Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products
Abstract
1. Introduction
2. Probiotics
2.1. Beneficial Claims of Probiotics
2.2. Probiotic Attributes
| Beneficial Claims | Probiotic Treatment | Main Findings | Reference |
|---|---|---|---|
| Prevention of infectious diarrhea | Bifidobacterium longum BORI and Lactobacillus acidophilus AD301 | Reduced duration of rotavirus diarrhea in young Korean children. | Park et al. [7] |
| Alleviate symptoms of type B gastritis and peptic ulcers and prevention of gastric cancer | Bifidobacterium lactis Bb12 | Growth inhibition of Helicobacter pylori leading to a decrease in urease activity, a key enzyme essential for survival of the pathogen in the stomach acid after 6 weeks of therapy. | Wang et al. [5] |
| Relieve inflammatory bowel disease syndromes | L. casei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, B. longum, B. breve, B. infantis, and Streptococcus salivarius subsp. thermophilus | Restoration of microbial flora to normal level through observation of increased lactobacilli, bifidobacterial, and Streptococcus salivarius in patient’s fecal matter, leading to a reduced inflammation and symptoms of chronic pouchitis. | Gionchetti et al. [4] |
| Lactobacillus GG | Significant reduction in Crohn’s disease activity and increased intestinal permeability after 4 weeks medication of Lactobacillus GG enterocoated tablets containing 1010 CFU/g. | Gupta et al. [56] | |
| Alternative prevention for cancer | Lactobacillus rhamnosus strain GG and LC-705 | Decrease in carcinogenic aflatoxin level in the chicken lumen after daily ingestion probiotic strains. | El-Nezami et al. [57] |
| Modulate host’s immunity | L. acidophilus, L. casei, L. reuteri, Bifidobacterium bifidum, and Streptococcus thermophilus | Induced hyporesponsiveness of T- and B-cells, non-apoptotic downregulation of T helper (Th)1, Th2, and Th17 cytokines, and generation and increased suppressor activity of CD4+CD25+Tregs. | Kwon et al. [6] |
| Allergy prevention and treatment | B. longum NCC 3001 and Lactobacillus paracasei NCC 2461 | Downregulation of allergen-specific immune responses contributing to airway inflammation in mucosal lining of polysensitize mouse. | Schabussova et al. [58] |
| Bifidobacterium lactis Bb-12 and Lactobacillus strain GG | Improved skin condition in infants suffering atopic eczema after 2 months supplementation of the probiotic formulas. | Isolauri et al. [59] | |
| Bacterial vaginosis treatment | Yoghurt (containing mostly Lactobacillus sp.) | Cured bacterial vaginosis after 1 to 2 months of intra-vaginal treatment through the increased lactobacilli flora and vaginal pH correction. | Neri et al. [60] |
2.3. The Market of Probiotic Drinks
3. Dairy and Non-Dairy Based Probiotic Drinks
3.1. Cholesterol and Fat Content
3.2. Allergens
3.3. Consumers Preference on Non-Dairy Products
3.4. Recent Development of Non-Dairy Probiotic Drinks
4. Comparison of Cereal and Fruit-Based Probiotic Beverages
5. Utilization of Fermentable Sugars in Non-Dairy Substrates
5.1. Production of Fermentable Sugar in Plants

5.2. Glucose
5.3. Fructose
5.4. Galactose
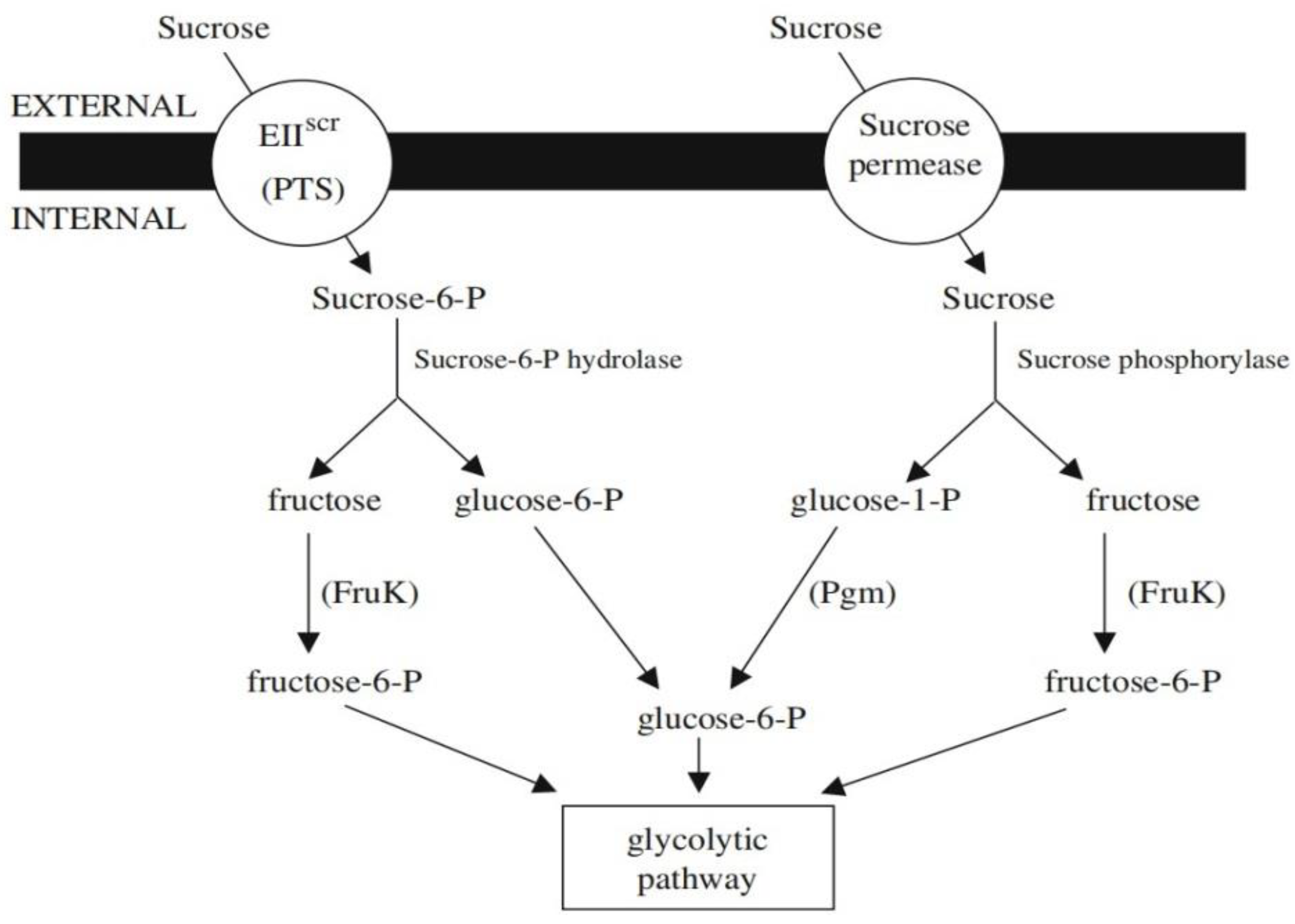
5.5. Sucrose
5.6. Fermentability of Vegetable and Fruit Juices by Lactic Acid Bacteria
6. Quality Indicators of Fermented Product
6.1. Total Soluble Solids and Sugar Consumption
6.2. Titratable Acidity and pH
6.3. Stability at Low Storage Temperature
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO/WHO. Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Bacteria; A Joint FAO/WHO Expert Consultation: Cordóba, Argentina, 2001; Available online: https://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf (accessed on 21 May 2021).
- Martins, E.M.F.; Ramos, A.M.; Martins, M.L.; Leite Junior, B.R.C. Fruit salad as a new vehicle for probiotic bacteria. Food Sci. Technol. 2016, 36, 540–548. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Geonchetti, P.; Rizzello, F.; Venturi, A.; Brigidi, P.; Matteuzi, D.; Bazzochi, G.; Campieri, M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology 2000, 119, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Li, S.N.; Liu, C.S.; Perng, D.S.; Su, Y.C.; Wu, D.C.; Jan, C.M.; Lai, C.H.; Wang, T.N.; Wang, W.M. Effect of ingesting Lactobacillus- and Bifidobacterium- containing yogurt in subjects with colonized Helicobacter pylori. Am. J. Clin. Nutr. 2004, 80, 737–741. [Google Scholar] [PubMed]
- Kwon, H.K.; Lee, C.G.; So, J.S. Generation of regulatory dendritic cells and CD4+Foxp3+ cells by probiotics administration suppresses immune disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kwon, B.; Ku, S.; Ji, G.E. The efficacy of Bifidobacterium longum BORI and Lactobacillus acidophilus AD301 probiotic treatment in infants with rotavirus infection. Nutrients 2017, 9, 887. [Google Scholar] [CrossRef]
- Mukisa, I.V.; Byaruhanga, Y.B.; Muyanja, C.M.B.K.; Langsurd, T.; Narvhus, J.A. Production of organic flavor compounds by dominant lactic acid bacteria and yeasts from Obushera, a traditional sorghum malt fermented beverage. Food Sci. Nutr. 2017, 5, 702–712. [Google Scholar] [CrossRef]
- Huang, H.; Qureshi, N.; Chen, M.H.; Liu, W.; Singh, V. Ethanol production from food waste at high solids content with vacuum recovery technology. J. Agric. Food Chem. 2015, 63, 2760–2766. [Google Scholar] [CrossRef]
- Market Analysis. Probiotic Drink Market Size, Share & Trends Analysis Report By Product (Dairy-Based, Plant-Based), By Distribution Channel (Offline, Online), By Region, And Segment Forecasts, 2020–2070. 2020, p. 80. Available online: //www.grandviewresearch.com/industry-analysis/probiotic-drink-market (accessed on 24 June 2020).
- Vasudha, S.; Mishra, H.N. Non dairy probiotic beverages. Int. Food Res. J. 2013, 20, 7–15. [Google Scholar]
- Cordle, T.C. Soy protein allergy: Incidence and relative severity. J. Nutr. 2004, 134, 1213S–1219S. [Google Scholar] [CrossRef]
- Thitiprasert, S.; Kodama, K.; Tanasupawat, S.; Prasitchoke, P.; Rampai, T.; Prasirtsak, B.; Tolieng, V.; Piluk, J.; Assabumrungrat, S.; Thongchul, N. A homofermentative Bacillus sp. BC-001 and its performance as a potential L-lactate industrial strain. Bioprocess. Biosyst. Eng. 2017, 40, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Blajman, J.; Vinderola, G.; Paez, R.; Signorini, M. The role of homofermentative and heterofermentative lactic acid bacteria for alfalfa silage: A meta-analysis. J. Agric. Sci. 2020, 158, 107–118. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic lactobacilli and bifidobacterial effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Guandilini, S. Probiotics for prevention and treatment of diarrhea. J. Clin. Gastroenterol. 2011, 45, S149–S153. [Google Scholar] [CrossRef]
- Aziz, M.F.A.; Abbasiliasi, S.; Jawan, R. Effects of carbon and nitrogen sources on the growth of bacteriocin-like inhibitory substances producing lactic acid bacterium, Lactobacillus farciminis TY1. In Proceeding of the 13th Seminar on Science & Technology, Faculty of Science & Natural Resources, University Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia, 6–7 October 2020; pp. 228–232. [Google Scholar]
- Yuliana, N.; Rangga, A.; Rakhmiati. Manufacture of fermented coco milk-drink containing lactic acid bacteria cultures. Afr. J. Food Sci. 2010, 4, 558–562. [Google Scholar]
- Yoon, Y.K.; Woodams, E.E.; Hang, Y.D. Production of probiotic cabbage juice by lactic acid bacteria. Bioresour. Technol. 2006, 97, 1427–1430. [Google Scholar] [CrossRef]
- Zimmermann, M.H.; Ziegler, H. List of sugars and sugar alcohols in sieve-tube exudates. In Transport in Plants I. Phloem Transport; Zimmermann, M.H., Milburn, J.A., Eds.; Springer: Berlin, Germany, 1975; pp. 480–503. [Google Scholar]
- Williams, B.A.; Deirdre, M.; Flanagan, B.M.; Gidley, M.J. “Dietary fibre”: Moving beyond the “soluble/insoluble” classification for monogastric nutrition, with an emphasis on humans and pigs. J. Anim. Sci. Biotechnol. 2019, 10, 45. [Google Scholar] [CrossRef]
- Arboleya, S.; Solis, G.; Fernandez, N.; de los Reyes-Gavilan, C.G.; Gueimonde, M. Facultative to strict anaerobes ratio in the preterm infant microbiota: A target for intervention? Gut Microbes 2012, 3, 583–588. [Google Scholar] [CrossRef]
- Van der Waaji, D.; Berghuis-de Vries, J.M.; Lekkerkerk-van der Wees, J.E.S. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 1971, 69, 405–411. [Google Scholar] [CrossRef]
- Vollard, E.J.; Clasener, H.A. Colonization resistance. Antimicrob. Agents. Chemother. 1944, 38, 409–414. [Google Scholar] [CrossRef]
- Collins, L.B.; Thomas, T.D. Pyruvate kinase of Streptococcus lactis. J. Bacteriol. 1982, 120, 52. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L. In vitro selection of probiotic lactobacilli: A critical appraisal. Curr. Issues Intest. Microbiol. 2000, 1, 59–67. [Google Scholar] [PubMed]
- Midolo, P.D.; Lambert, J.R.; Hull, R.; Grayson, M.L. In vitro inhibition of Helicobacter pylori NCTC 11637 by organic acids and lactic acid bacteria. J. Appl. Bacteriol. 1995, 79, 475–479. [Google Scholar] [CrossRef]
- Coconnier, M.H.; Lievin, V.; Hemery, E.; Servin, A.L. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl. Environ. Microbiol. 1998, 64, 4573–4580. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E. Probiotics. Food Technol. 1999, 53, 67–77. [Google Scholar]
- Gangwar, A.S.; Bhardwaj, A.; Sharma, V. Fermentation of tender coconut water by probiotic bacterial Bacillus coagulans. Int. J. Food Stud. 2018, 7, 100–110. [Google Scholar] [CrossRef]
- Basu, S.; Paul, D.K.; Ganguly, S.; Chatterjee, M.; Chandra, P.K. Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: A randomized controlled trial. J. Clin. Gastroenterol. 2009, 43, 208–213. [Google Scholar] [CrossRef]
- Guandalini, S. Probiotics for children with diarrhea: An update. J. Clin. Gastroenterol. 2008, 42, S53–S57. [Google Scholar] [CrossRef]
- Fang, S.B.; Lee, H.C.; Hu, J.J.; Hou, S.Y.; Liu, H.L.; Fang, H.W. Dose-dependent effect of Lactobacillus rhamnosus on quantitative reduction of faecal rotavirus shedding in children. J. Trop. Pediatr. 2009, 55, 297–301. [Google Scholar] [CrossRef]
- Mao, M.; Yu, T.; Xiong, Y.; Wang, Z.; Liu, H.; Gotteland, M.; Brunser, O. Effect of a lactose-free milk formula supplemented with bifidobacterial and streptococci on the recovery from acute diarrhoea. Asia Pac. J. Clin. Nutr. 2008, 17, 30–34. [Google Scholar]
- Liu, Q.F.; Kim, H.M.; Lim, S.; Chung, M.J.; Lim, C.Y.; Koo, B.S.; Kang, S.S. Effect of probiotic administration on gut microbiota and depressive behaviors in mice. DARU J. Pharm. Sci. 2020, 28, 181–189. [Google Scholar] [CrossRef] [PubMed]
- McGinn, P.N. Does Lactobacillus reuteri Probiotic Treatment Improve Sleep Quality in Rhesus Macaques (Macaca mulatta) Displaying the Self-Injurious Phenotype? Master’s Thesis, University of Massachutes Amherst, Amherst, MA, USA, 2019. [Google Scholar]
- Sensoy, I. A review on the food digestion in the digestive tract and the use in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.C.K.; Walsh, J.H. Regulation of acid secretion in vivo. In Gastrin; Walsh, L.J.H., Ed.; Raven Press: New York, NY, USA, 1993; pp. 221–243. [Google Scholar]
- Giannella, R.A.; Broitman, S.A.; Zamcheck, N. Gastric acid barrier to ingested microorganisms in man. Gut 1972, 13, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaluddin, S. Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult. Sci. 1998, 77, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Ronka, E.; Malinen, E.; Saarela, M.; Rinta-Koski, M.; Aarnikunnas, J.; Palva, A. Probiotic and milk technological properties of Lactobacillus brevis. Int. J. Food Microbiol. 2003, 83, 63–74. [Google Scholar] [CrossRef]
- Tennant, S.M.; Hartland, E.L.; Phumoonna, T.; Lyras, D.; Rood, J.I.; Robins-Browne, R.M.; van Driel, I.R. Influence of gastric acid on the susceptibility to infection with ingested bacterial pathogens. Infect. Immun. 2007, 76, 639–645. [Google Scholar] [CrossRef]
- Sethi, S. How Long Does Food Stay in Your Stomach? 2020. Available online: https://www.healthline.com/health/how-long-does-it-take-for-your-stomach-to-empty (accessed on 22 April 2021).
- Jacobsen, C.N.; Nielsen, V.R.; Hayford, A.E.; Moller, P.L.; Michaelsen, K.F.; Paerregaard, A.; Sandstorm, B.; Tvede, M.; Jakobsen, M. Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl. Environ. Microbiol. 1999, 65, 4949–4956. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C. Surviving the acid test: Responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef]
- Sebald, W.; Friedl, P.; Schairer, H.U.; Hoppe, J. Structure and genetics of the H+-conducting F0 portion of the ATP synthase. Ann. N. Y. Acad. Sci. 1982, 402, 28–44. [Google Scholar] [CrossRef]
- Fortier, L.C.; Tourdot-Marechal, R.; Divies, C.; Lee, B.H.; Guzzo, J. Induction of Oenococcus oeni H+-ATPase activity and mRNA transcription under acidic conditions. FEMS Microbiol. Lett. 2003, 222, 165–169. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R. Bile salts: Chemistry, papthochemistry, biology, pathobiology, and therapeutics. Cell Mol. Life Sci. 2008, 65, 2461–2468. [Google Scholar] [CrossRef]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [PubMed]
- Leverrier, P.; Dimova, D.; Pichereau, V.; Auffray, Y.; Boyaval, P.; Jan, G. Susceptibility and adaptive response to bile salts in Propionibacterium fredenreichiii: Physiological and proteomic analysis. Appl. Environ. Microbiol. 2003, 69, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Kandell, R.L.; Bernstein, C. Bile salt/acid induction of DNA damage in bacterial and mammalian cells: Implications for colon cancer. Nutr. Cancer 1991, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Flahaut, S.; Frere, J.; Boutibonnes, P.; Auffray, Y. Comparison of the bile salts and sodium dodecyl sulfate stress responses in Enterococcus faecalis. Appl. Environ. Microbial. 1996, 62, 2416–2420. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, A.; Marangos, M. Iron and microbial growth. Insight Control Infect. Dis. Glob. Scenar. 2012, 16, 289–330. [Google Scholar]
- Rajagopalan, N.; Lindenbaum, S. The binding of Ca2+ to taurine and glycine-conjugated bile salt micelles. Biochem. Biophys. Acta 1982, 11, 66–74. [Google Scholar] [CrossRef]
- Gupta, P.; Andrew, H.; Krischner, B.S.; Guandalini, S. Is Lactobacillus GG helpful in children with Chron’s disease? Results of a preliminary, open-label study. J. Pediatric Gastroenterol. Nutr. 2000, 31, 453–457. [Google Scholar] [CrossRef]
- El-Nazemi, H.; Mykkanen, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B1 from chicken duodenum. J. Food Protect. 2000, 63, 549–552. [Google Scholar] [CrossRef]
- Schabussova, I.; Hufnagl, K.; Wild, C. Distinctive anti-allergy properties of two probiotic bacterial strains in a mouse model of allergic poly-sensitization. Vaccine 2011, 29, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Isolauri, E.; Arvola, T.; Sutas, T.; Moilanen, E.; Salminen, S. Probiotics in the management of atomic eczema. Clin. Exp. Allergy 2000, 30, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Neri, A.; Sabah, G.; Samra, Z. Bacterial vaginosis in pregnancy treated with yoghurt. Acta Obstet. Gynecol. Scand. 1993, 72, 17–19. [Google Scholar] [CrossRef]
- Crawford, R.W.; Keestra, A.M.; Winter, S.E.; Xavier, M.N.; Tsolis, R.M.; Tolstikov, V. Very long O-antigen chains enhance fitness during Salmonella-induced colitis by increasing bile resistance. PLoS Patho. 2012, 8, e1002918. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, E.; Yamaguchi, A.; Nishino, K. AcrAB multidrug efflux pump regulation in Salmonella enterica serovar typhimurium by RamA in response to environmental signals. J. Biol. Chem. 2008, 283, 24245–24253. [Google Scholar] [CrossRef] [PubMed]
- Zgurskaya, H.I.; Nikaido, H. Bypassing the periplasm: Reconstruction of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. USA 1999, 96, 7190–7195. [Google Scholar] [CrossRef]
- Prieto, A.I.; Ramos-Morales, F.; Casadesus, J. Repair DNA damage induces by bile salts in Salmonella enterica. Genetics 2006, 174, 575–584. [Google Scholar] [CrossRef]
- Davati, N.; Yazdi, F.T.; Zibaee, S.; Shahidi, F.; Edalatian, M.R. Study of lactic acid bacteria community from raw milk of Iranian one humped camel and evaluation of their probiotic properties. Jundishapur. J. Microbiol. 2015, 8, e16750. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Woodams, E.E.; Hang, Y.D. Probiotication of tomato juice lactic acid bacteria. J. Microbiol. 2004, 42, 315–318. [Google Scholar]
- Dogahe, M.K.; Khosravi-Darani, K.; Tofighi, A.; Dadgar, M.; Mortazavian, A.M. Effect of process variables on survival of bacteria in probiotics enriched pomegranate juice. Biotechnol. J. Int. 2014, 5, 37–50. [Google Scholar] [CrossRef]
- Kumar, B.V.; Sreedharamurthy, M.; Reddy, O.V.S. Probiotication of mango and sapota juice using Lactobacillus plantarum NCDC LP 20. Nutrifoods 2015, 14, 97–106. [Google Scholar] [CrossRef]
- Gao, Y.; Hamis, N.; Gutierrez-Maddox, N.; Kantono, K.; Kitundu, E. Development of a probiotic beverage using breadfruit flour as a substrate. Foods 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Tourila, H.; Cardello, A.V. Consumer responses to an off-flavor in juice in the presence of specific health claims. Food Qual. Prefer. 2002, 13, 561–569. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2006, 61, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Virtual Medical Centre. Milk and Milk Products (Dairy Products). 2020. Available online: https://www.myvmc.com/lifestyles/milk-and-milk-products-dairy-products/ (accessed on 24 June 2021).
- Chaudhary, A. Probiotic fruit and vegetable juices: Approach towards a healthy gut. Int. J. June Curr. Microbiol. App. Sci. 2019, 8, 1265–1279. [Google Scholar] [CrossRef]
- Bovine Alliance on Management and Nutrition. A Guide to Calf Milk Replacers: Types, Use and Quality. 2008. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/bamn/BAMN08_GuideMilkRepl.pdf (accessed on 24 June 2021).
- Lim, L.B.L.; Chieng, H.I.; Wimmer, F.L. Nutrient composition of artocarppus champeden and its hybrid (nanchem) in negara brunei darussalam. ASEAN J. Sci. Technol. Dev. 2011, 28, 122–138. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Know Your Risk for Heart Disease. 2019. Available online: https://www.cdc.gov/heartdisease/risk_factors.htm?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fheartdisease%2Fbehavior.htm (accessed on 24 June 2021).
- World Health Organization. Cardiovascular Diseases. 2021. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 24 June 2021).
- Bard, J.M.; Drouet, L.; Lairon, D.; Cazaubiel, M.; Marmonier, C.; Ninio, E.; dit Sollier, C.B.; Martin, J.C.; Boyer, C.; Bobin-Dubigeon, C. Effect of milk fat on LDL cholesterol and other cardiovascular risk markers in healthy humans: The INNOVALAIT project. Eur. J. Clin. Nutr. 2019, 74, 285–296. [Google Scholar] [CrossRef]
- Faye, B.; Bengoumi, M.; Al-Masaud, A.; Konuspayeva, G. Comparative milk and serum cholesterol content in dairy cow and camel. J. King Saud Univ.–Sci. 2015, 27, 169–175. [Google Scholar] [CrossRef]
- Afonso, M.S.; Machado, R.M.; Lavrador, M.S.; Quintao, E.C.R.; Moore, K.J.; Lottenberg, A.M. Molecular pathways underlying cholesterol homestasis. Nutrients 2018, 10, 760. [Google Scholar] [CrossRef]
- University of California San Francisco Health. Cholesterol Content of Foods. 2021. Available online: https://www.ucsfhealth.org/education/cholesterol-content-of-foods (accessed on 24 June 2021).
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High cholesterol/low cholesterol: Effects in biological membranes review. Cell Biochem. Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, indicators risk factors and new hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Celermajer, D.S.; Chow, C.K.; Marijon, E.; Anstey, N.M.; Woo, K.S. Cardiovascular disease in the developing world: Prevalences, patterns, and the potential of early disease detection. J. Am. Coll. Cardiol. 2012, 60, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- World Health Rankings. Malaysia: Coronary Heart Disease. 2018. Available online: https://www.worldlifeexpectancy.com/malaysia-coronary-heart-disease (accessed on 24 June 2021).
- US Department of Health and Human Services. What Causes Obesity & Overweight? 2016. Available online: https://www.nichd.nih.gov/health/topics/obesity/conditioninfo/cause (accessed on 24 June 2021).
- Cleveland Clinic. Fat: What You Need to Know. 2014. Available online: https://health.clevelandclinic.org/all-about-fats-why-you-need-them-in-your-diet/ (accessed on 24 June 2021).
- Malnick, S.D.H.; Knobler, H. The medical complications of obesity. QJM Int. J. Med. 2006, 99, 565–579. [Google Scholar] [CrossRef]
- Coulston, A.M. The role of dietary fats in plant-based diets. Am. J. Clin. Nutr. 1999, 70, 512s–515s. [Google Scholar] [CrossRef] [PubMed]
- Jenness, R. Comparative aspects of milk proteins. J. Dairy Res. 1979, 46, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Wal, J.M. Cow’s milk proteins/allergens. Ann. Allergy Asthma Immunol. 2002, 89 (Suppl. 1), 3–10. [Google Scholar] [CrossRef]
- Wal, J.M. Bovine milk allergenicity. Ann. Allergy Asthma Immunol. 2004, 93 (Suppl. 3), S2–S11. [Google Scholar] [CrossRef]
- Arnberg, K.; Molgaard, C.; Michaelsen, K.M.; Jensen, S.M.; Trolle, E.; Larnkjaer, A. Skim milk, whey, and casein increase body weight and whey and casein increase the plasma C-peptide concentration in overweight adolescents. J. Nutr. 2012, 142, 2083–2090. [Google Scholar] [CrossRef]
- Schulmeister, U.; Hochwallner, H.; Swoboda, I.; Focke-Tejkl, M.; Geller, B.; Nystrand, M.; Harlin, A.; Thalhamer, J.; Scheiblhofer, S.; Keller, W.; et al. Cloning, expression, and mapping of allergenic determinants of alhpha S1-casein, a major cow;s milik allergen. J. Immunol. 2009, 182, 7019–7029. [Google Scholar] [CrossRef]
- Docena, G.H.; Fernandez, R.; Chirdo, F.G.; Fossati, C.A. Identification of casein as the major allergenic and antigenic protein of cow’s milk. Allergy 1996, 51, 412–416. [Google Scholar] [CrossRef]
- Restani, P.; Ballabio, C.; Lorenzo, C.D.; Tripodi, S.; Fiocchi, A. Molecular aspects of milk allergens and their role in clinical events. Anal. Bioanal. Chem. 2009, 395, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Niemi, M.; Jylha, S.; Laukkanen, M.L.; Soderlund, H.; Makinen-Kiljunen, S.; Kallio, J.M.; Hakulinen, N.; Haatela, T.; Takkinen, K.; Rouvinen, J. Molecular interactions between a recombinant IgE antibody and the beta-lactoglobulin allergen. Structure 2007, 15, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- Wong, S.Y.; Hartel, R.W. Crystallization in lactose refining—A review. J. Food Sci. 2014, 79, R257–R272. [Google Scholar] [CrossRef]
- Fosgard, R.A. Lactose digestion in humans: Intestinal lactase appears to be consecutive whereas the colonic microbiome is adaptable. Am. J. Clincal Nutr. 2019, 110, 273–279. [Google Scholar] [CrossRef]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Definition & Facts for Lactose Intolerance. 2018. Available online: https://www.niddk.nih.gov/health-information/digestive-diseases/lactose-intolerance/definition-facts (accessed on 24 June 2021).
- Rollin, B.E. Animal rights as a mainstream phenomenon. Animals 2011, 1, 102–115. [Google Scholar] [CrossRef]
- People For The Ethical Treatment Of Animals (PETA). Cow’s Milk: A Cruel and Unhealthy Product. 2021. Available online: https://www.peta.org/issues/animals-used-for-food/animals-used-food-factsheets/cows-milk-cruel-unhealthy-product/ (accessed on 24 June 2021).
- Stevenson, J. Heifers Are Still Too Old When They Calve. 2011. Available online: https://hoards.com/article-2204-heifers-are-still-too-old-when-they-calve.html (accessed on 24 June 2021).
- Wagner, K.; Setner, D.; Barth, K.; Palme, R.; Futschik, A.; Waiblinger, S. Effects of mother versus artificial rearing during the first 12 weeks of life on challenge reponses of dairy cows. Appl. Anim. Behav. Sci. 2015, 164, 1–11. [Google Scholar] [CrossRef]
- Cheng, W.N.; Han, S.G. Bovine mastitis: Risk factors, therapeutic strategies, and alternative treatments—A review. Asian-Aust. J. Anim. Sci. 2020, 33, 1699–1713. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.; Bowman, D.D. Viability of pathogens in the environment. In Pathogens in the Environment Workshop Proceeedings, Kansas City, Missouri, 23–25 February 2004; Hargrove, W.L., Ed.; U.S. Department of Agriculture: Washington, DC, USA, 2004; pp. 30–33. [Google Scholar]
- United States Environmental Protection Agency. Nonpoint Source: Agriculture. 2021. Available online: https://www.epa.gov/nps/nonpoint-source-agriculture (accessed on 24 June 2021).
- Global Dairy Alternatives Market Research Report. 2020. Available online: https://www.marketstudyreport.com/reports/global-dairy-alternatives-market-research-report2020?gclid=Cj0KCQjw2tCGBhCLARIsABJGmZ7gfwdGDpuJHK5ePqWx6k1vQqxMLsF2FOY1Kf2iuIv58UwHqP4OE3QaArv_EALw_wcB (accessed on 24 June 2021).
- Lifeway. 2021. Available online: https://lifewaykefir.com/ (accessed on 25 June 2021).
- GoodBelly. 2019. Available online: https://goodbelly.com/ (accessed on 25 June 2021).
- Griffin, J.N.; Silliman, B.R. Resource partitioning and why it matters. Nat. Educ. Knowl. 2011, 3, 49. [Google Scholar]
- Lifeway Foods. Lifeway Foods, Inc. Announces Results for First Quarter Ended 31 March 2021. 2021. Available online: https://www.globenewswire.com/en/search/organization/Lifeway%2520Foods%CE%B4%2520Inc%C2%A7 (accessed on 25 June 2021).
- Hume, M.E.; Donskey, C.J. Effect of vanomycin, tylosin, and chlortetracycline on vanomycin-resistant Enterococcus faecium colonization of broiler chickens during grow-out. Foodborne Patho. Dis. 2017, 14, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Melo-Bolivar, J.F.; Pardo, R.Y.R.; Hume, M.E.; Nisbet, D.J.; Rodriguez-Villamizar, F.; Alzate, J.F.; Junca, H.; Diaz, L.M.V. Establishment and characterization of a competitive exclusion bacterial culture derived from Nile tilapia (Oreochromis niloticus) gut microbiomes showing antimicrobial activity against pathogenic Streptococcus agalactiae. PLoS ONE 2019, 14, e0215375. [Google Scholar] [CrossRef]
- Biomel. 2021. Available online: https://www.biomel.life/ (accessed on 25 June 2021).
- GT’s. 2021. Available online: https://gtslivingfoods.com/ (accessed on 25 June 2021).
- KeVita. 2021. Available online: https://www.kevita.com/products/sparkling-probiotic-tonics/ (accessed on 25 June 2021).
- VitaCup. 2021. Available online: https://www.vitacup.com/products/immunity-coffee-pods (accessed on 25 June 2021).
- Cao, J.; Yu, Z.; Liu, W.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Probiotic characteristics of Bacillus coagulans and associated implications for human health and diseases. J. Funct. Foods 2020, 64, 103643. [Google Scholar] [CrossRef]
- Leroy, F.; Vuyst, L.D. Functional lactic acid bacteria starter cultures for the food fermentation industry. Trend Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Hassan, A.A.; Aly, M.A.A.; El-Hadidie, S.T. Production of cereal-based probiotic beverages. World Appl. Sci. J. 2012, 19, 1376–1380. [Google Scholar]
- de Jesus Silva, J.R.; Cairo, P.A.R.; do Bomfim, R.A.A.; Barbosa, M.P.; Souza, M.O.; Leite, T.C. Morphological and physiological changes during leaf ontogeny in genotypes of Eucalyptus young plants. Trees 2020, 34, 759. [Google Scholar] [CrossRef]
- Clifford, P. The pressure-flow hypothesis of phloem transport: Misconceptions in the A-level textbooks. J. Biol. Educ. 2010, 36, 110–112. [Google Scholar] [CrossRef]
- Zamski, E. Structure and function of beta vulgaris parenchyma cells: Ultrastructure and sugar uptake characteristics of tissue and cell in suspension. Bot. Gaz. 1986, 147, 20–27. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Ma, F.; Dandekar, A.M.; Cheng, L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Komamine, A. Turnover of cell-wall polysaccharides of a vinca-rosea suspension-culture, III turnover of arabinogalactan. Physiol. Plant. 1980, 50, 113–118. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodelling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, S. Metabolism of sugars translocated to fruit and their regulation. J. Jpn. Soc. Hort. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef]
- Bren, A.; Park, J.O.; Towbin, B.D.; Dekel, E.; Rabinowitz, J.D.; Alon, U. Glucose becomes one of the worst carbon sources for E. coli on poor nitrogen sources due to suboptimal levels of cAMP. Sci. Rep. 2016, 6, 24834. [Google Scholar] [CrossRef]
- Chaudry, R.; Varacallo, M. Biochemistry, glycolysis. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing: Tampa, FL, USA, 2020. [Google Scholar]
- Narayanan, N.; Roychoudhury, P.K.; Srivastava, A. L (+) lactic acid fermentation and its product polymerization. J. Biotechnol. 2004, 7, 168–179. [Google Scholar]
- Kodama, R. Studies on the nutrition of lactic acid bacteria. Part 1. On the nutrition of a strain, no. 353, of lactic acid bacteria (1). Nippon. Nougei Kagaku Kaishi 1955, 30, 224–228. [Google Scholar] [CrossRef][Green Version]
- Koo, O.K.; Jeong, D.W.; Lee, J.M.; Kim, M.J.; Lee, J.H.; Chang, H.C. Cloning and characterization of the bifunctional alcohol/acetaldehyde dehydrogenase gene (adhE) in Leuconostoc mesenteroides isolated from kimchi. Biotechnol. Lett. 2005, 27, 505–510. [Google Scholar] [CrossRef]
- Endo, A. Fructophilic lactic acid bacteria inhabit fructose-rich niches in nature. Microb. Ecol. Health Dis. 2012, 23, 18563. [Google Scholar] [CrossRef]
- Fillanino, P.; Cagno, R.D.; Addante, R.; Pontonio, E.; Gobbetti, M. Metabolism of fructophilic lactic acid bacteria isolated from the Apis mellifera L. bee gut: Phenolic acids as external electron acceptors. Appl. Environ. Microbiol. 2016, 82, 6899–6911. [Google Scholar] [CrossRef]
- Endo, A.; Maeno, S.; Tanizawa, Y.; Kneifel, W.; Arita, M.; Dicks, L.; Salminen, S. Fructophilic lactic acid bacteria, a unique group of fructose-fermenting microbes. Appl. Environ. Microbiol. 2018, 84, e01290–e01318. [Google Scholar] [CrossRef] [PubMed]
- Grossiord, B.; Vaughan, E.E.; Luesink, E.; de Vos, W.M. Genetics of galactose utilisation via the Leloir pathway in lactic acid bacteria. Lait 1998, 78, 77–84. [Google Scholar] [CrossRef][Green Version]
- Zheng, L.; Das, S.; Burne, R.A. Utilization of lactose and galactose by Streptococcus mutans: Transport, toxicity, and carbon catabolite repression. J. Bacteriol. 2010, 192, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.A.; McDonald, T.R.R.; Robertson, J.H.; Stern, F. The crystal structure of sucrose. Acta Crystallogr. 1952, 5, 689–690. [Google Scholar] [CrossRef]
- Shanton, J.R.; Valerie, R.A. Sucrose utilisation in bacteria: Genetic organisation and regulation. Appl. Microbiol. Biotechnol. 2005, 67, 312–321. [Google Scholar]
- Vijayakumar, J.; Aravindan, R.; Viruthagiri, T. Recent trends in the production, purification, and application of lactic acid. Chem. Biochem. Eng. Q. 2008, 22, 245–264. [Google Scholar]
- Crater, J.S.; Lievense, J.C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 2018, 365, fny138. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Arendt, E.; Hill, C.; Stanton, C.; Ross, R.P. Recent advances in microbial fermentation for dairy and health. F1000Research 2017, 6, 751. [Google Scholar] [CrossRef]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Sablayrolles, J.M. Control of alcoholic fermentation in winemaking: Current situation and prospect. Food Res. Int. 2009, 42, 418–424. [Google Scholar] [CrossRef]
- Vengadaramana, A.; Balakumar, S.; Arasaratnam, V. Optimization of fermentation medium components to improve α-amylase production by submerged fermentation technology. Sch. Acad. J. Pharm. 2013, 2, 180–186. [Google Scholar]
- Boontawan, P.; Kanchanathawee, S.; Boontawan, A. Extractive fermentation of L-(+)-lactic acid by Pediococcus pentosaceus using electrodeionization (EDI) technique. Biochem. Eng. J. 2011, 54, 192–199. [Google Scholar] [CrossRef]
- Reece, J.B.; Urry, L.A.; Cain, M.L.; Wasserman, S.A.; Minorsky, P.V.; Jackson, R.B. Cellular respiration and fermentation. In Campbell Biology, 10th ed.; Pearson: San Francisco, CA, USA, 2011; pp. 162–184. [Google Scholar]
- Madigan, M.; Martinko, J.; Bender, K.; Buckley, D.; Stahl, D. Brock Biology of Microorganisms, 4th ed.; Pearson Education Limited: Edinburgh, UK, 2005. [Google Scholar]
- Corona-Hernandez, R.I.; Alvarez-Parrilla, E.; Lizardi-Mendoza, J. Tructural stability and viability of microencapsulated probiotic bacteria: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 614–628. [Google Scholar] [CrossRef]
- Kasimin, M.E.; Ainol, A.M.F.; Jani, J.; Abbasiliasi, S.; Arriff, A.B.; Jawan, R. Probiotic properties of antimicrobial-producing lactic acid bacteria isolated from dairy products and raw milk of Sabah (Northern Borneo), Malaysia. Malays. Appl. Biol. 2020, 49, 95–106. [Google Scholar] [CrossRef]
- Lee, P.R.; Boo, C.X.; Liu, S.Q. Fermentation of coconut water by probiotic strains Lactobacillus acidophilus L10 and Lactobacillus casei L26. Ann. Microbiol. 2013, 63, 1441–1450. [Google Scholar] [CrossRef]
- Kiesling, Y.G.; Farinazzo, F.S.; Fernandes, M.T.C.; Mauro, C.S.I.; Ioro, A.B.D.; Garcia, S. Coconut water fermentation by Lactobacillus plantarum with inulin addition: Development of a potentially symbiotic beverage. Braz. J. Dev. 2020, 6, 42324–42337. [Google Scholar] [CrossRef]
- Praia, A.B.; Junior, G.C.A.C.; dos Santos Guimaraes, G.; Rodriguez, F.L.; Ferreira, N.R. Coconut water-based probiotic drink proposal: Evaluation of microbiological stability and lactic acid estimation. HSOA J. Food Sci. Nutr. 2020, 6, 062. [Google Scholar] [CrossRef]
- Sivudu, S.N.; Umamahesh, K.; Reddy, O.V.S. A comparative study on probiotication of mixed watermelon and tomato juice by using probiotic strains of lactobacilli. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 977–984. [Google Scholar]
- Elgin, R. Why Is High Refractive Index Important? 2015. Available online: https://focenter.com/why-is-high-refractive-index-important/ (accessed on 24 June 2021).
- Tyl, C.; Sadler, G.D. pH and Titratable Acidity. In Food Analysis; Food Science Text Series; Nielsen, S., Ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Titration of Fruit Juices. 2021. Available online: https://chem.libretexts.org/Courses/Saint_Marys_College_Notre_Dame_IN/Chem_122L%3A_Principles_of_Chemistry_II_Laboratory_(Under_Construction__)/07%3A_Titration_of_Fruit_Juices (accessed on 24 June 2021).
- GEA Niro Method No. A 19 A—Titratable Acidity. 2006. Available online: https://www.gea.com/en/binaries/A%2019%20a%20-%20Titratable%20Acidity_tcm11-30930.pdf (accessed on 24 June 2021).
- Amrane, A.; Prigent, Y. Effect of the main culture parameters on the growth and production coupling lactic acid bacteri. Appl. Microbiol. 1999, 2, 101–108. [Google Scholar]
- National Research Council (US) Panel on the Applications of Biotechnology to Traditional Fermented Foods. Applications of Biotechnology to Fermented Foods: Report of an Ad Hoc Panel of the Board on Science and Pearce, 1973; National Academies Press: Washington, DC, USA, 1992. [Google Scholar]
- Pearce, L.E. A survey of bulk starter preparation and handling in New Zealand cheese factories. N. Z. Dairy Sci. Technol. 1973, 8, 17. [Google Scholar]
- Lawrance, R.C.; Thomas, T.D.; Terzaghi, B.E. Reviews of the progress of dairy science: Cheese starters. J. Dairy Res. 1976, 43, 141. [Google Scholar] [CrossRef]
- Lawrance, R.C.; Thomas, T.D. The fermentation of milk by lactic acid bacteria. Proc. Microbial Technology. Symp. Soc. Gen. Microbiol. Cambridge Engl. 1979, 187. [Google Scholar]
- Hutkins, R.W.; Nannen, N.L. pH homeostasis in lactic acid bacteria. J. Dairy Sci. 1993, 76, 2354–2365. [Google Scholar] [CrossRef]
- Le Bras, G.; Deville-Bonne, D.; Garel, J.R. Purification and properties of the phosphofructokinase from Lactobacillus bulgaricus. Eur. J. Biochem. 1991, 198, 683. [Google Scholar] [CrossRef]
- El Soda, M.; Desmazeaud, M.J.; Bergere, J.L. Peptide hydrolases of Lactobacillus casei: Isolation and general properties of various peptidase activities. J. Dairy Res. 1978, 45, 445. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Murakami, N.; Unemoto, T. Regulation of the cytoplasmic pH in Streptococcus faecalis. J. Biol. Chem. 1982, 257, 13246. [Google Scholar] [CrossRef]
- Konings, W.N.; Poolman, B.; Driessen, A.J.M. Bioenergetics and solute transport in lactococci. CRC Crit. Rev. Microbiol. 1989, 16, 419. [Google Scholar] [CrossRef]
- Gatje, G.; Muller, V.; Gottschalk, G. Lactic acid excretion via carrier-mediated facilitated diffusion in Lactobacillus helveticus. Appl. Microbiol. Biotechnol. 1991, 34, 778. [Google Scholar] [CrossRef]
- Mitchell, P. Performance and conservation of osmotic work by proton-coupled solute porter systems. J. Bioenerg. 1973, 4, 63. [Google Scholar] [CrossRef]
- Harold, F.M.; Pavlasova, E.; Baarda, J.R. A transmembrane pH gradient in Streptococcus faecalis: Origin and dissipation by proton conductors and N’N’-dicyclohexylcarbodiimide. Biochem. Biophys. Acta 1970, 196, 235. [Google Scholar] [CrossRef]
- Morita, R.Y. Psychrophilic bacteria. Bacteriol. Rev. 1975, 39, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Feller, G. Psychrophilic enzymes: From folding to function and biotechnology. Scientifica 2013, 2013, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef]
- Casanueva, A.; Tuffin, M.; Cary, C.; Cowan, D.A. Molecular adaptations to psychrophily: The impact of “omic” technologies. Trends Microbiol. 2010, 18, 374–381. [Google Scholar] [CrossRef]
- Kato, Y.; Sakala, R.M.; Hayashidani, H.; Kiuchi, A.; Kaneuchi, C.; Ogawa, M. Lactobacillus algidus sp. nov., a psychrophilic lactic acid bacterium isolated from vacuum-packaged refrigerated beef. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 3, 1143–1149. [Google Scholar] [CrossRef]



| Product Name | Manufacturer | Probiotic Strain (s) | Non-Dairy Substrate |
|---|---|---|---|
| Biomel | Biomel, UK | B. bifidum, B. coagulans, and L. plantarum | Coconut milk and grape extract |
| Califia Farms | Califia Farms, California | Bifidobacterium BB-2, S. thermophilus, and L. bulgaricus | Almond milk, coconut cream, and oat fiber |
| GT’s Organic Kombucha | GT, Los Angeles | Lactobacillus bacterium (species not specified) and Bacillus coagulans GBI-30 6086 | Black and green tea (to make Kombucha) and kiwi juice |
| KeVita Apple Cider Vinegar Tonics | KeVita, California | Water kefir (starter culture) and Bacillus coagulans GBI-30 6086 | Apple juice (to make apple cider), apple juice, and lemon extract. |
| Plantiful | Lifeway Foods Inc., Illinois, U.S. | L. casei, L. plantarum, B. bifidum, B. animalis subsp. lactis, B. longum subsp. longum, L. acidophilus, L. paracasei, L. rhamnosus, L. lactis subsp. lactis, and S. thermophilus. | Non-GMO pea protein |
| GoodBelly JuiceDrink | NextFoods, Boulder, Colorado | Lactobacillus plantarum LP299V | Mango: pear juice, mango puree, banana puree, oat flour, barley malt Cranberry watermelon: grape juice, pear juice, cranberry juice, strawberry juice, oat flour, watermelon juice, barley malt, vegetable juice |
| Harmless Harvest Dairy-Free Yogurt | Harmless Harvest, Thailand | L. acidophilus, B. lactis, S. thermophilus, L. casei, L. bulgaricus, B. bifidum, L. rhamnosus, and Bifidobacterium lactis HN019 | Young Thai coconut milk and water |
| Tropicana Essentials Probiotics® Pineapple Mango | PepsiCo, U.S. | Bifidobacterium lactis | Mango puree, pineapple, banana puree, and vegetable juice |
| VitaCup Immunity Coffee Pods | VitaCup, San Diego | Bacillus coagulans | Coffee, Inulin |
| Gut Shot® | Farmhouse Culture | Naturally occurring bacteria in the cabbage (Not specified) | Sauerkraut brine (fermented cabbage) and apple |
| Dee-V Drinks | Dates Valley, Malaysia | Not specified | Khal dates cider with four optional flavors (honey, berry, ginger, lemon) |
| Gut Kulture | Steve’s PaleoGoods, New Jersey | Naturally occurring probiotics culture (Not specified) | Beet, carrot, sarsaparilla, turmeric, ginger, burdock root, kudzu root, astragalus root, shatavari root, dandelion root, white ginseng, ashwaganda, rhodiola root |
| Probiotic Beverages | Advantages | Disadvantages | |||
|---|---|---|---|---|---|
| Healthier Alternative | Wider Taste Selection | Stability/Shelf Life | Nutrition | Production Process | |
| Cereal-based | Contains carbohydrates, proteins, dietary fibre, minerals, and vitamins with lesser fat content and cholesterol | Can be made from different source of cereal such as rice, millet, oat, and barley. | Stability in refrigeration period depends on strain of probiotic and type of cereal. | Exposed to starch gelatinization and increased viscosity | Complex: Involving various step in preparation of cereal milk (soaking, draining, wet milling, heat treatment, colling) followed by fermentation with probiotic strain and products formulation. |
| Fruit-based | Different types of fruit contain handful amount of carbohydrates, proteins, dietary fibres, minerals, and vitamins. Most fruit contain bioactive compound with antimicrobial, antioxidant, and anticancer properties | Can be made from different type of fruit ranging from sweet to citrusy fruit. Taste and aroma of each fruit differs depending on type and maturation stage. | Stability in refrigeration period depends on strain of probiotic and type of fruit. | Exposed to oxidation of the antioxidant (ascorbic acid) | Depends on type and parts of fruit: Fruit such as coconut with hard shell need to be de-husked and cracked open to obtain coconut water. The flesh needs to be grated and pressed to obtain coconut milk. Easier fruit such as grapes are only pressed to release the juice and introduced into heat treatment before inoculation and fermentation by probiotic strain. |
| Non-Dairy Substrate | Probiotic | Change in Cell Density/Viable Cell Count | Reference |
|---|---|---|---|
| Coconut water | Bacillus coagulans MTCC 5856 spore | After 2 days of fermentation the cell density of Bacillus coagulans at 540 nm increased from 0.121 to 0.683, corresponding to viable cell count of 109 CFU/mL (cell density higher than 0.600). | [30] |
| Lactobacillus acidophilus L10 and Lactobacillus casei L26 | Both strains showed increase in viable cell count during the 2 days’ fermentation where L. acidophilus showed higher growth at 3.58 × 108 CFU/mL compared to L. casei at 1.41 × 108 CFU/mL on day 2. | [157] | |
| Coconut water + inulin | Lactobacillus plantarum BG 112 | The viable cell count of 9 log CFU/mL of the starting inoculum dropped to a range of 6.00 to 8.70 log CFU/mL depending on the temperature after 16 h of fermentation. Fermentation at temperature 32 °C supplied with 0.5% (w/v) inulin retained the highest viable cells at 8.85 log CFU/mL. | [158] |
| Industrialized and fresh coconut water | Lactobacillus casei shirota | After 48 h of fermentation at 36 °C, Lactobacillus casei shirota experienced an increase in viable cell count from 4.15 × 107 CFU/mL to 7.56 × 108 CFU/mL in industrialized coconut water but in fresh coconut water, the cell count increased from 5.4 × 107 CFU/mL (0 h) to 2.5 × 109 CFU/mL (6 h) but no observable cell colony from 18 h to 48 h incubation period. | [159] |
| Coconut milk | Lactobacillus acidophilus | The viability of L. acidophilus at initial viable cell count log 4.32 CFU/mL increased after 4 h of fermentation in coconut milk at 37 °C and reached maximum viable cell count of log 9.89 CFU/mL at 20 h and remain constant until 24 h. | [18] |
| Breadfruit supernatant | L. acidophilus, L. casei, and L. plantarum DPC 206 | L. acidophilus, L. casei, and L. plantarum DPC 206 showed increased in viable cell count from 5.275 to 8.029 log CFU/mL, 6.055 to 7.952 log CFU/mL, and 5.555 to 7.764 log CFU/mL, respectively, after 72 h of fermentation in breadfruit supernatant at 37 °C. | [69] |
| Mango juice and sapota juice | Lactobacillus plantarum NCDC LP 20 | Increased viable cell count from 105 CFU/mL to 8.1 × 108 CFU/mL in mango juice and to 8.0 × 108 CFU/mL in sapota juice after 72 h incubation at 30 °C. | [68] |
| Cabbage juice | L. casei A4, L. debrueckii D7 and L. plantarum C3 | L. casei A4, L. debrueckii D7, and L. plantarum C3 showed increase in viable cell count from 3.0 × 106 to 11 × 108 CFU/mL, 4.3 × 105 to 11 × 108 CFU/mL, and 8.0 × 105 to 7.05 × 108 CFU/mL, respectively, after 48 h incubation at 30 °C. | [19] |
| Watermelon and tomato juice | L. fermentum and L. casei | Each probiotic shows excellent growth at 37 °C in watermelon and tomato juice combination where L. fermentum and L. casei showed increase in viable cell count from 4.6 × 107 to 2.3 × 108 CFU/mL and 2.7 × 107 to 9.4 × 108 CFU/mL, respectively, after 72 h of fermentation. | [160] |
| Pomegranate juice + grape juice + tomato juice + pomegranate peel extract | L. plantarum and L. delbrueckii | When the pomegranate juice is combined with 10% (v/v) grape juice, 5% (v/v) tomato juice, 0.1% (v/v) pomegranate peel extract and added with 2.0 g/L glucose, both strains achieved the highest survival rate with cell count 4.74 × 106 CFU/mL and 4 × 106 CFU/mL of L. plantarum and L. delbrueckii, respectively. | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojikon, F.D.; Kasimin, M.E.; Molujin, A.M.; Gansau, J.A.; Jawan, R. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients 2022, 14, 3457. https://doi.org/10.3390/nu14173457
Mojikon FD, Kasimin ME, Molujin AM, Gansau JA, Jawan R. Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients. 2022; 14(17):3457. https://doi.org/10.3390/nu14173457
Chicago/Turabian StyleMojikon, Floyd Darren, Melisa Elsie Kasimin, Arnold Marshall Molujin, Jualang Azlan Gansau, and Roslina Jawan. 2022. "Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products" Nutrients 14, no. 17: 3457. https://doi.org/10.3390/nu14173457
APA StyleMojikon, F. D., Kasimin, M. E., Molujin, A. M., Gansau, J. A., & Jawan, R. (2022). Probiotication of Nutritious Fruit and Vegetable Juices: An Alternative to Dairy-Based Probiotic Functional Products. Nutrients, 14(17), 3457. https://doi.org/10.3390/nu14173457







