern California Evidence-based Practice Center: San Diego, CA, USA, 2016. [Google Scholar]
- European Food Safety Authority (EFSA). Panel on Dietetic Products, Nutrition, and Allergies. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Jsińska-Melon, E.; Mojska, H. Dietary trans fatty acids influence on allergic disease prevalence and development. Med. Stand. Pediatr. 2013, 10, 662–675. [Google Scholar]
- European Food Safety Authority (EFSA). Dietary Reference Values for nutrients. Summ. Rep. EFSA Supporting Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO); World Health Organization (WHO). Fats and Fatty Acids in Human Nutrition: Report of an Expert Consultation. FAO Food and Nutrition Paper No. 91. 2010. Available online: https://www.fao.org/3/i1953e/i1953e00.pdf (accessed on 20 April 2022).
- Bobiński, R.; Bobińska, J. Fatty acids of human milk—A review. Int. J. Vitam. Nutr. Res. 2020, 21, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed Med. 2017, 12, 517–527. [Google Scholar] [CrossRef]
- Mueller, A.; Thijs, C.; Rist, L.; Simões-Wüst, A.P.; Huber, M.; Steinhart, H. Trans fatty acids in human milk are an indicator of different maternal dietary sources containing trans fatty acids. Lipids 2010, 43, 245–251. [Google Scholar] [CrossRef]
- Larque, E.; Zamoraa, S.; Gilb, A. Dietary trans fatty acids in early life: A review. Early Hum. Dev. 2001, 65, 31–41. [Google Scholar] [CrossRef]
- Craig-Schmidt, M.C. Isomeric Fatty Acids: Evaluating Status and Implications for Maternal and Child Health. Lipids 2001, 36, 997–1006. [Google Scholar] [CrossRef]
- Butts, C.A.; Hedderley, D.I.; Herath, T.D.; Paturi, G.; Glyn-Jones, S.; Wiens, F.; Stahl, B.; Gopal, P. Human Milk Composition and Dietary Intakes of Breastfeeding Women of Different Ethnicity from the Manawatu-Wanganui Region of New Zealand. Nutrients 2018, 10, 1231. [Google Scholar] [CrossRef]
- Floris, L.M.; Stahl, B.; Abrahamse-Berkeveld, M.; Teller, I.C. Human milk fatty acid profile across lactational stages after term and preterm delivery: A pooled data analysis. Prostaglandins Leukot. Essent. Fat. Acids 2020, 156, 102023. [Google Scholar] [CrossRef]
- Dlouhý, P.; Tvrzická, E.; Stanková, B.; Buchtiková, M.; Pokorný, R.; Wiererová, O.; Bílková, D.; Rambousková, J.; Andel, M. Trans Fatty Acids in Subcutaneous Fat of Pregnant Women and in Human Milk in the Czech Republic. Ann. N. Y. Acad. Sci. 2002, 967, 544–547. [Google Scholar] [CrossRef]
- Mojska, H.; Socha, P.; Socha, J.; Soplińska, E.; Jaroszewska-Balicka, W.; Szponar, L. Trans fatty acid in human milk in Poland and their association with breastfeeding mother’s diet. Acta Paediatr. 2003, 92, 1381–1387. [Google Scholar] [CrossRef]
- Friesen, R.; Innis, S.M. Trans Fatty Acids in Human Milk in Canada Declined with the Introduction of Trans Fat Food Labeling. J. Nutr. 2006, 136, 2558–2561. [Google Scholar] [CrossRef]
- Marhol, P.; Dlouhý, P.; Rambousková, J.; Pokorný, R.; Wiererová, O.; Hrncírová, D.; Procházka, B.; Andel, M. Higher Content of C18:1 Trans Fatty Acids in Early Human Milk Fat of Roma Breast-Feeding Women. Ann. Nutr. Metab. 2007, 51, 461–467. [Google Scholar] [CrossRef]
- Samur, G.; Topcu, A.; Turan, S. Trans fatty acids and fatty acid composition of mature breast milk in Turkish women and their association with maternal diet’s. Lipids 2009, 44, 405–413. [Google Scholar] [CrossRef]
- Szabó, É.; Boehm, G.; Beermann, C.; Weyermann, M.; Brenner, H.; Rothenbacher, D.; Decsi, T. Fatty Acid Profile Comparisons in Human Milk Sampled From the Same Mothers at the Sixth Week and the Sixth Month of Lactation. JPGN 2010, 50, 316–320. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Żóralska, K.; Zagierski, M.; Szlagatys-Sidorkiewicz, A. Fatty acid composition in breast milk of women from gdańsk and the surrounding district in the course of lactation. Dev. Period Med. 2011, 2, 167–177. [Google Scholar]
- Daud, A.Z.; Mohd-Esa, N.; Azlan, A.; Chan, Y.M. The trans fatty acid content in human milk and its association with maternal diet among lactating mothers in Malaysia. Asia Pac. J. Clin. Nutr. 2013, 22, 431–442. [Google Scholar] [CrossRef]
- De Souza Santos da Costa, R.; da Silva Santos, F.; de Barros Mucci, D.; de Souza, T.V.; de Carvalho Sardinha, F.L.; Moutinho de Miranda Chaves, C.R.; das Graças Tavares do Carmo, M. trans Fatty Acids in Colostrum, Mature Milk and Diet of Lactating Adolescents. Lipids 2016, 51, 1363–1373. [Google Scholar] [CrossRef]
- Aumeistere, L.; Beluško, A.; Ciproviˇca, I.; Zavadska, D. Trans Fatty Acids in Human Milk in Latvia: Association with Dietary Habits during the Lactation Period. Nutrients 2021, 13, 2967. [Google Scholar] [CrossRef]
- Nishimura, R.Y.; Castro, G.S.F.; Jordão Junior, A.A.; Sartorelli, D.S. Breast milk fatty acid composition of women living far from the coastal area in Brazil. J. Pediatr. 2013, 89, 263–268. [Google Scholar] [CrossRef]
- Bahrami, G.; Rahimi, Z. Fatty acid composition of human milk in Western Iran. Eur. J. Clin. Nutr. 2005, 59, 494–497. [Google Scholar] [CrossRef]
- Sun, Q.; Ma, J.; Campos, H.; Hankinson, S.E.; Hu, F.B. Comparison between plasma and erythrocyte fatty acids content as biomarkers of fatty acid intake in US women. Am. J. Clin. Nutr. 2007, 86, 74–81. [Google Scholar] [CrossRef]
- Li, C.; Cobb, L.K.; Vesper, H.W.; Asma, S. Global Surveillance of trans-Fatty Acids. Prev. Chronic Dis. 2019, 16, 147–152. [Google Scholar] [CrossRef]
- Polish Committee for Standardization. PN ISO 1444:2000 Meat and Meat Products-Determination of Free Fat Content; Polish Committee for Standardization: Warsaw, Poland, 2013. [Google Scholar]
- Mojska, H.; Gielecinska, I.; Balas, J.; Pawlicka, M.; Szponar, L. Trans fatty acids in foods in Poland: Monitoring study. Pol. J. Hum. Nutr. 2006, 33, 2. [Google Scholar]
- Bzikowska-Jura, A.; Czerwonogrodzka-Senczyna, A.; Jasińska-Melon, E.; Mojska, H.; Olędzka, G.; Wesołowska, A.; Szostak-Węgierek, D. The Concentration of Omega-3 Fatty Acids in Human Milk Is Related to Their Habitual but Not Current Intake. Nutrients 2019, 11, 1585. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, 2272–2292. [Google Scholar] [CrossRef]
- Murray, C. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis forthe Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Ziemlański, Ś.; Budzyńska–Topolowska, J. Rola izomerów trans kwasów tłuszczowych w metabolizmie lipidów ze szczególnym uwzględnieniem układu krążenia. Czynniki Ryzyka 1995, 3/4, 5–18. [Google Scholar]
- Barylko-Pikielna, N.; Jacorzynski, B.; Mielniczuk, E.; Pawlicka, M.; Daniewski, M.; Kostyra, E. Dzienne spożycie izomerów trans w polskiej racji pokarmowej. Pol. J. Hum. Nutr. 1998, 25, 28–46. [Google Scholar]
- Daniewski, M.; Mielniczuk, E.; Jacorzynski, B.; Pawlicka, M.; Balas, J.; Filipek, A.; Cierpikowska, M. Oszacowanie dziennego spożycia kwasów tłuszczowych w przeciętnej polskiej racji pokarmowej. Pol. J. Hum. Nutr. 1999, XXVI, 23–33. [Google Scholar]
- European Commission (EC). Commission Staff Working Document Impact Assessment Accompanying the Document Commission Regulation (EU) Amending Annex III to Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards Trans Fat, Other than Trans Fat Naturally Occurring in Animal Fat, in Foods Intended for the Final Consumer; 162 Final, Part 3/6; SWD: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:e259bb15-667c-11e9-b6eb-01aa75ed71a1.0001.02/DOC_3&format=PDF (accessed on 20 April 2022).
- World Health Organization. Draft Guidelines on Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2018; Available online: https://extranet.who.int/dataform/upload/surveys/666752/files/Draft%20WHO%20SFA-TFA%20guidelines_04052018%20Public%20Consultation(1).pdf (accessed on 21 April 2022).
- Innis, S.M.; King, D.J. Trans fatty acids in human milk are inversely associated with concentration of essential all-cis n-6 and n-3 fatty acids and determine trans, but not n-6 and n-3, fatty acids in plasma lipids of breast-fed infants. Am. J. Clin. Nutr. 1999, 70, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Innis, S.M. Trans fatty intakes during pregnancy, infancy and early childhood. Atheroscler. Suppl. 2006, 7, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.W.; Rifas-Shiman, S.L.; Rimm, E.B.; Oken, E.; Gillman, M.W. Maternal trans fatty acid intake and fetal growth. Am. J. Clin. Nutr. 2011, 94, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Kresic, G.; Dujmovic, M.; Mandic, M.L.; Delaš, I. Dietary and Breast Milk Trans Fatty Acids Seen in Croatian Breastfeeding Women from Adriatic Region. J. Food Nutr. Res. 2013, 52, 156–163. [Google Scholar]
- Zupanič, N.; Hribar, M.; Hristov, H.; Lavriša, Ž.; Kušar, A.; Gregorič, M.; Blaznik, U.; Koroušić Seljak, B.; Golja, P.; Vidrih, R.; et al. Dietary Intake of trans Fatty Acids in the Slovenian Population. Nutrients 2021, 13, 207. [Google Scholar] [CrossRef]
- National Institute of Public Health-National Institute of Hygiene. Nutrition Standards for the Polish Population and Their Application; National Institute of Public Health-National Institute of Hygiene: Warsaw, Poland, 2020. Available online: https://www.pzh.gov.pl/wp-content/uploads/2020/12/Normy_zywienia_2020web-1.pdf (accessed on 20 April 2022).
- Mojska, H.; Jasińska-Melon, E.; Bobiński, R.; Mikulska, M. Nutrient level in the diet of breastfeeding women from Silesia region of Poland. Probl. Hig. Epidemiol. 2014, 95, 325–330. [Google Scholar]
- Ministry of Health. Regulation of the Council of Ministers of 4 August 2016 on the National Health Program for 2016–2020. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20160001492/O/D20161492.pdf (accessed on 20 April 2022).
- Ministry of Health. Regulation of the Council of Ministers of 30 March 2021 on the National Health Program for 2021–2025. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20210000642/O/D20210642.pdf (accessed on 20 April 2022).
- Ministry of Health. Regulation of the Minister of Health of 9 August 2019 on the Pilot Program “Standard of Hospital Nutrition for Pregnant and Postpartum Women–Mum’s Diet”. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20190001537/O/D20191537.pdf (accessed on 20 April 2022).
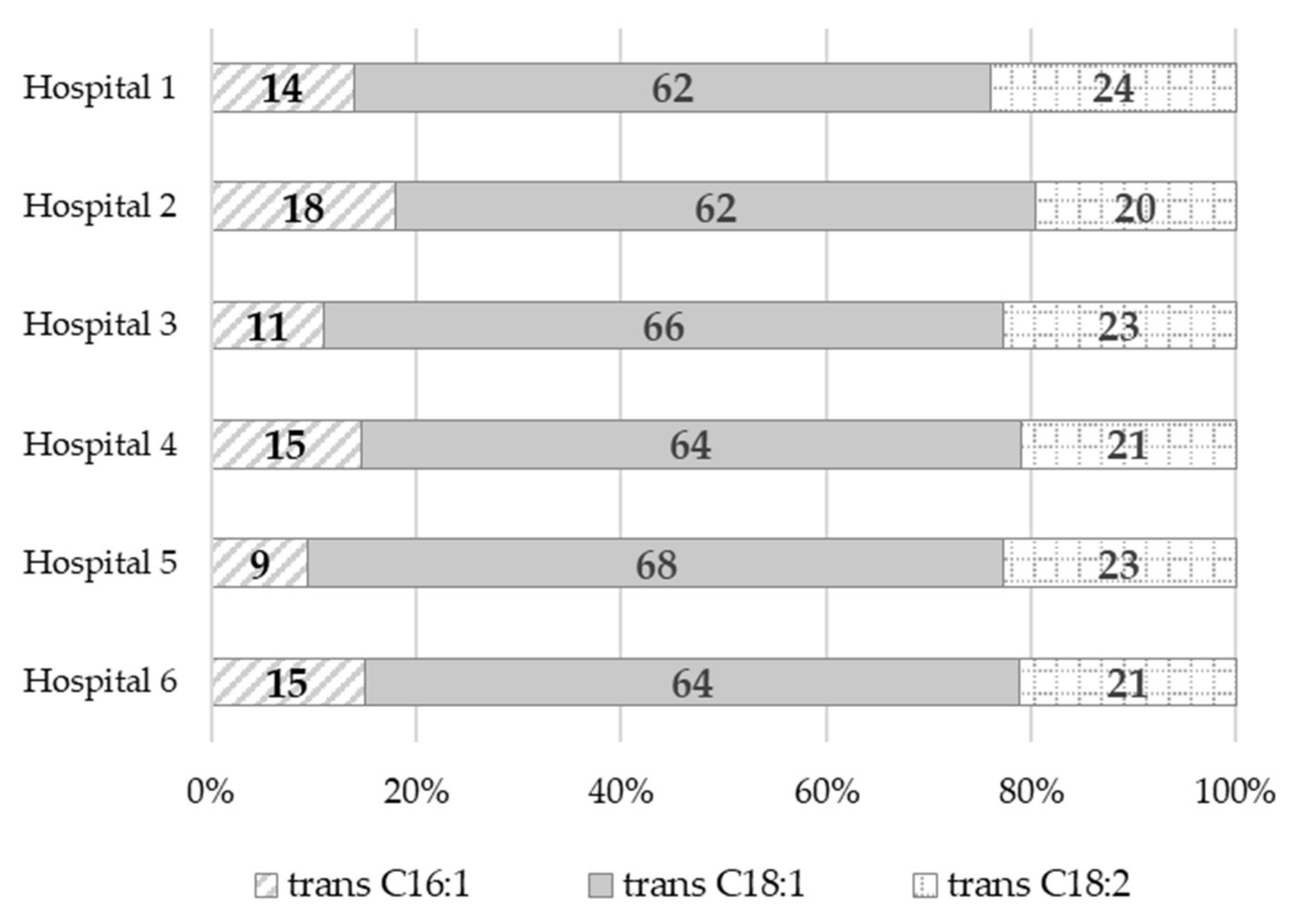


| Hospital 1 a | Hospital 2 a | Hospital 3 a | Hospital 4 a | Hospital 5 a | Hospital 6 a | Average Value (Me, Median) | |
|---|---|---|---|---|---|---|---|
| Breakfast | |||||||
| Weight (g/meal) | 538.8 | 751.4 | 829.5 | 906.5 | 476.0 | 803.3 | 777.4 |
| Energy value (kcal/meal) | 481 | 687 | 672 | 592 | 646 | 801 | 659 |
| Fat (g/meal) | 12.4 | 15.0 | 23.2 | 14.5 | 14.3 | 29.7 | 14.8 |
| Elevenses | |||||||
| Weight (g/meal) | 200.0 | 96.6 | 128.9 | 62.5 | 315.9 | 447.2 | 164.5 |
| Energy value (kcal/meal) | 77 | 233 | 382 | 163 | 95 | 253 | 198 |
| Fat (g/meal) | 0.4 | 4.1 | 18.3 | 5.1 | 4.7 | 7.2 | 4.9 |
| Dinner | |||||||
| Weight (g/meal) | 918.8 | 1184.6 | 1201.3 | 1283.4 | 1005.5 | 1261.4 | 1193.0 |
| Energy value (kcal/meal) | 745 | 483 | 1256 | 613 | 334 | 796 | 679 |
| Fat (g/meal) | 38.6 | 17.8 | 49.3 | 11.6 | 6.0 | 25.2 | 21.5 |
| Afternoon snack | |||||||
| Weight (g/meal) | 245.0 | 282.3 | 102.5 | 153.9 | 150.8 | 383.1 | 199.5 |
| Energy value (kcal/meal) | 127 | 242 | 163 | 79 | 66 | 241 | 145 |
| Fat (g/meal) | 2.9 | 6.2 | 5.3 | 3.8 | 0.6 | 6.5 | 4.6 |
| Supper | |||||||
| Weight (g/meal) | 278.5 | 694.3 | 514.8 | 550.0 | 267.0 | 715.5 | 532.4 |
| Energy value (kcal/meal) | 576 | 700 | 467 | 499 | 652 | 750 | 614 |
| Fat (g/meal) | 17.5 | 20.8 | 16.0 | 14.3 | 15.5 | 27.9 | 16.8 |
| Weight of whole-day diet (g/whole-day diet) | 2181.1 | 3009.2 | 2777.0 | 2956.3 | 2215.2 | 3610.5 | 2866.7 |
| Energy value of whole-day diet (kcal/whole-day diet) | 2006 | 2345 | 2940 | 1946 | 1793 | 2841 | 2176 |
| Fat content in whole-day diet (g/whole-day diet) | 71.8 | 63.9 | 112.1 | 49.3 | 41.1 | 96.5 | 67.9 |
| Meal Type | Fat [g/100 g] 1 | Saturated Fatty Acids (SFAs) [% wt/wt] 2 | Monounsaturated Fatty Acids (MUFAs) [% wt/wt] 2 | Polyunsaturated Fatty Acids (PUFAs) [% wt/wt] 2 | Trans Fatty Acids (TFAs) [% wt/wt] 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C18:0 | Total SFAs | C16:1 cis | C18:1 cis | Total MUFAs | C18:3 n-3 | C22:6 n-3 | Total LC-PUFAs n-3 | C18:2 n-6 linoleic acid, LA | C20-4 n-6 | Total LC-PUFAs n-6 | Total PUFAs | Total TFAs | ||
| Hospital 1 a | ||||||||||||||||
| Breakfast | 2.3 | 9.86 | 44.91 | 7.44 | 66.79 | 1.31 | 22.23 | 24.57 | 0.71 | 0.04 | 0.86 | 6.11 | <LOQ *** | 6.11 | 6.97 | 1.67 |
| Elevenses | <LOQ * | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** | - ** |
| Dinner | 4.2 | 0.16 | 6.26 | 2.41 | 9.73 | 0.27 | 68.79 | 70.06 | 4.37 | 0.01 | 4.51 | 15.36 | 0.07 | 15.46 | 20.11 | 0.08 |
| Afternoon snack | 1.2 | 10.83 | 45.87 | 7.98 | 70.72 | 1.39 | 22.08 | 24.69 | 0.32 | 0.03 | 0.50 | 2.07 | <LOQ *** | 2.13 | 2.70 | 1.89 |
| Supper | 6.3 | 1.18 | 10.85 | 2.94 | 16.74 | 0.40 | 42.75 | 43.81 | 3.37 | 0.02 | 3.49 | 35.46 | <LOQ *** | 35.49 | 39.19 | 0.22 |
| Hospital 2 a | ||||||||||||||||
| Breakfast | 2.0 | 6.58 | 34.92 | 7.83 | 54.19 | 1.35 | 26.64 | 29.10 | 1.24 | 0.04 | 1.35 | 13.51 | <LOQ *** | 13.54 | 15.33 | 1.30 |
| Elevenses | 4.2 | 5.90 | 39.41 | 8.29 | 57.47 | 1.63 | 27.04 | 29.74 | 0.72 | 0.06 | 0.98 | 10.26 | <LOQ *** | 10.32 | 11.41 | 1.38 |
| Dinner | 1.5 | 2.69 | 32.38 | 9.57 | 46.85 | 1.89 | 40.26 | 43.07 | 0.93 | <LOQ *** | 1.39 | 7.76 | <LOQ *** | 7.87 | 9.42 | 0.66 |
| Afternoon snack | 2.2 | 10.93 | 41.91 | 10.40 | 70.56 | 1.33 | 22.34 | 25.00 | 0.48 | 0.05 | 0.67 | 1.50 | <LOQ *** | 1.55 | 2.30 | 2.13 |
| Supper | 3.0 | 5.00 | 35.02 | 8.56 | 52.20 | 1.92 | 31.97 | 34.80 | 1.12 | 0.17 | 1.88 | 9.62 | <LOQ *** | 9.68 | 11.74 | 1.26 |
| Hospital 3 a | ||||||||||||||||
| Breakfast | 2.8 | 8.23 | 47.17 | 7.25 | 66.81 | 1.33 | 24.04 | 26.18 | 0.26 | 0.04 | 0.30 | 4.82 | <LOQ *** | 4.82 | 5.47 | 1.46 |
| Elevenses | 14.2 | 0.03 | 3.61 | 0.86 | 5.17 | 0.08 | 61.55 | 62.35 | 3.20 | <LOQ *** | 3.20 | 29.23 | <LOQ *** | 29.23 | 32.48 | <LOQ *** |
| Dinner | 4.1 | 3.47 | 43.80 | 4.10 | 53.79 | 0.82 | 36.27 | 37.76 | 0.80 | 0.03 | 0.82 | 6.92 | <LOQ *** | 6.95 | 7.77 | 0.69 |
| Afternoon snack | 5.2 | 11.46 | 50.92 | 7.10 | 74.66 | 1.11 | 19.66 | 21.72 | 0.25 | 0.03 | 0.31 | 1.68 | <LOQ *** | 1.69 | 2.02 | 1.56 |
| Supper | 3.1 | 6.34 | 49.20 | 6.75 | 65.72 | 1.51 | 25.37 | 27.57 | 0.26 | <LOQ *** | 0.30 | 4.77 | <LOQ *** | 4.79 | 5.36 | 1.36 |
| Hospital 4 a | ||||||||||||||||
| Breakfast | 1.6 | 10.16 | 38.41 | 9.25 | 65.31 | 1.45 | 22.83 | 25.78 | 0.86 | 0.08 | 0.98 | 5.68 | 0.12 | 5.87 | 7.02 | 1.89 |
| Elevenses | 8.2 | 7.69 | 39.61 | 8.47 | 61.00 | 1.89 | 27.18 | 30.28 | 0.49 | <LOQ *** | 0.57 | 6.23 | <LOQ *** | 6.28 | 7.20 | 1.50 |
| Dinner | 0.9 | 1.71 | 29.60 | 5.84 | 40.70 | 2.30 | 39.89 | 43.30 | 0.98 | 0.03 | 1.36 | 12.85 | 0.12 | 13.11 | 15.48 | 0.42 |
| Afternoon snack | 2.5 | 11.91 | 40.77 | 10.33 | 71.77 | 1.35 | 20.88 | 23.76 | 0.37 | 0.05 | 0.45 | 1.41 | 0.12 | 1.61 | 2.10 | 2.35 |
| Supper | 2.6 | 5.86 | 33.41 | 8.02 | 51.84 | 1.92 | 33.79 | 37.05 | 0.85 | 0.02 | 1.02 | 7.95 | 0.24 | 8.25 | 9.85 | 1.23 |
| Hospital 5 a | ||||||||||||||||
| Breakfast | 3.0 | 4.68 | 28.25 | 7.22 | 43.91 | 1.11 | 34.25 | 36.37 | 0.97 | 0.03 | 1.06 | 16.87 | 0.12 | 16.99 | 18.50 | 1.22 |
| Elevenses | 1.5 | 10.04 | 42.05 | 8.85 | 67.38 | 1.34 | 24.35 | 27.06 | 0.48 | <LOQ *** | 0.63 | 2.78 | <LOQ *** | 2.83 | 3.46 | 2.09 |
| Dinner | 0.6 | 5.27 | 39.38 | 9.36 | 58.64 | 2.13 | 23.84 | 27.14 | 2.17 | 0.08 | 2.44 | 9.32 | 0.51 | 10.08 | 12.76 | 1.43 |
| Afternoon snack | 0.4 | 8.07 | 37.69 | 10.04 | 63.71 | 1.21 | 23.74 | 26.38 | 2.33 | 0.09 | 2.79 | 4.77 | <LOQ *** | 4.90 | 7.90 | 2.00 |
| Supper | 5.8 | 9.09 | 40.47 | 7.26 | 61.40 | 1.62 | 27.72 | 30.47 | 0.77 | 0.03 | 0.89 | 5.48 | 0.07 | 5.59 | 6.48 | 1.63 |
| Hospital 6 a | ||||||||||||||||
| Breakfast | 3.7 | 9.11 | 38.95 | 9.60 | 63.88 | 1.74 | 24.81 | 27.85 | 0.56 | 0.04 | 0.83 | 5.43 | <LOQ *** | 5.48 | 6.68 | 1.74 |
| Elevenses | 1.6 | 11.54 | 37.77 | 9.29 | 66.87 | 1.46 | 21.30 | 24.82 | 3.14 | 0.05 | 3.20 | 2.74 | <LOQ *** | 2.83 | 6.06 | 2.24 |
| Dinner | 2.0 | 2.35 | 20.65 | 4.93 | 30.79 | 2.16 | 46.34 | 49.64 | 2.56 | 0.06 | 2.63 | 15.63 | 0.37 | 16.09 | 19.23 | 0.34 |
| Afternoon snack | 1.7 | 10.25 | 38.49 | 9.19 | 65.60 | 1.27 | 21.38 | 24.33 | 2.05 | 0.06 | 2.17 | 5.43 | <LOQ *** | 5.64 | 7.89 | 2.17 |
| Supper | 3.9 | 5.02 | 24.83 | 6.39 | 40.48 | 1.15 | 43.15 | 45.71 | 2.71 | 0.04 | 2.82 | 9.96 | <LOQ *** | 10.02 | 12.89 | 0.91 |
| Meal Type | Total Fat (g/100 g) | TFAs (g/100 g) | TFAs (g/Meal) | TFAs (g/Whole-Day Diet) | |
|---|---|---|---|---|---|
| Hospital 1a | Breakfast | 2.3 | 0.04 | 0.21 | 0.34 |
| Elevenses | <LOQ * | ND ** | ND ** | ||
| Dinner | 4.2 | 0.003 | 0.03 | ||
| Afternoon snack | 1.2 | 0.02 | 0.06 | ||
| Supper | 6.3 | 0.01 | 0.04 | ||
| Hospital 2a | Breakfast | 2.0 | 0.03 | 0.20 | 0.77 |
| Elevenses | 4.2 | 0.06 | 0.06 | ||
| Dinner | 1.5 | 0.01 | 0.12 | ||
| Afternoon snack | 2.2 | 0.05 | 0.13 | ||
| Supper | 3.0 | 0.04 | 0.26 | ||
| Hospital 3a | Breakfast | 2.8 | 0.04 | 0.34 | 0.98 |
| Elevenses | 14.2 | 0.001 *** | 0.001 *** | ||
| Dinner | 4.1 | 0.03 | 0.34 | ||
| Afternoon snack | 5.2 | 0.08 | 0.08 | ||
| Supper | 3.1 | 0.04 | 0.22 | ||
| Hospital 4a | Breakfast | 1.6 | 0.03 | 0.27 | 0.67 |
| Elevenses | 8.2 | 0.12 | 0.08 | ||
| Dinner | 0.9 | 0.004 | 0.05 | ||
| Afternoon snack | 2.5 | 0.06 | 0.09 | ||
| Supper | 2.6 | 0.03 | 0.18 | ||
| Hospital 5a | Breakfast | 3.0 | 0.04 | 0.17 | 0.62 |
| Elevenses | 1.5 | 0.03 | 0.10 | ||
| Dinner | 0.6 | 0.01 | 0.09 | ||
| Afternoon snack | 0.4 | 0.01 | 0.01 | ||
| Supper | 5.8 | 0.09 | 0.25 | ||
| Hospital 6a | Breakfast | 3.7 | 0.06 | 0.52 | 1.16 |
| Elevenses | 1.6 | 0.04 | 0.16 | ||
| Dinner | 2.0 | 0.01 | 0.09 | ||
| Afternoon snack | 1.7 | 0.04 | 0.14 | ||
| Supper | 3.9 | 0.04 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasińska-Melon, E.; Mojska, H.; Przygoda, B.; Stoś, K. Trans Fatty Acids Content in Whole-Day Diets Intended for Pregnant and Breastfeeding Women in Gynaecological and Obstetric Wards: Findings from the Study under the “Mum’s Diet” Pilot Program in Poland. Nutrients 2022, 14, 3360. https://doi.org/10.3390/nu14163360
Jasińska-Melon E, Mojska H, Przygoda B, Stoś K. Trans Fatty Acids Content in Whole-Day Diets Intended for Pregnant and Breastfeeding Women in Gynaecological and Obstetric Wards: Findings from the Study under the “Mum’s Diet” Pilot Program in Poland. Nutrients. 2022; 14(16):3360. https://doi.org/10.3390/nu14163360
Chicago/Turabian StyleJasińska-Melon, Edyta, Hanna Mojska, Beata Przygoda, and Katarzyna Stoś. 2022. "Trans Fatty Acids Content in Whole-Day Diets Intended for Pregnant and Breastfeeding Women in Gynaecological and Obstetric Wards: Findings from the Study under the “Mum’s Diet” Pilot Program in Poland" Nutrients 14, no. 16: 3360. https://doi.org/10.3390/nu14163360
APA StyleJasińska-Melon, E., Mojska, H., Przygoda, B., & Stoś, K. (2022). Trans Fatty Acids Content in Whole-Day Diets Intended for Pregnant and Breastfeeding Women in Gynaecological and Obstetric Wards: Findings from the Study under the “Mum’s Diet” Pilot Program in Poland. Nutrients, 14(16), 3360. https://doi.org/10.3390/nu14163360








