The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Drugs, and Solutions Used in This Work
2.2. Cell Preparations
2.3. Electrophysiological Measurements
2.4. Potential and Current Recordings
2.5. Data Analyses
2.6. Curve-Fitting Procedures and Statistical Analyses
3. Results
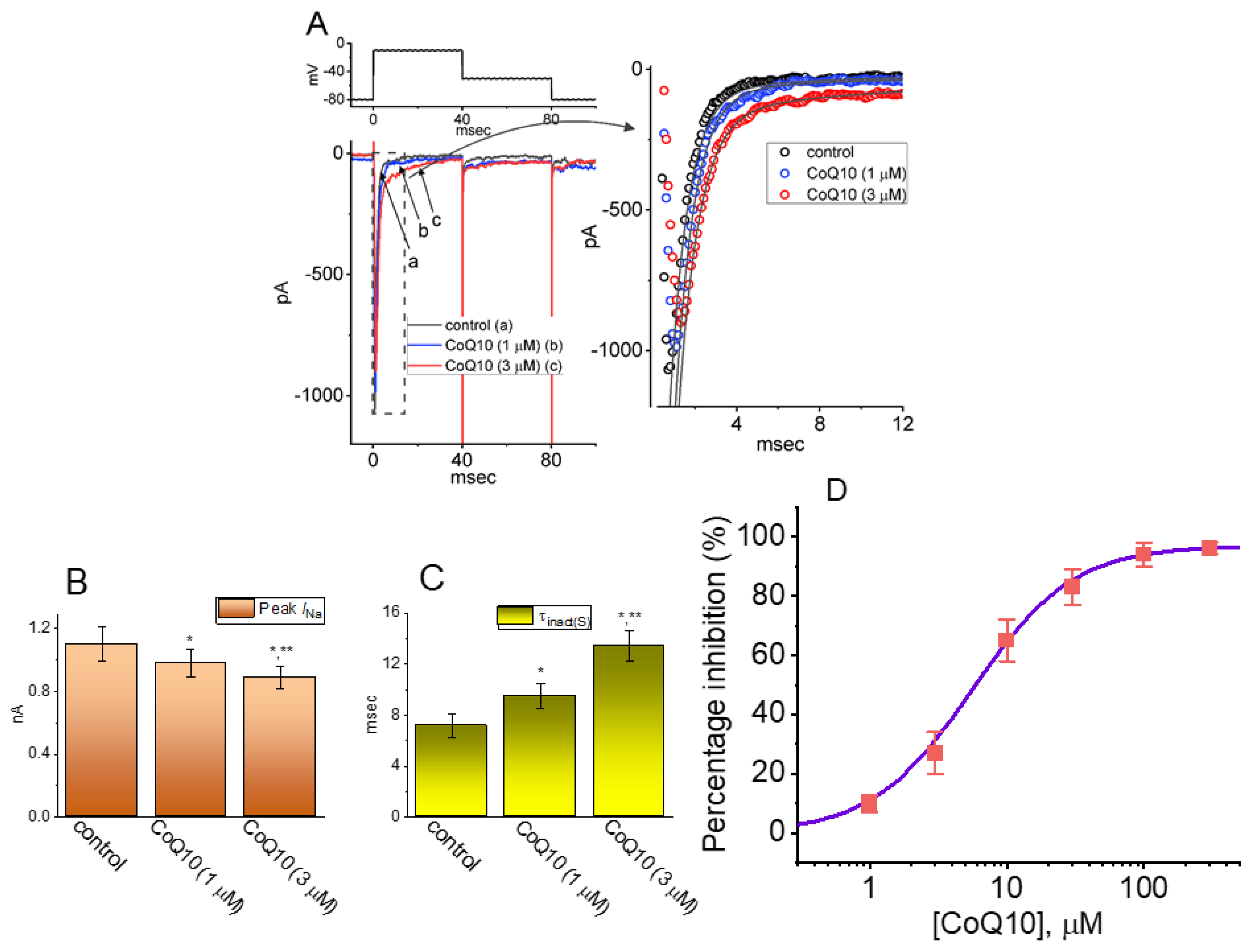
3.1. Effects of Ubiquinone on INa in GH3 Pituitary Cells
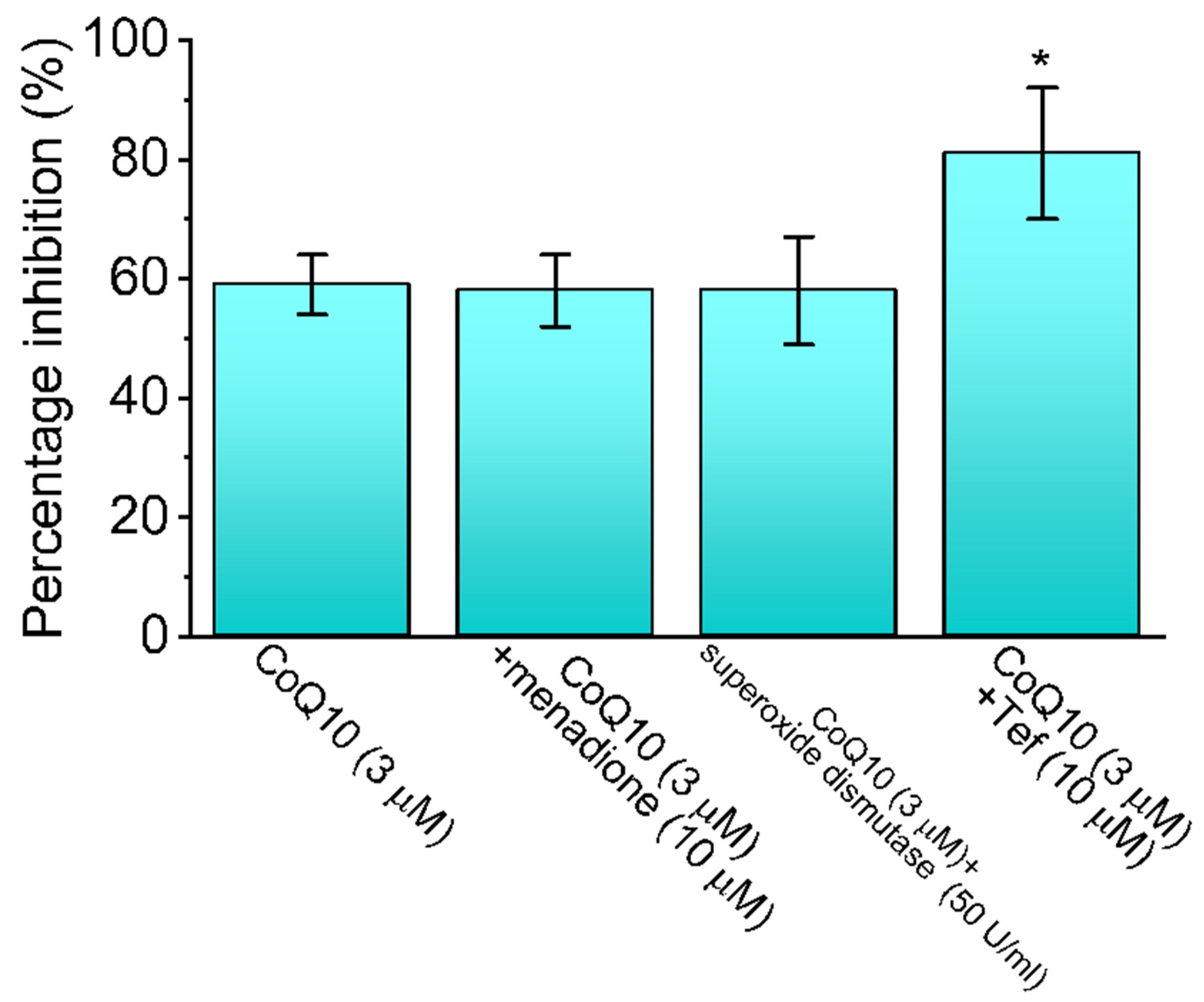
3.2. Comparison between the Effects of Ubiquinone Alone and Those of Ubiquinone plus Menadione, Ubiquinone plus Superoxide Dismutase, and Ubiquinone plus Tefluthrin (Tef) on the Peak INa
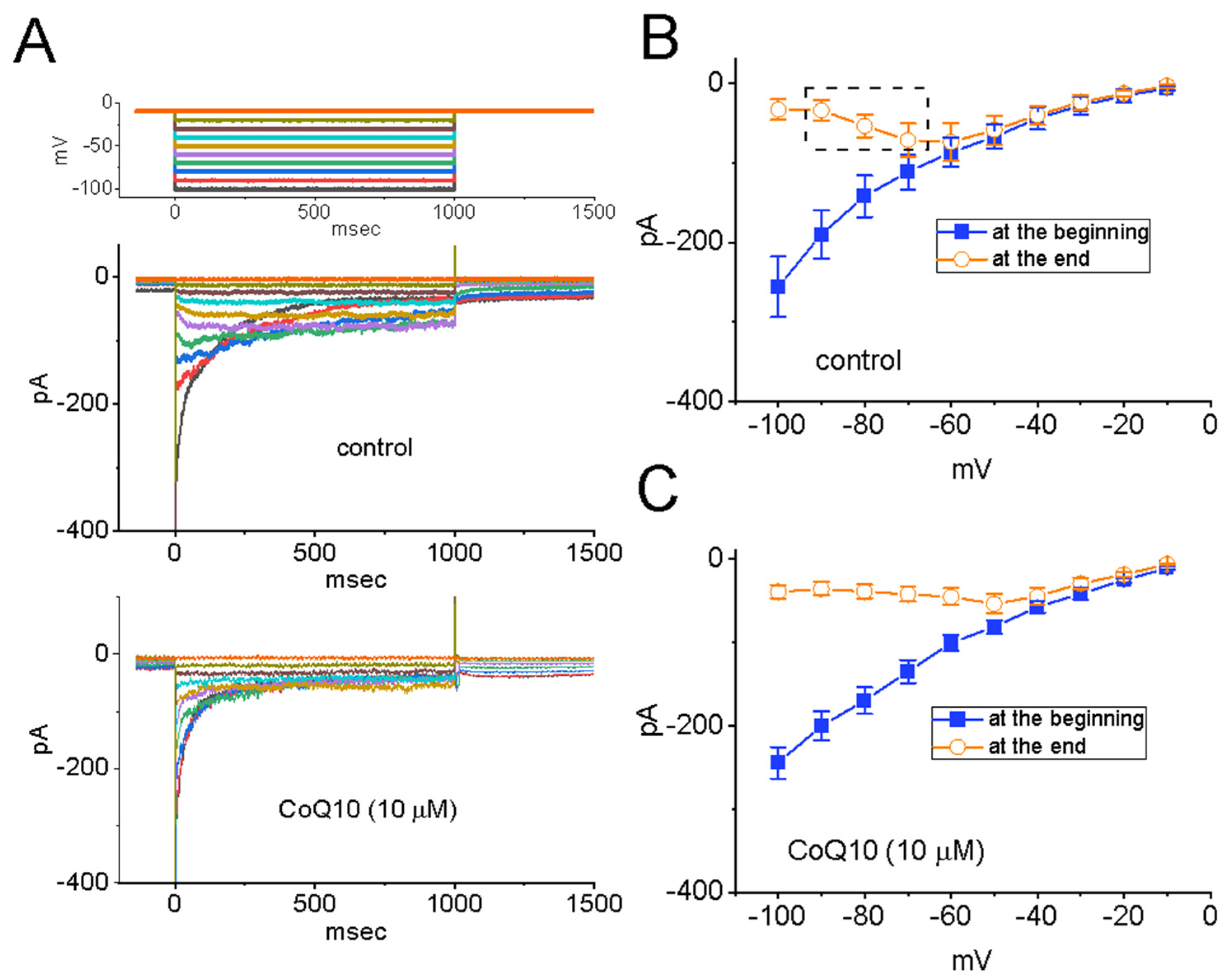
3.3. Effects of Ubiquinone on the Voltage-Dependent Hysteresis of the Persistent INa (INa(P)) in GH3 Cells
3.4. Mild Inhibition of Erg-Mediated K+ Current (IK(erg)) Produced by Ubiquinone in GH3 Cells
3.5. Inability of Ubiquinone to Modify the Amplitude and Gating of Hyperpolarization-Activated Cation Current (Ih) in GH3 Cells
3.6. Inhibitory Effects of Ubiquinone on INa in MMQ Pituitary Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Digiesi, V.; Cantini, F.; Oradei, A.; Bisi, G.; Guarino, G.C.; Brocchi, A.; Bellandi, F.; Mancini, M.; Littarru, G.P. Coenzyme Q10 in essential hypertension. Mol. Asp. Med. 1994, 15, s257–s263. [Google Scholar] [CrossRef]
- Hidaka, T.; Fujii, K.; Funahashi, I.; Fukutomi, N.; Hosoe, K. Safety assessment of coenzyme Q10 (CoQ10). Biofactors 2008, 32, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, A.; Diaz-Casiro, J.; Pulido-Moran, M.; Kajarabille, N.; Guisado, R.; Ochoa, J.J. Coenzyme Q10 supplementation and exercise in healthy humans: A systematic review. Curr. Drug Metab. 2016, 17, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Fonarow, G.C.; Butler, J.; Ezekowitz, J.A.; Felker, G.M. Coenzyme Q10 and heart failure: A state-of-the-art review. Circ. Heart Fail. 2016, 9, e002639. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Casado, M.E.; Quiles, J.L.; Barriocanal-Casado, E.; González-Garcia, P.; Battino, M.; López, L.C.; Varela-López, A. The paradox of coenzyme Q(10) in aging. Nutrients 2019, 11, 2221. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef]
- Valero, T. Mitochondrial biogenesis: Pharmacological approaches. Curr. Pharm. Des. 2014, 20, 5507–5509. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Mantle, D. Coenzyme Q10 supplementation in fibrosis and aging. Adv. Exp. Med. Biol. 2019, 1178, 103–112. [Google Scholar]
- Baggio, E.; Gandini, R.; Plancher, A.C.; Passeri, M.; Carmosino, G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. CoQ10 drug surveillance investigators. Mol. Asp. Med. 1994, 15, s287–s294. [Google Scholar] [CrossRef]
- Molyneux, S.; Florkowski, C.M.; George, P.M.; Pilbrow, A.P.; Frampton, C.M.; Lever, M.; Richards, A.M. Coenzyme Q10: An independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008, 52, 1435–1441. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Santa, K.M. Treatment options for mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome. Pharmacotherapy 2010, 30, 1179–1196. [Google Scholar] [CrossRef] [PubMed]
- Sándor, P.S.; Di Clemente, L.; Coppola, G.; Saenger, U.; Fumal, A.; Magis, D.; Seidel, L.; Agosti, R.M.; Schoenen, J. Efficacy of coenzyme Q10 in migraine prophylaxis: A randomized controlled trial. Neurology 2005, 64, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Xekouki, P.; Stratakis, C.A. Succinate dehydrogenase (SDHx) mutations in pituitary tumors: Could this be a new role for mitochondrial complex II and/or Krebs cycle defects? Endocr.-Related Cancer 2012, 19, C33–C40. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Bianchi, A.; Fusco, A.; Sacco, E.; Leone, E.; Tilaro, L.; Porcelli, T.; Giampietro, A.; Principi, F.; De Marinis, L.; et al. Coenzyme Q10 evaluation in pituitary-adrenal axis disease: Preliminary data. Biofactors 2005, 25, 197–199. [Google Scholar] [CrossRef]
- Mancini, A.; Festa, R.; Di Donna, V.; Leone, E.; Littarru, G.P.; Silvestrini, A.; Meucci, E.; Pontecorvi, A. Hormones and antioxidant systems: Role of pituitary and pituitary-dependent axes. J. Endocrinol. Investig. 2010, 33, 422–433. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Nwangwa, E.K.; Ben-Azu, B.; Edesiri, T.P.; Emojevwe, V.; Igweh, J.C. Taurine and coenzyme Q10 synertistically prevent and reverse chloropromazine induced psycho-neuoendocrine changes and cataleptic behavior in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 717–734. [Google Scholar] [CrossRef]
- Asl, F.R.; Khosravi, M.; Hajikhani, R.; Solati, J.; Fahimi, H. Complementary effects of coenzyme Q10 and Lepidium sativum supplementation on the reproductive function of mice: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 607–618. [Google Scholar]
- Huang, M.H.; Wu, S.N.; Chen, C.P.; Shen, A.Y. Inhibition of Ca2+-activated and voltage-dependent K+ currents by 2-merceptophenyl-1,4-naphthoquinone in pituitary GH3 cells: Contribution to its antiproliferative effect. Life Sci. 2002, 70, 1185–1203. [Google Scholar] [CrossRef]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH3 cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Tseng, Y.T.; Liu, C.M.; Wu, B.N.; Wu, S.N. Actions of KMUP-1, a xanthine and piperazine derivative on voltage-gated Na+ and Ca2+-activated K+ currents in GH3 pituitary tumour cells. Br. J. Pharmacol. 2015, 172, 5110–5122. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, S.N. Inhibitory effectiveness of gomisin A, a dibenzocyclooctadiene lignan isolated from Schizandra chinensis, on the amplitude and gating of voltage-gated Na+ current. Int. J. Mol. Sci. 2020, 21, 8816. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Activation of voltage-gated sodium current and inhibition of erg-mediated potassium current caused by telmisartan, an antagonist of angiotensin II type-1 receptor, in HL-1 atrial cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2018, 45, 797–807. [Google Scholar] [CrossRef] [PubMed]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef]
- Lai, M.C.; Wu, S.N.; Huang, C.W. Telmisartan, an antagonist of angiotensin II receptors, accentuates voltage-gated Na+ currents and hippocampal neuronal excitability. Front. Neurosci. 2020, 14, 902. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Bezanilla, F. Currents related to movement of the gating particles of the sodium channels. Nature 1973, 242, 459–461. [Google Scholar] [CrossRef]
- Sheets, M.F.; Fozzard, H.A.; Hanck, D.A. Important role of asparagines in coupling the pore and voltage-sensor domain in voltage-gated sodium channels. Biophys. J. 2015, 109, 2277–2286. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A. Hysteresis in voltage-gated channels. Channels 2017, 11, 140–155. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A.; Chiem, A.T. Hysteretic behavior in voltage-gated channels. Front. Pharmacol. 2020, 11, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, P.; Messinger, A.; Blatt, Y.; Gopher, A.; Kleinfeld, D. Charge displacements in interfacial layers containing reaction centers. J. Membr. Biol. 1998, 165, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Roginsky, V.A.; Pashkovskaya, A.A.; Rokitskaya, T.I.; Kotova, E.A.; Zaspa, A.A.; Chernyak, B.V.; Skulachev, V.P. Protective effects of mitochondria-targeted antioxidant SkQ in aqueous and lipid membrane environments. J. Membr. Biol. 2008, 222, 141–149. [Google Scholar] [CrossRef]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell. Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Zhang, Y. Effects of an estrogen receptor antagonist on proliferation, prolactin secretion and growth factor expression in the MMQ pituitary prolactinoma cell line. J. Clin. Neurosci. 2011, 18, 1694–1698. [Google Scholar] [CrossRef]
- Milton, R.L.; Caldwell, J.H. Na current in membrane blebs: Implications for channel mobility and patch clamp recordings. J. Neurosci. 1990, 10, 885–893. [Google Scholar] [CrossRef]
- Hung, T.Y.; Wu, S.N.; Huang, C.W. The integrated effects of brivaracetam, a selective analog of levetiracetam, on ionic currents and neuronal excitability. Biomedicines 2021, 9, 369. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Effectiveness of columbianadin, a bioactive coumarin derivative, in perturbing transient and persistent INa. Int. J. Mol. Sci. 2021, 22, 621. [Google Scholar] [CrossRef]
- Kourie, J.I. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol. 1998, 275, C1–C24. [Google Scholar] [CrossRef]
- Stark, G. Functional consequences of oxidative membrane damage. J. Membr. Biol. 2005, 205, 1–16. [Google Scholar] [CrossRef]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [PubMed]
- Cannio, R.; D’Angelo, A.; Rossi, M.; Bartolucci, S. A superoxide dismutase from the archaeon Sulfolobus solfataricus is an extracellular enzyme and prevents the deactivation by superoxide of cell-bound proteins. Eur. J. Biochem. 2000, 267, 235–243. [Google Scholar] [CrossRef]
- Chiang, H.T.; Wu, S.N. On the mechanism of selective action of probucol on the inwardly rectifying potassium current in GH3 lactotrophs. Drug Dev. Res. 2001, 54, 1–11. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F.; Jan, C.R.; Chen, I.J.; Lo, Y.C. Selective block by glyceryl nonivamide of inwardly rectifying K+ current in rat anterior pituitary GH3 cells. Life Sci. 1998, 63, PL281–PL288. [Google Scholar] [CrossRef]
- Simasko, S.M.; Sankaranarayanan, S. Characterization of a hyperpolarization-activated cation current in rat pituitary cells. Am. J. Physiol. 1997, 272, E405–E414. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.L.; Lu, T.J.; Wu, S.N. Effectiveness in block by dexmedetomidine of hyperpolarization-activated cation current, independent of its antagonistic effect on alpha 2-adrenergic receptors. Int. J. Mol. Sci. 2020, 21, 9110. [Google Scholar] [CrossRef]
- Banyasz, T.; Szentandrássy, N.; Magyar, J.; Szabo, Z.; Nánási, P.P.; Chen-Izu, Y.; Izu, L.T. An emerging antiarrhythmic target: Late sodium current. Curr. Pharm. Des. 2015, 21, 1073–1090. [Google Scholar] [CrossRef]
- Yu, S.; Li, G.; Huang, C.L.; Lei, M.; Wu, L. Late sodium current associated cardiac electrophysiological and mechanical dysfunction. Pflug. Arch. 2018, 470, 461–469. [Google Scholar] [CrossRef]
- Samori, B.; Lenaz, G.; Battino, M.; Marconi, G.; Domini, I. On coenzyme Q orientation in membrane: A linear dichroism study of ubiquinones in a model bilayer. J. Membr. Biol. 1992, 128, 193–203. [Google Scholar] [CrossRef]
- Lenaz, G. Role of mobility of redox components in the inner mitochondrial membrane. J. Membr. Biol. 1988, 104, 193–209. [Google Scholar] [CrossRef]
- Belardinelli, L.; Giles, W.; West, A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J. Physiol. 1988, 405, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.J. Stimulation of insulin release from pancreatic islets by quinines. Biosci. Rep. 1991, 11, 165–170. [Google Scholar] [CrossRef]
- Rosenfeldt, F.L.; Haas, S.J.; Krum, H.; Hadj, A.; Ng, K.; Leong, J.Y.; Watts, G.F. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J. Hum. Hypertens. 2007, 21, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Grocin, A.G.; da Silva, T.N.X.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef]
- Gutierrez-Mariscal, F.M.; Arenas-de Larriva, A.P.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q(10) supplementation for the reduction of oxidative stress: Clinical implications in the treatment of chronic diseases. Int. J. Mol. Sci. 2020, 21, 7870. [Google Scholar] [CrossRef]
- Kang, L.; Liu, S.; Li, J.; Tian, Y.; Xue, Y.; Liu, X. The mitochondria-targeted anti-oxidant MitoQ protects against intervertebral disc degeneration by ameliorating mitochondrial dysfunction and redox imbalance. Cell Prolif. 2020, 53, e12779. [Google Scholar] [CrossRef]
- Sattarinezhad, E.; Shafaroodi, H.; Sheikhnouri, K.; Mousavi, Z.; Moezi, L. The effects of coenzyme Q10 on seizures in mice: The involvement of nitric oxide. Epilepsy Behav. 2014, 37, 36–42. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Roghani, M. Coenzyme Q10 ameliorates neurodegeneration, mossy fiber sprouting, and oxidative stress in intrahippocampal kainate model of temporal lobe epilepsy in rat. J. Mol. Neurosci. 2013, 49, 194–201. [Google Scholar] [CrossRef]
- Tawfik, M.K. Coenzyme Q10 enhances the anticonvulsant effect of phenytoin in pilocarpine-induced seizures in rats and ameliorates phenytoin-induced cognitive impairment and oxidative stress. Epilepsy Behav. 2011, 22, 671–677. [Google Scholar] [CrossRef]
- Chang, H.H.; Sung, P.S.; Liao, W.C.; Chang, A.Y.W.; Hsiao, Y.H.; Fu, T.F.; Huang, C.Y.; Huang, C.W. An open pilot study of the effect and tolerability of add-on multivitamin therapy in patients with intractable focal epilepsy. Nutrients 2020, 12, 2359. [Google Scholar] [CrossRef]
- Liao, W.C.; Huang, C.W.; Hsiao, Y.H.; Sung, P.S.; Fu, T.F.; Chang, A.Y.W.; Chang, H.H. Association between the Serum Coenzyme Q10 Level and Seizure Control in Patients with Drug-Resistant Epilepsy. Healthcare 2021, 9, 1118. [Google Scholar] [CrossRef]
- Lee, D.; Shim, M.S.; Kim, K.Y.; Noh, Y.H.; Kim, H.; Kim, S.Y.; Weinreb, R.N.; Ju, W.K. Coenzyme Q10 inhibits glutamate excitotoxicity and oxidative stress-mediated mitochondrial alteration in a mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 993–1005. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, S.K.; Wang, S.J. Coenzyme Q10 inhibits the release of glutamate in rat cerebrocortical nerve terminals by suppression of voltage-dependent calcium influx and mitogen-activated protein kinase signaling pathway. J. Agric. Food Chem. 2012, 60, 11909–11918. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, T.-Y.; Wu, S.-N.; Huang, C.-W. The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current. Nutrients 2022, 14, 3393. https://doi.org/10.3390/nu14163393
Hung T-Y, Wu S-N, Huang C-W. The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current. Nutrients. 2022; 14(16):3393. https://doi.org/10.3390/nu14163393
Chicago/Turabian StyleHung, Te-Yu, Sheng-Nan Wu, and Chin-Wei Huang. 2022. "The Modulation of Ubiquinone, a Lipid Antioxidant, on Neuronal Voltage-Gated Sodium Current" Nutrients 14, no. 16: 3393. https://doi.org/10.3390/nu14163393





