Co-Microencapsulation of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Lactic Acid Bacteria into Antioxidant and Anti-Proliferative Derivative Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Fresh Fruits Processing
2.3. Phytochemical Extractions
2.4. Co-Microencapsulation of the Anthocyanins and Lactic Acid Bacteria
2.5. Phytochemical Profile of the Extract and Freeze-Dried Powders
2.6. Viability of L. casei 431®
2.7. Co-Microencapsulation Efficiency
2.8. Structure and Morphology of the Powders
2.9. In Vitro Simulated Digestion of Anthocyanins
2.10. Colorimetric Analysis
2.11. Anti-Proliferative Activity and Cytocompatibility of the Powders
2.11.1. Cell Culture and Treatment
2.11.2. Cells’ Viability
2.11.3. Cell Morphology
2.12. Storage Stability
2.13. Kinetics of Anthocyanins and Antioxidant Activity Degradation during Storage
2.14. Statistical Analysis
3. Results
3.1. Phytochemical Characterization of the Extract
3.2. Co-Microencapsulation Efficiency and Phytochemical Profile of the Powders
3.3. Structural and Morphological Analysis of the Powders
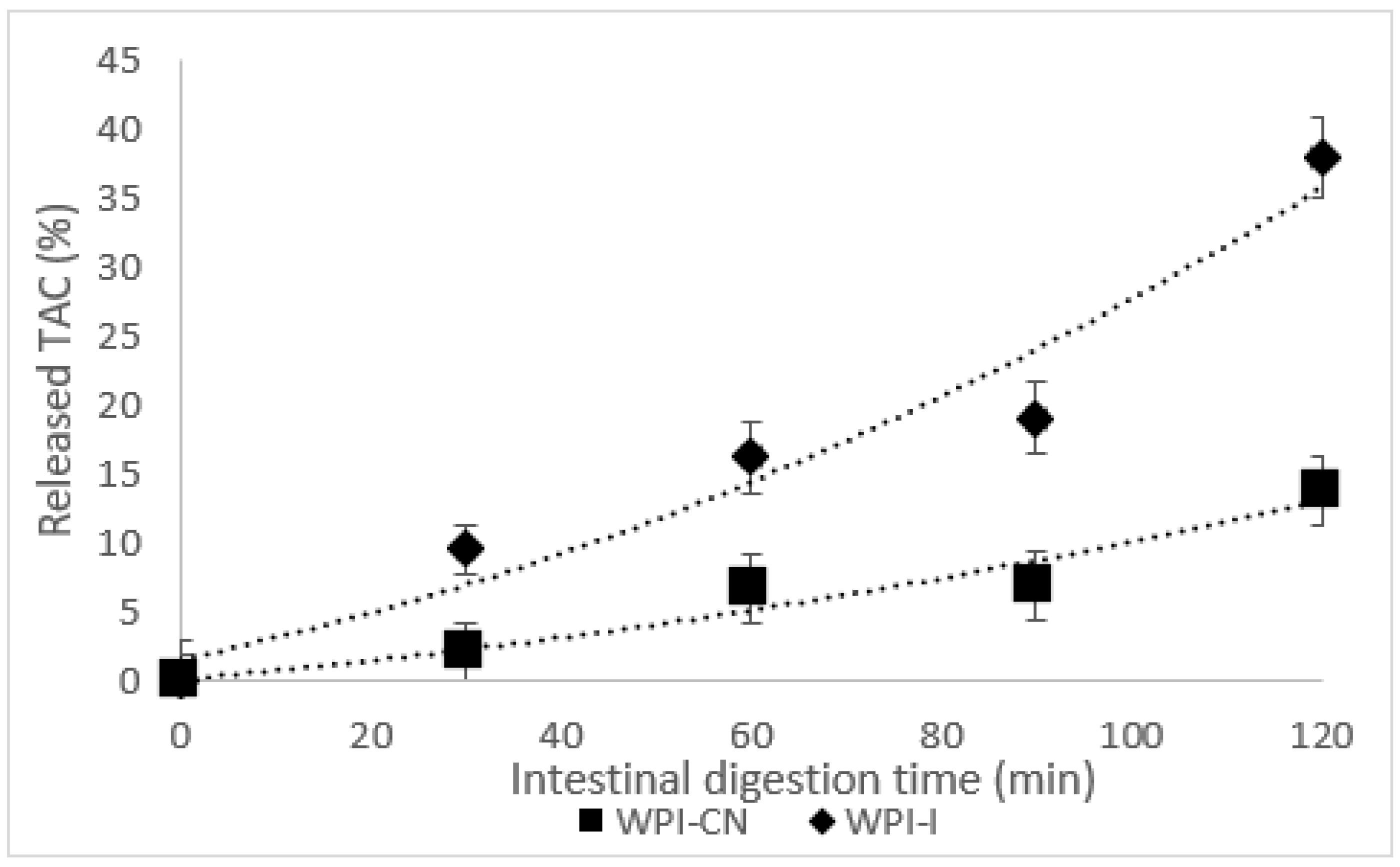
3.4. In Vitro Digestion of Anthocyanins
3.5. Colorimetric Analysis
3.6. Viability of Lactic Acid Bacteria
3.7. Storage Stability of the Bioactive Compounds in the Co-Microencapsulated Powders
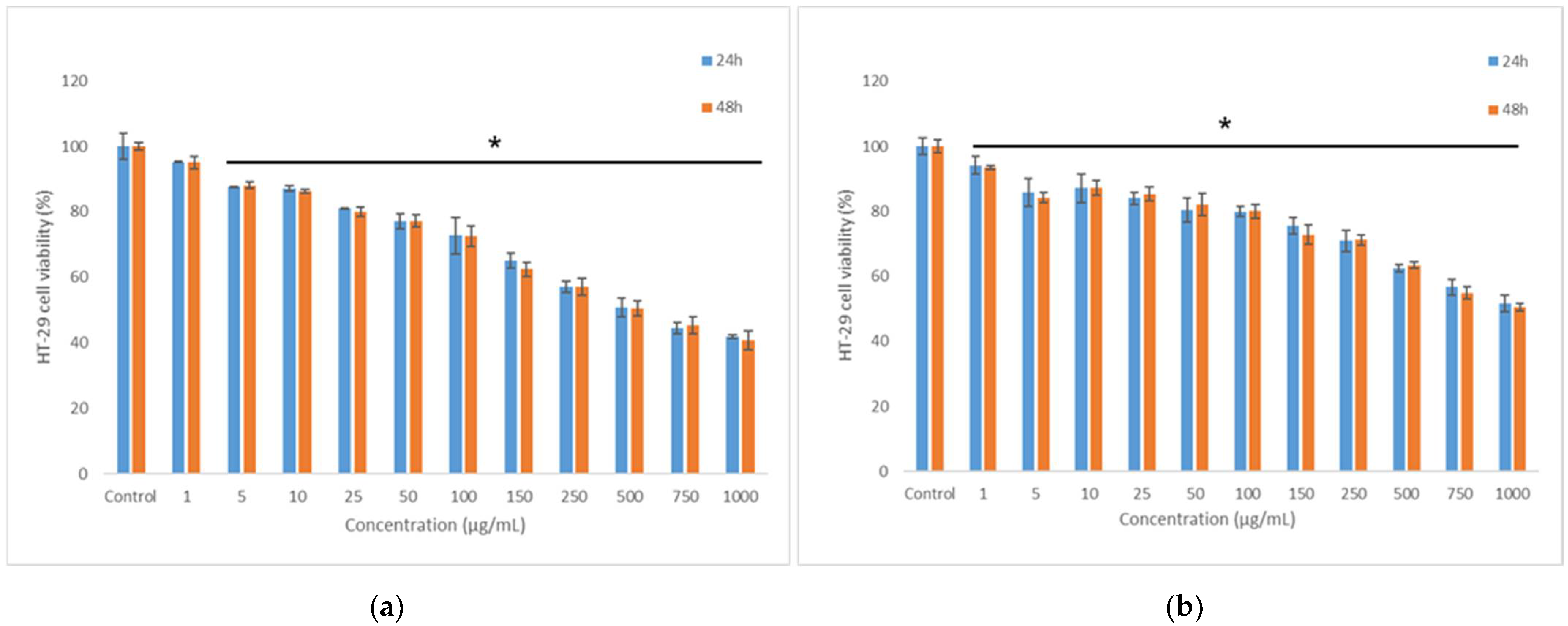
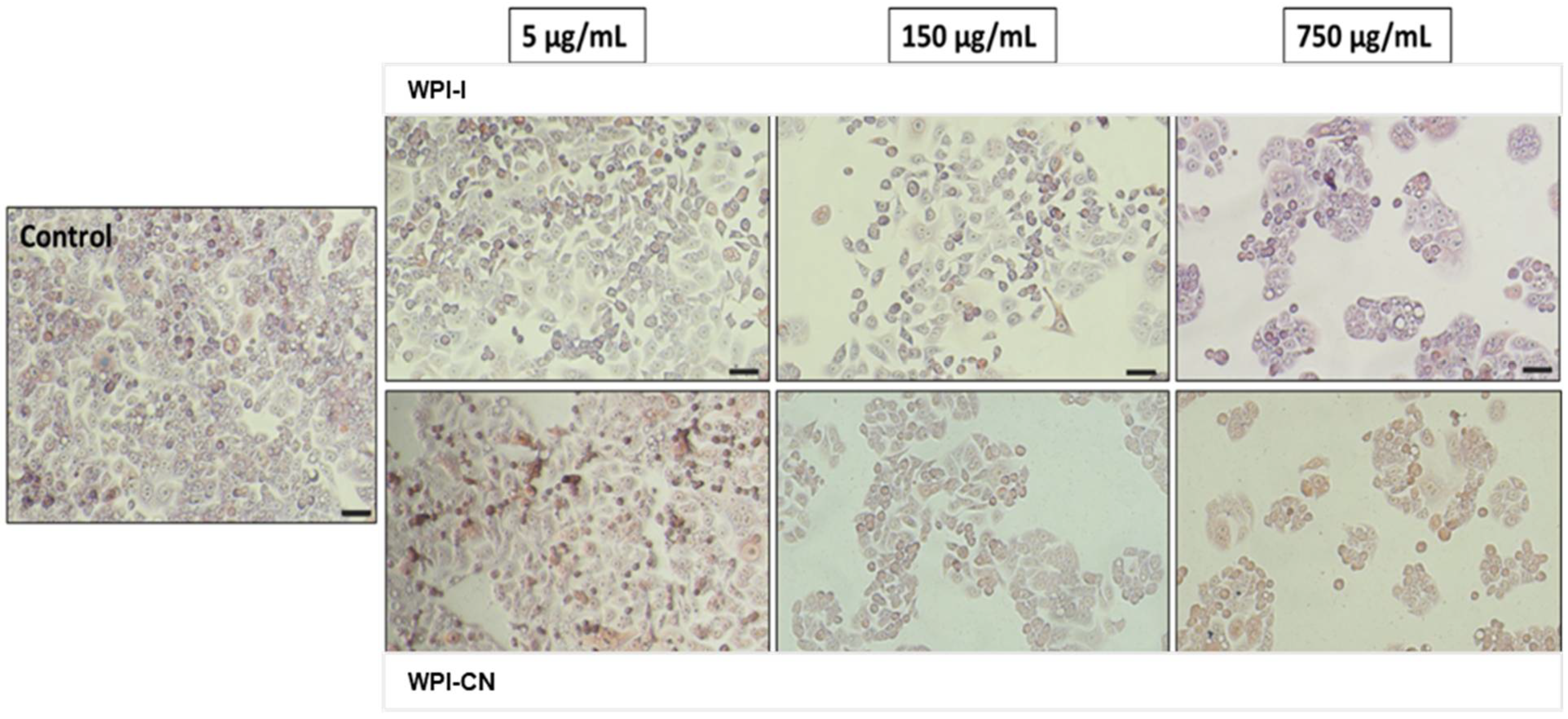
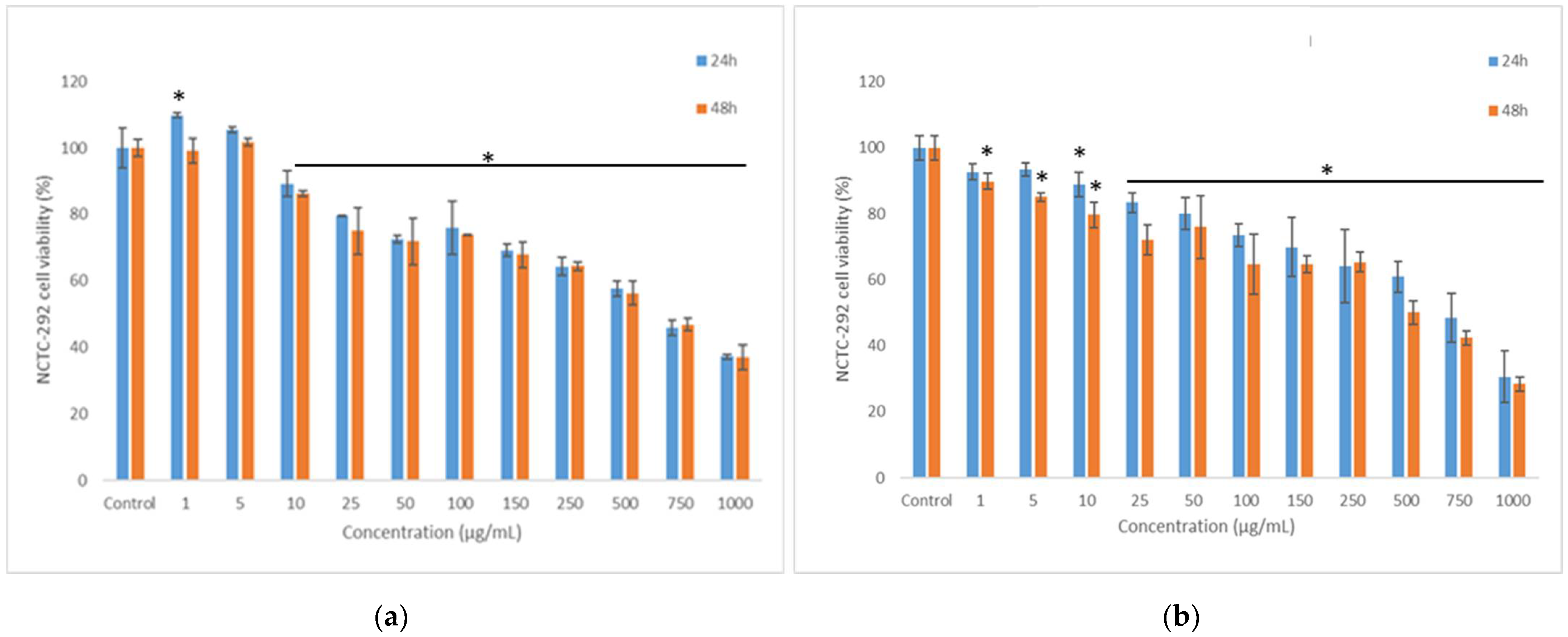
3.8. In Vitro Anti-Proliferative Activity of the Co-Microencapsulated Powders
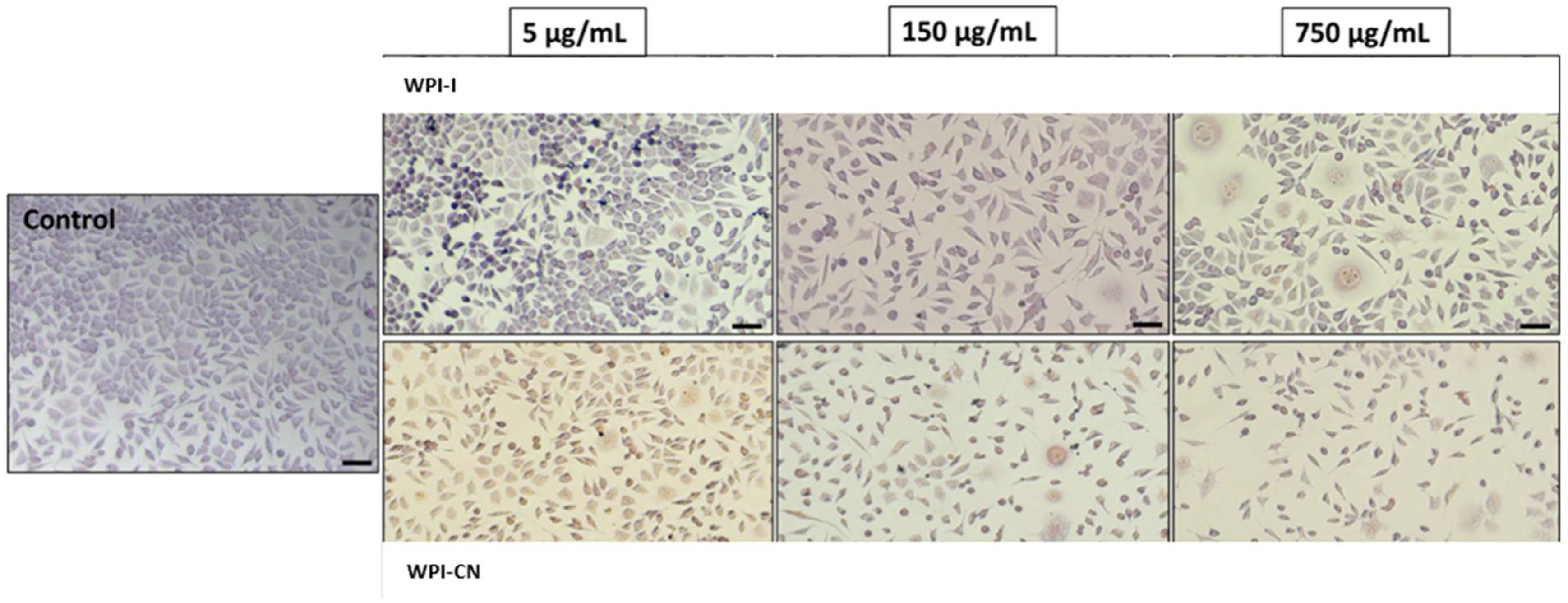
3.9. In Vitro Cytocompatibility of the Co-Microencapsulated Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.S.; Ali, M.N.; Hamid, R.; Ganie, S.A. Genotoxic effect of two commonly used food dyes metanil yellow and carmoisine using Allium cepa L. as indicator. Toxicol. Rep. 2020, 7, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinha, A.F.; Rodrigues, F.; Nunes, M.A.; Oliveira, M.B.P.P. Natural pigments and colorants in foods and beverages. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 363–391. [Google Scholar]
- Buchweitz, M. Natural solutions for blue colors in food. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Reinhold, C., Schweiggert, R.M., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 355–384. [Google Scholar]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, 43, 12974. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Micro- and nanoencapsulation of natural colors: A holistic view. Appl. Biochem. Biotechnol. 2021, 193, 3787–3811. [Google Scholar] [CrossRef]
- Neuenfeldt, N.H.; Almeida Farias, C.A.; de Oliveira Mello, R.; Sasso Robalo, S.; Smanioto Barin, J.; Picolli da Silva, L.; Müller, E.I.; Moraes Flores, E.M.; Teixeira Barcia, M.; Ragagnin de Menezes, C. Effects of blueberry extract co-microencapsulation on the survival of Lactobacillus rhamnosus. LWT 2022, 155, 112886. [Google Scholar] [CrossRef]
- Quintana, G.; Simoes, M.G.; Hugo, A.; Alves, P.; Ferreira, P.; Gerbino, E.; Simoes, P.N.; Gomez-Zavaglia, A. Layer-by-layer encapsulation of Lactobacillus delbrueckii subsp. bulgaricus using block-copolymers of poly (acrylic acid) and pluronic for safe release in gastro-intestinal conditions. J. Funct. Foods 2017, 35, 408–417. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Pan, P.; Lam, V.; Salzman, N.; Huang, Y.; Yu, J.; Zhang, J.; Wang, L. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr. Cancer 2017, 69, 943–951. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of dark sweet cherry powder consumption on the gut microbiota, short-chain fatty acids, and biomarkers of gut health in obese db/db mice. PeerJ 2018, 6, e4195. [Google Scholar] [CrossRef] [Green Version]
- Bayram, H.M.; Ozturkcan, S.A. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Szczepaniak, O.M.; Ligaj, M.; Kobus-Cisowska, J.; Maciejewska, P.; Tichoniuk, M.; Szulc, P. Application for novel electrochemical screening of antioxidant potential and phytochemicals in Cornus mas extracts. CyTA J. Food 2019, 17, 781–789. [Google Scholar] [CrossRef] [Green Version]
- Kazimierski, M.; Regula, J.; Molska, M. Cornelian cherry (Cornus mas L.)—Characteristics, nutritional and pro-health properties. Acta Sci. Pol. Technol. Aliment. 2019, 18, 5–12. [Google Scholar] [PubMed]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2019, 193, 670–690. [Google Scholar] [CrossRef]
- Hill, D.; Sugrue, I.; Tobin, C.; Hill, C.; Stanton, C.; Ross, R.P. The Lactobacillus casei group: History and health related applications. Front. Microbiol. 2018, 9, 2107. [Google Scholar] [CrossRef] [Green Version]
- Sidira, M.; Galanis, A.; Ypsilantis, P.; Karapetsas, A.; Progaki, Z.; Simopoulos, C.; Kourkoutas, Y. Effect of probiotic-fermented milk administration on gastrointestinal survival of Lactobacillus casei ATCC 393 and modulation of intestinal microbial flora. J. Mol. Microbiol. Biotechnol. 2010, 19, 224–230. [Google Scholar]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei exerts anti-proliferative effects accompanied by apoptotic cell death and up-regulation of TRAIL in colon carcinoma cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Romeih, E.; Gamba, R.R.; Nagai, E.; Suzuki, T.; Koyanagi, T.; Enomoto, T. The biological activity of fermented milk produced by Lactobacillus casei ATCC 393 during cold storage. Int. Dairy J. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Trachootham, D.; Chupeerach, C.; Tuntipopipat, S.; Pathomyok, L.; Boonnak, K.; Praengam, K.; Promkam, C.; Santivarangkn, C. Drinking fermented milk containing Lactobacillus paracasei 431 (IMULUSTM) improves immune response against H1N1 and cross-reactive H3N2 viruses after influenza vaccination: A pilot randomized triple-blinded placebo controlled trial. J. Funct. Foods 2017, 33, 1–10. [Google Scholar] [CrossRef]
- Matalanis, A.; Jones, O.G.; MacClements, D.J. Structured biopolymer based delivery systems for encapsulation, protection, and release of lipophilic compounds. Food Hydrocol. 2011, 25, 1865–1880. [Google Scholar] [CrossRef] [Green Version]
- McIntosh, G.H.; Royle, P.J.; Le Leu, R.K.; Regester, G.O.; Johnson, M.A.; Grinstead, R.L.; Kenward, R.S.; Smithers, G.W. Whey proteins as functional food ingredients? Int. Dairy J. 1996, 8, 425–434. [Google Scholar] [CrossRef]
- Tarifa, M.C.; Piqueras, C.M.; Genovese, D.B.; Brugnoni, L.I. Microencapsulation of Lactobacillus casei and Lactobacillus rhamnosus in pectin and pectin-inulin microgel particles: Effect on bacterial survival under storage conditions. Int. J. Biol. Macromol. 2021, 179, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Enache, I.M.; Vasile, A.M.; Enachi, E.; Barbu, V.; Stănciuc, N.; Vizireanu, C. Co-microencapsulation of anthocyanins from black currant extract and lactic acid bacteria in biopolymeric matrices. Molecules 2020, 25, 1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 8261 IDF122:2001; Milk and Milk Products—General Guidance for the Preparation of Test Samples, Initial Suspensions and Decimal Dilutions for Microbiological Examination. ISO: Geneva, Switzerland, 2001.
- AOAC Official Method 2005.02. Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines—pH Differential Method. In Official Methods of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005; Method 935.14 and 992.24. [Google Scholar]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Voicescu, M.; Ionescu, S.; Manoiu, V.S.; Anastasescu, M.; Craciunescu, O.; Moldovan, L. Synthesis and biophysical characteristics of riboflavin/HSA protein system on silver nanoparticles. Mat. Sci. Eng. C 2019, 96, 30–40. [Google Scholar] [CrossRef]
- Azarpazhooh, E.; Sharayei, P.; Zomorodi; Ramaswamy, H.S. Physicochemical and phytochemical characterization and storage stability of sreeze-dried encapsulated pomegranate peel anthocyanin and in vitro evaluation of its antioxidant activity. Food Bioproc. Technol. 2019, 12, 199–210. [Google Scholar] [CrossRef]
- Gąstoł, M.; Krośniak, M.; Derwisz, M.; Dobrowolska-Iwanek, J. Cornelian cherry (Cornus mas L.) juice as a potential source of biological compounds. J. Med. Food 2013, 16, 728–732. [Google Scholar] [CrossRef]
- Okan, O.T.; Serencam, H.; Baltas, N.; Can, Z. Some edible forest fruits their in vitro antioxidant activities, phenolic compounds and some enzyme inhibition effects. Fresenius Environ. Bull. 2019, 28, 6090–6098. [Google Scholar]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 16, 6459–6472. [Google Scholar] [CrossRef] [Green Version]
- Kutlu, N.; Isci, A.; Sakiyan, O.; Yilmaz, A.M. Extraction of phenolic compounds from cornelian cherry (Cornus mas L.) using microwave and ohmic heating assisted microwave methods. Food Bioproc. Technol. 2020, 14, 650–664. [Google Scholar] [CrossRef]
- Oancea, A.M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional evaluation of microencapsulated anthocyanins from sour cherries skins extract in whey proteins isolate. LWT 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining various wall materials for encapsulation of blueberry anthocyanin extracts: Optimization by artificial neural network and genetic algorithm and a comprehensive analysis of anthocyanin powder properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Peñas, E.; Hernandez-Ledesma, B.; Frias, J.; Martínez-Villaluenga, C. A comparative study on the phenolic bioaccessibility, antioxidant and inhibitory effects on carbohydrate-digesting enzymes of maca and mashua powders. LWT 2020, 131, 109798. [Google Scholar] [CrossRef]
- Kaderides, K.; Mourtzinos, I.; Goula, A.M. Stability of pomegranate peel polyphenols encapsulated in orange juice industry by-product and their incorporation in cookies. Food Chem. 2020, 310, 125849. [Google Scholar] [CrossRef]
- Moser, P.; Telis, V.R.N.; de Andrade Neves, N.; García-Romero, E.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Storage stability of phenolic compounds in powdered BRS Violeta grape juice microencapsulated with protein and maltodextrin blends. Food Chem. 2017, 214, 308–318. [Google Scholar] [CrossRef]
- Champagne, C.P.; Raymond, Y.; Guertin, N.; Belanger, G. Effects of storage conditions, microencapsulation and inclusion in chocolate particles on the stability of probiotic bacteria in ice cream. Int. Dairy J. 2015, 47, 109–117. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Fitsiou, E.; Spyridopoulou, K.; Vasileiadis, S.; Iliopoulos, C.; Galanis, A.; Vekiari, S.; Pappa, A.; Chlichlia, K. Evaluation of antioxidant and antiproliferative properties of Cornus mas L. fruit juice. Antioxidants 2019, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Blagojević, B.; Agić, D.; Serra, A.M.; Matić, S.; Matovina, M.; Bijelić, S.; Popović, B.M. An in vitro and in silico evaluation of bioactive potential of cornelian cherry (Cornus mas L.) extracts rich in polyphenols and iridoids. Food Chem. 2021, 335, 127619. [Google Scholar] [CrossRef]
- Popović, B.M.; Blagojević, B.; Latković, D.; Cetojević-Simin, D.; Kucharska, A.Z.; Parisi, F.; Lazzara, G. A one-step enhanced extraction and encapsulation system of cornelian cherry (Cornus mas L.) polyphenols and iridoids with β-cyclodextrin. LWT 2021, 141, 110884. [Google Scholar] [CrossRef]


| Analyzed Parameter | WPI-CN | WPI-I |
|---|---|---|
| Anthocyanins’ co-microencapsulation efficiency (%) | 77.97 ± 0.57 | 79.03 ± 0.72 |
| Lactobacillus casei 431® co-microencapsulation efficiency (%) | 90.03 ± 0.56 a | 90.07 ± 0.34 a |
| Total monomeric anthocyanins content (mg C3R/g DM) | 32.14 ± 0.97 a | 32.29 ± 0.26 a |
| Total polyphenolic content (mg GAE/g DM) | 9.67 ± 0.12 a | 9.79 ± 0.15 a |
| Total flavonoids content (mg CE/g DM) | 5.59 ± 0.51 a | 5.94 ± 0.24 a |
| Antioxidant activity (mMol Trolox/g DM) | 50.05 ± 0.94 a | 47.62 ± 1.43 b |
| Lactobacillus casei 431® (CFU/g DM) | 8.02 × 109 a | 8.10 × 109 a |
| Variants | L* | a* | b* | c* | h* | ΔE |
|---|---|---|---|---|---|---|
| WPI-CN | 37.17 ± 0.24 a | 21.45 ± 0.18 a | 6.67 ± 0.01 a | 22.47 ± 0.17 a | 0.30 ± 0.01 a | 43.43 ± 0.29 a |
| WPI-I | 35.35 ± 0.06 b | 21.30 ± 0.12 a | 6.00 ± 0.02 b | 22.13 ± 0.12 a | 0.27 ± 0.01 b | 41.71 ± 0.11 b |
| Variants | Storage (Days) | Antioxidant Activity (mMol/g DM) | TPC (mg GAE/g DM) | TFC (mg CE/g DM) | TAC (mg C3R/g DM) |
|---|---|---|---|---|---|
| WPI-CN | 0 | 50.05 ± 0.94 a | 9.67 ± 0.12 a | 5.59 ± 0.51 a | 32.14 ± 0.97 a |
| 21 | 49.73 ± 2.35 b | 9.65 ± 0.07 a | 5.65 ± 0.31 a | 14.62 ± 1.12 b | |
| 90 | 46.33 ± 1.01 a | 9.57 ± 0.08 a | 5.85 ± 0.65 a | 10.64 ± 0.66 b | |
| WPI-I | 0 | 47.62 ± 1.43 b | 9.79 ± 0.15 a | 5.94 ± 0.24 a | 32.29 ± 0.26 a |
| 21 | 46.03 ± 0.24 a | 9.75 ± 0.02 a | 5.81 ± 0.41 a | 17.26 ± 0.81 a | |
| 90 | 45.87 ± 1.11 a | 9.54 ± 0.02 a | 5.67 ± 0.33 a | 13.35 ± 0.37 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enache, I.M.; Vasile, M.A.; Crăciunescu, O.; Prelipcean, A.M.; Oancea, A.; Enachi, E.; Barbu, V.V.; Stănciuc, N.; Vizireanu, C. Co-Microencapsulation of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Lactic Acid Bacteria into Antioxidant and Anti-Proliferative Derivative Powders. Nutrients 2022, 14, 3458. https://doi.org/10.3390/nu14173458
Enache IM, Vasile MA, Crăciunescu O, Prelipcean AM, Oancea A, Enachi E, Barbu VV, Stănciuc N, Vizireanu C. Co-Microencapsulation of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Lactic Acid Bacteria into Antioxidant and Anti-Proliferative Derivative Powders. Nutrients. 2022; 14(17):3458. https://doi.org/10.3390/nu14173458
Chicago/Turabian StyleEnache, Iuliana Maria, Mihaela Aida Vasile, Oana Crăciunescu, Ana Maria Prelipcean, Anca Oancea, Elena Enachi, Viorica Vasilica Barbu, Nicoleta Stănciuc, and Camelia Vizireanu. 2022. "Co-Microencapsulation of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Lactic Acid Bacteria into Antioxidant and Anti-Proliferative Derivative Powders" Nutrients 14, no. 17: 3458. https://doi.org/10.3390/nu14173458
APA StyleEnache, I. M., Vasile, M. A., Crăciunescu, O., Prelipcean, A. M., Oancea, A., Enachi, E., Barbu, V. V., Stănciuc, N., & Vizireanu, C. (2022). Co-Microencapsulation of Anthocyanins from Cornelian Cherry (Cornus mas L.) Fruits and Lactic Acid Bacteria into Antioxidant and Anti-Proliferative Derivative Powders. Nutrients, 14(17), 3458. https://doi.org/10.3390/nu14173458








