Dietary Xenobiotics Derived from Food Processing: Association with Fecal Mutagenicity and Gut Mucosal Damage
Abstract
:1. Introduction
2. Materials and Methods
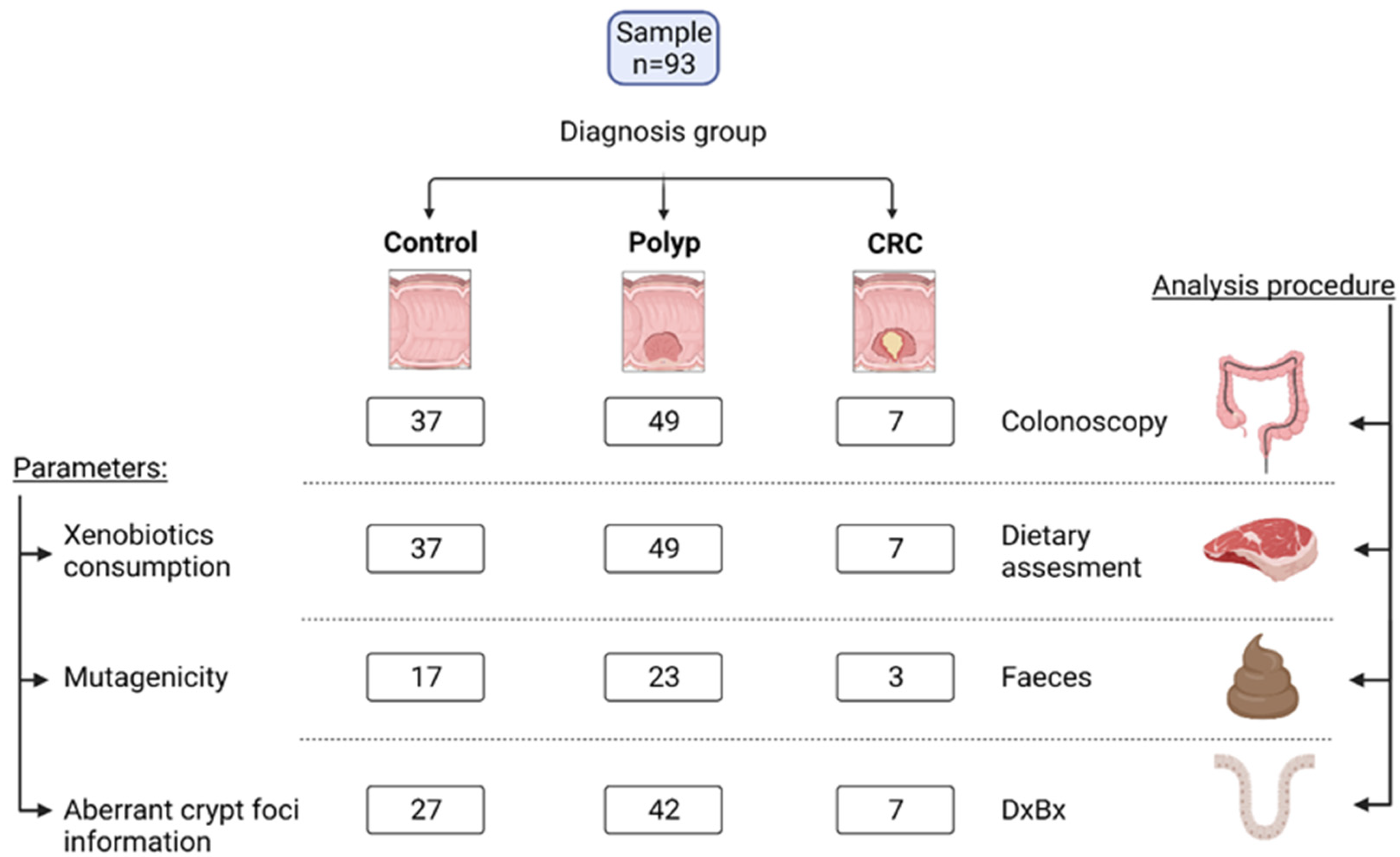
2.1. Study Design and Volunteers
2.2. Nutritional Assessment
2.3. Xenobiotics Derived from Food Processing
2.4. Anthropometrical Determinations
2.5. Pathological Assessment
2.6. Fecal Samples and Mutagenicity
2.7. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Red Española de Registros de Cáncer (REDECAN), 2021, Estimaciones de la Incidencia del Cáncer en España. Available online: https://www.redecan.org/es/proyectos/3/estimaciones-de-la-incidencia-del-cancer-en-espana-2021 (accessed on 18 August 2022).
- Bultman, S.J. Interplay between Diet, Gut Microbiota, Epigenetic Events, and Colorectal Cancer. Mol. Nutr. Food Res. 2017, 61, 1500902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Bardou, M.; Barkun, A.N.; Martel, M. Obesity and Colorectal Cancer. Gut 2013, 62, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Pischon, T.; Lahmann, P.H.; Boeing, H.; Friedenreich, C.; Norat, T.; Tjønneland, A.; Halkjaer, J.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Body Size and Risk of Colon and Rectal Cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). J. Natl. Cancer Inst. 2006, 98, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Pearson, J.R.; Gill, C.I.R.; Rowland, I.R. Diet, Fecal Water, and Colon Cancer—Development of a Biomarker. Nutr. Rev. 2009, 67, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Mai, V. Dietary Modification of the Intestinal Microbiota. Nutr. Rev. 2004, 62, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R. An Epidemiologic Approach to Studying Heterocyclic Amines. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2002, 506–507, 197–204. [Google Scholar] [CrossRef]
- World Health Organization; International Agency for Research on Cancer. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Geneva, Switzerland, 1993; Volume 56, pp. 211–242, ISBN 92 832 1256-0; Available online: https://publications.iarc.fr/74 (accessed on 20 May 2022).
- World Health Organization (WHO). Environmental Health Criteria 202: Selected Non-Heterocyclic Polycyclic Aromatic Hydrocarbons. 1998. ISBN 9241572027. Available online: https://apps.who.int/iris/handle/10665/41958 (accessed on 21 May 2022).
- Tricker, A.R.; Preussmann, R. Carcinogenic N-Nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential. Mutat. Res. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Ferrucci, L.M.; Sinha, R.; Huang, W.Y.; Berndt, S.I.; Katki, H.A.; Schoen, R.E.; Hayes, R.B.; Cross, A.J. Meat Consumption and the Risk of Incident Distal Colon and Rectal Adenoma. Br. J. Cancer 2012, 106, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Chiavarini, M.; Bertarelli, G.; Minelli, L.; Fabiani, R. Dietary Intake of Meat Cooking-Related Mutagens (HCAs) and Risk of Colorectal Adenoma and Cancer: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Chan, D.S.M.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary Fibre, Whole Grains, and Risk of Colorectal Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Br. Med. J. 2011, 343, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Núñez-Sánchez, M.A.; González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Selma, M.V.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Dietary Phenolics against Colorectal Cancer-From Promising Preclinical Results to Poor Translation into Clinical Trials: Pitfalls and Future Needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; et al. Dietary Fibre Intake and Risks of Cancers of the Colon and Rectum in the European Prospective Investigation into Cancer and Nutrition (EPIC). PLoS ONE 2012, 7, e39361. [Google Scholar] [CrossRef] [Green Version]
- DellaValle, C.T.; Xiao, Q.; Yang, G.; Ou Shu, X.; Aschebrook-Kilfoy, B.; Zheng, W.; Li, H.L.; Ji, B.-T.; Rothman, N.; Chow, W.-H.; et al. Dietary Nitrate and Nitrite Intake and Risk of Colorectal Cancer in the Shanghai Women’s Health Study. Int. J. Cancer 2014, 134, 2091–2926. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, P.P.; Zhao, J.; Green, R.; Sun, Z.; Roebothan, B.; Squires, J.; Buehler, S.; Dicks, E.; Zhao, J.; et al. Dietary N-Nitroso Compounds and Risk of Colorectal Cancer: A Case-Control Study in Newfoundland and Labrador and Ontario, Canada. Br. J. Nutr. 2014, 111, 1109–1117. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.C.; Ferguson, L.R.; Tannock, G.W. Dietary Fibres as “Prebiotics”: Implications for Colorectal Cancer. Mol. Nutr. Food Res. 2005, 49, 609–619. [Google Scholar] [CrossRef]
- O’Keefe, S.J.D.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, Fibre and Cancer Risk in African Americans and Rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, L.R.; Harris, P.J. The Dietary Fibre Debate: More Food for Thought. Lancet 2003, 361, 1487–1488. [Google Scholar] [CrossRef]
- Bultman, S.J. Molecular Pathways: Gene-Environment Interactions Regulating Dietary Fiber Induction of Proliferation and Apoptosis via Butyrate for Cancer Prevention. Clin. Cancer Res. 2014, 20, 799–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.N.; Almoyad, M.; Huq, F. Polyphenols in Colorectal Cancer: Current State of Knowledge Including Clinical Trials and Molecular Mechanism of Action. BioMed Res. Int. 2018, 2018, 4154185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapper, M.L.; Chang, W.-C.L.; Cooper, H.S. Dysplastic Aberrant Crypt Foci: Biomarkers of Early Colorectal Neoplasia and Response to Preventive Intervention. Cancer Prev. Res. 2020, 13, 229–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk, M.; Orłowski, M.; Klepacki, Ł.; Zinkiewicz, K.; Kurpiewski, W.; Kaczerska, D.; Pesta, W.; Zieliński, E.; Siermontowski, P. Rectal Aberrant Crypt Foci (ACF) as a Predictor of Benign and Malignant Neoplastic Lesions in the Large Intestine. BMC Cancer 2020, 20, 133. [Google Scholar] [CrossRef]
- Ocké, M.; de Boer, E.; Brants, H.; van der Laan, J.; Niekerk, M.; van Rossum, C.; Temme, L.; Freisling, H.; Nicolas, G.; Casagrande, C.; et al. PANCAKE—Pilot Study for the Assessment of Nutrient Intake and Food Consumption Among Kids in Europe. EFSA Supporting Publ. 2012, 9, 339E. [Google Scholar] [CrossRef]
- Zapico, A.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de los Reyes-Gavilán, C.G.; González, S. Pilot Study for the Dietary Assessment of Xenobiotics Derived from Food Processing in an Adult Spanish Sample. Foods 2022, 11, 470. [Google Scholar] [CrossRef]
- Centro de Enseñanza Superior de Nutrición Humana y Dietética (CESNID). Tablas de Composición de Alimentos por Medidas Caseras de Consumo Habitual en España; McGrawHill, Ed.; Publicaciones y Ediciones de la Universidad de Barcelona: Barcelona, Spain, 2008. [Google Scholar]
- United States Department of Agriculture (USDA). Food Composition Databases. Available online: https://fdc.nal.usda.gov/ (accessed on 29 June 2021).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Marlett, J.; Cheung, T. Database and Quick Methods of Assessing Typical Dietary Fiber Intakes Using Data for 228 Commonly Consumed Foods. J. Am. Diet. Assoc. 1997, 1151, 1139–1148. [Google Scholar] [CrossRef]
- Jakszyn, P.; Agudo, A.; Ibãñez, R.; García-Closas, R.; Pera, G.; Amiano, P.; González, C.A. Development of a Food Database of Nitrosamines, Heterocyclic Amines, and Polycyclic Aromatic Hydrocarbons. J. Nutr. 2004, 134, 2011–2014. [Google Scholar] [CrossRef]
- National Cancer Institute: Division of Cancer Epidemiology & Genetics. CHARRED: Computerized Heterocyclic Amines Resource for Research in Epidemiology of Disease. 2006. Available online: http://dceg.cancer.gov/neb/tools/charred (accessed on 21 May 2021).
- European Food Safety Authority (EFSA) Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the European Comission to Perform a Scientific Risk Assessment on Nitrate in Vegetables. EFSA J. 2008, 689, 1–79. [CrossRef]
- Food and Drug Administration (FDA). Survey Data on Acrylamide in Food. Available online: https://www.fda.gov/food/chemical-contaminants-food/survey-data-acrylamide-food-total-diet-study-results (accessed on 21 May 2021).
- Svensson, K.; Abramsson, L.; Becker, W.; Glynn, A.; Hellenäs, K.E.; Lind, Y.; Rosén, J. Dietary Intake of Acrylamide in Sweden. Food Chem. Toxicol. 2003, 41, 1581–1586. [Google Scholar] [CrossRef]
- Hellenäs, K.-E.; Fohgelberg, P.; Fäger, U.; Busk, L.; Zetterberg, A.; Ionescu, C.; Färnstrand, J.S. Acrylamide in Swedish Food—Targeted Sampling 2011 and 2012. 2013. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2013/2013_national_food_agency_25_acrylamide_in_swedish_food_targeted_sampling_2011_and_2012.pdf (accessed on 20 May 2022).
- Konings, E.J.M.; Baars, A.J.; van Klaveren, J.D.; Spanjer, M.C.; Rensen, P.M.; Hiemstra, M.; van Kooij, J.A.; Peters, P.W.J. Acrylamide Exposure from Foods of the Dutch Population and an Assessment of the Consequent Risks. Food Chem. Toxicol. 2003, 41, 1569–1579. [Google Scholar] [CrossRef]
- Falcó, G.; Domingo, J.L.; Llobet, J.M.; Teixidó, A.; Casas, C.; Müller, L. Polycyclic Aromatic Hydrocarbons in Foods: Human Exposure through the Diet in Catalonia, Spain. J. Food Prot. 2003, 66, 2325–2331. [Google Scholar] [CrossRef]
- Palacios Colón, L.; Rascón, A.J.; Ballesteros, E. Trace-Level Determination of Polycyclic Aromatic Hydrocarbons in Dairy Products Available in Spanish Supermarkets by Semi-Automated Solid-Phase Extraction and Gas Chromatography–Mass Spectrometry Detection. Foods 2022, 11, 713. [Google Scholar] [CrossRef]
- de Mey, E.; de Klerck, K.; de Maere, H.; Dewulf, L.; Derdelinckx, G.; Peeters, M.C.; Fraeye, I.; vander Heyden, Y.; Paelinck, H. The Occurrence of N-Nitrosamines, Residual Nitrite and Biogenic Amines in Commercial Dry Fermented Sausages and Evaluation of Their Occasional Relation. Meat Sci. 2014, 96, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Viñas, P.; Martínez-Castillo, N.; Hernández-Córdoba, M. Determination of Volatile Nitrosamines in Meat Products by Microwave-Assisted Extraction and Dispersive Liquid-Liquid Microextraction Coupled to Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2011, 1218, 1815–1821. [Google Scholar] [CrossRef]
- Lee, H.S. Literature Compilation of Volatile N-Nitrosamines in Processed Meat and Poultry Products—An Update. Food Addit. Contam.—Part A 2019, 36, 1491–1500. [Google Scholar] [CrossRef]
- Park, J.E.; Seo, J.E.; Lee, J.Y.; Kwon, H. Distribution of Seven N-Nitrosamines in Food. Toxicol. Res. 2015, 31, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Navarro, T.; Díaz, I.; Gutiérrez-Díaz, I.; Rodríguez-Carrio, J.; Suárez, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M.; Salazar, N.; González, S. Exploring the Interactions between Serum Free Fatty Acids and Fecal Microbiota in Obesity through a Machine Learning Algorithm. Food Res. Int. 2019, 121, 533–541. [Google Scholar] [CrossRef]
- Foz, M.; Barbany, M.; Remesar, X.; Carrillo, M.; Aranceta, J.; García-Luna, P.; Alemany, M.; Vázquez, C.; Palou, A.; Picó, C.; et al. Sociedad Española Para El Estudio de La Obesidad. Consenso SEEDO 2000 Para La Evaluación Del Sobrepeso y La Obesidad y El Establecimiento de Criterios de Intervención Terapéutica. Med. Clin. 2000, 115, 587–597. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenoglio-preiser, C.M.; Noffsinger, A. Aberrant Crypt Foci: A Review. Toxicol. Pathol. 1999, 27, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Pretlow, T.P.; Schoen, R.E. Aberrant Crypt Foci: What We Know and What We Need to Know. Clin. Gastroenterol. Hepatol. 2007, 5, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, C.D.; Losi, L.; Fante, R.; Modico, S.; Ghidoni, M.; Pedroni, M.; Tamassia, M.G.; Gafà, L.; Ponz de Leon, M.; Roncucci, L. Histology of Aberrant Crypt Foci in the Human Colon. Histopathology 1997, 30, 328–334. [Google Scholar] [CrossRef]
- Redruello, B.; Ladero, V.; Cuesta, I.; Álvarez-Buylla, J.R.; Martín, M.C.; Fernández, M.; Alvarez, M.A. A Fast, Reliable, Ultra High Performance Liquid Chromatography Method for the Simultaneous Determination of Amino Acids, Biogenic Amines and Ammonium Ions in Cheese, Using Diethyl Ethoxymethylenemalonate as a Derivatising Agent. Food Chem. 2013, 139, 1029–1035. [Google Scholar] [CrossRef]
- Salazar, N.; Ponce-Alonso, M.; Garriga, M.; Sánchez-Carrillo, S.; Hernández-Barranco, A.M.; Redruello, B.; Fernández, M.; Botella-Carretero, J.I.; Vega-Piñero, B.; Galeano, J.; et al. Fecal Metabolome and Bacterial Composition in Severe Obesity: Impact of Diet and Bariatric Surgery. Gut Microbes 2022, 14, 2106102. [Google Scholar] [CrossRef]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Aghaie Meybodi, M.; et al. Global, Regional, and National Burden of Colorectal Cancer and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Castelló, A.; Rodríguez-Barranco, M.; Fernández de Larrea, N.; Jakszyn, P.; Dorronsoro, A.; Amiano, P.; Chirlaque, M.D.; Colorado-Yohar, S.; Guevara, M.; Moreno-Iribas, C.; et al. Adherence to the Western, Prudent and Mediterranean Dietary Patterns and Colorectal Cancer Risk: Findings from the Spanish Cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). Nutrients 2022, 14, 3085. [Google Scholar] [CrossRef]
- Jonoush, M.; Fathi, S.; Hassanpour Ardekanizadeh, N.; Khalatbari Mohseni, G.; Majidi, N.; Keshavarz, S.A.; Shekari, S.; Nemat Gorgani, S.; Torki, S.A.; Sotoudeh, M.; et al. The Association Between Different Types of Dietary Carbohydrates and Colorectal Cancer: A Case-Control Study. Front. Nutr. 2022, 12, 898337. [Google Scholar] [CrossRef]
- GBD 2019 Colorectal Cancer Collaborators; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Willet, W.C. Diet and Cancer. Oncol. 2000, 5, 393–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manson, M.M.; Benford, D.J. Factors Influencing the Carcinogenicity of Food Chemicals. Toxicology 1999, 134, 93–108. [Google Scholar] [CrossRef]
- Rohrmann, S.; Hermann, S.; Linseisen, J. Heterocyclic Aromatic Amine Intake Increases Colorectal Adenoma Risk: Findings from a Prospective European Cohort Study. Am. J. Clin. Nutr. 2009, 89, 1418–1424. [Google Scholar] [CrossRef] [Green Version]
- Sander, A.; Linseisen, J.; Rohrmann, S. Intake of Heterocyclic Aromatic Amines and the Risk of Prostate Cancer in the EPIC-Heidelberg Cohort. Cancer Causes Control 2011, 22, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dybing, E.; Farmer, P.B.; Andersen, M.; Fennell, T.R.; Lalljie, S.P.D.; Müller, D.J.G.; Olin, S.; Petersen, B.J.; Schlatter, J.; Scholz, G.; et al. Human Exposure and Internal Dose Assessments of Acrylamide in Food. Food Chem. Toxicol. 2005, 43, 365–410. [Google Scholar] [CrossRef] [PubMed]
- Lodovici, M.; Dolara, P.; Casalini, C.; Ciappellano, S.; Testolin, G. Polycyclic Aromatic Hydrocarbon Contamination in the Italian Diet. Food Addit. Contam. 1995, 12, 703–713. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhou, Q.Y.; Zhu, J.Z.; Zhu, K.F.; Yu, C.H.; Li, Y.M. Systematic Review with Meta-Analysis: Alcohol Consumption and Risk of Colorectal Serrated Polyp. Dig. Dis. Sci. 2015, 60, 1889–1902. [Google Scholar] [CrossRef]
- Lee, K.; Kim, Y.H. Colorectal Polyp Prevalence According to Alcohol Consumption, Smoking and Obesity. Int. J. Environ. Res. Public Health 2020, 17, 2387. [Google Scholar] [CrossRef] [Green Version]
- World Cancer Research Fund (WCRF); American Institute for Cancer Research (AICR). Imperial College London WCRF/AICR Systematic Literature Review—Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: https://www.wcrf.org/wp-content/uploads/2021/02/Colorectal-cancer-report.pdf (accessed on 23 May 2022).
- Organisation for Economic Co-Operation Development (OECD). Meat Consumption (Indicator). Available online: https://data.oecd.org/agroutput/meat-consumption.htm (accessed on 23 May 2022).
- Gaesser, G.A. Whole Grains, Refined Grains, and Cancer Risk: A Systematic Review of Meta-Analyses of Observational Studies. Nutrients 2020, 12, 3756. [Google Scholar] [CrossRef]
- Zhang, X.F.; Wang, X.K.; Tang, Y.J.; Guan, X.X.; Guo, Y.; Fan, J.M.; Cui, L.L. Association of Whole Grains Intake and the Risk of Digestive Tract Cancer: A Systematic Review and Meta-Analysis. Nutr. J. 2020, 19, 52. [Google Scholar] [CrossRef]
- Katsidzira, L.; Laubscher, R.; Gangaidzo, I.T.; Swart, R.; Makunike-Mutasa, R.; Manyanga, T.; Thomson, S.; Ramesar, R.; Matenga, J.A.; Rusakaniko, S. Dietary Patterns and Colorectal Cancer Risk in Zimbabwe: A Population Based Case-Control Study. Cancer Epidemiol. 2018, 57, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.M.H.; Hughes, J.; Grafenauer, S. Whole Grain Intakes Are Associated with Healthcare Cost Savings Following Reductions in Risk of Colorectal Cancer and Total Cancer Mortality in Australia: A Cost-of-Illness Model. Nutrients 2021, 13, 2982. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Slattery, M.L.; Sweeney, C.; Herrick, J.; Wolff, R.K.; Albertsen, H. APC Mutations and Other Genetic and Epigenetic Changes in Colon Cancer. Mol. Cancer Res. 2007, 5, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojep, D.N.A.; Seng, P.K. Histomorphology of Aberrant Crypt Foci in Colorectal Carcinoma. Malays. J. Pathol. 2010, 32, 111–116. [Google Scholar]
- Orlando, F.A.; Tan, D.; Baltodano, J.D.; Khoury, T.; Gibbs, J.F.; Hassid, V.J.; Ahmed, B.H.; Alrawi, S.J. Aberrant Crypt Foci as Precursors in Colorectal Cancer Progression. J. Surg. Oncol. 2008, 98, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Bruce, R.W.; Giacca, A.; Medline, A. Possible Mechanisms Relating Diet and Risk of Colon Cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1271–1279. [Google Scholar]





| Control (n = 37) | Polyps (n = 49) | CRC (n = 7) | |
|---|---|---|---|
| Male gender | 17 (45.95) a | 30 (61.22) a | 7 (100.00) b |
| Age (years) | 60 (54–66) a | 63 (56–66) a | 63 (61–70) a |
| Energy intake (kcal/d) | 1974.87 (1492.99–2463.87) a | 1926.43 (1691.48–2675.80) a | 2070.43 (1915.76–2830.29) a |
| BMI (kg/m2) | 25.70 (23.67–28.94) a | 27.56 (25.14–31.22) b | 24.68 (24.21–29.32) a |
| CRC history (1st grade) | 9 (24.32) a | 11 (22.45) a | 1 (14.29) a |
| Physical activity (min/d) | 75.00 (37.50–75.00) a | 50.00 (37.50–75.00) a | 90.00 (75.00–90.00) b |
| Sleeping (hours/d) | 7.00 (6.00–7.25) a | 7.00 (6.00–8.00) a | 7.00 (6.00–8.00) a |
| Current smoker | 6 (16.22) a | 13 (26.53) a | 1 (14.29) a |
| Gastrointestinal functionality | |||
| Deposition/week | 8.50 (6.00–8.50) a | 7.00 (6.00–8.50) a | 8.50 (8.50–8.50) a |
| Liquid feces | 0 (0.00) a | 1 (2.04) a | - |
| Soft feces | 27 (72.97) a | 32 (65.31) a | 5 (71.43) a |
| Hard feces | 10 (27.03) a | 16 (32.65) a | 2 (28.57) a |
| Pathological analysis (DxBx) | |||
| HP | 4 (10.81) a | 7 (14.29) a | 0 (0.00) a |
| TA | 0 (0.00) a | 22 (44.90) b | - |
| TVA | 0 (0.00) a | 5 (10.20) b | - |
| SSA | 0 (0.00) a | 1 (2.04) a | - |
| HGD | 0 (0.00) a | 6 (12.24) b | - |
| AC | 0 (0.00) a | 1 (2.04) a | 7 (100.00) b |
| LSC | 24 (64.86) a | 3 (6.12) b | 0 (0.00) b |
| Not available | 9 (24.32) a | 4 (8.16) b | 0 (0.00) a |
| Variables | Total Sample (n = 93) | Diagnosis Group | ||
|---|---|---|---|---|
| Control (n = 37) | Polyps (n = 49) | CRC (n = 7) | ||
| Bioactive | ||||
| Total fiber (g/d) | 20.88 (14.77–25.15) | 21.89 (14.77–26.87) a | 20.41 (15.22–23.75) a | 22.33 (13.80–29.55) a |
| Insoluble fiber (g/d) | 12.50 (8.66–15.08) | 12.50 (8.66–16.57) a | 12.29 (9.25–14.64) a | 13.27 (8.04–17.70) a |
| Soluble fiber (g/d) | 2.40 (1.87–3.06) | 2.62 (1.86–3.17) a | 2.32 (1.88–2.85) a | 2.77 (1.91–3.03) a |
| Cellulose (g/d) | 5.01 (3.62–6.39) | 5.01 (3.50–6.40) a | 4.93 (3.64–6.24) a | 5.35 (2.97–8.02) a |
| Insoluble hemicellulose (g/d) | 3.88 (2.80–4.90) | 4.02 (2.86–5.38) a | 3.63 (2.68–4.51) a | 4.01 (2.69–5.48) a |
| Soluble hemicellulose (g/d) | 1.65 (1.15–2.27) | 1.77 (1.09–2.32) a | 1.57 (1.19–2.04) a | 1.90 (1.04–2.35) a |
| Insoluble pectin (g/d) | 1.34 (1.01–1.96) | 1.53 (1.11–2.02) a | 1.29 (0.94–1.81) a | 1.58 (1.20–2.06) a |
| Soluble pectin (g/d) | 0.66 (0.51–0.89) | 0.70 (0.55–0.92) a | 0.62 (0.45–0.88) a | 0.69 (0.58–1.29) a |
| Klason lignin (g/d) | 1.63 (1.22–2.26) | 1.63 (1.30–2.26) a | 1.69 (1.22–2.11) a | 1.42 (1.13–2.44) a |
| Total polyphenols (mg/d) | 1482.46 (963.00–1951.48) | 1509.00 (1074.04–1877.40) a | 1376.33 (904.37–1951.48) a | 1454.97 (1000.45–2051.10) a |
| Flavonoids (mg/d) | 128.09 (72.89–302.17) | 136.93 (78.28–251.34) a | 122.87 (53.65–331.58) a | 174.05 (80.98–498.80) a |
| Lignans (mg/d) | 46.95 (26.29–74.22) | 47.39 (28.61–85.26) a | 40.18 (22.28–60.34) a | 55.39 (30.43–92.01) a |
| Other polyphenols (mg/d) | 24.27 (15.19–42.26) | 27.32 (16.37–45.15) a | 19.93 (14.86–35.08) a | 29.48 (19.32–60.48) a |
| Phenolic acids (mg/d) | 496.08 (211.63–836.53) | 609.92 (222.81–958.37) a | 386.62 (188.52–781.00) a | 496.08 (262.91–1223.65) a |
| Stilbenes (mg/d) | 0.11 (0.04–0.76) | 0.09 (0.04–0.36) a | 0.16 (0.03–1.87) a | 0.11 (0.04–2.10) a |
| Carcinogens | ||||
| Ethanol (g/d) | 2.18 (0.19–10.56) | 1.88 (0.28–8.80) a | 8.13 (1.76–22.93) b | 6.02 (0.00–24.46) a |
| Xenobiotics | ||||
| B(a)P (µg/d) | 0.06 (0.04–0.08) | 0.06 (0.05–0.08) a | 0.06 (0.04–0.08) a | 0.07 (0.03–0.08) a |
| DiB(a)A (µg/d) | 0.03 (0.01–0.10) | 0.03 (0.00–0.04) a | 0.05 (0.01–0.15) b | 0.03 (0.00–0.32) a |
| Total PAH (µg/d) | 1.09 (0.66–1.44) | 0.93 (0.58–1.44) a | 1.15 (0.75–1.43) a | 1.22 (1.07–1.46) a |
| PhlP (ng/d) | 82.56 (25.14–232.97) | 77.78 (24.45–182.10) a | 82.64 (23.79–329.91) a | 83.12 (36.77–222.53) a |
| DiMelQx (ng/d) | 6.67 (3.29–14.72) | 5.13 (3.00–13.83) a | 6.90 (3.47–16.83) a | 9.96 (4.53–18.22) a |
| MelQx (ng/d) | 23.50 (13.44–61.12) | 22.15 (13.44–61.12) a | 23.24 (13.42–56.27) a | 25.27 (16.64–69.07) a |
| MelQ (ng/d) | 0.81 (0.00–1.68) | 0.93 (0.34–1.82) a | 0.81 (0.00–1.30) a | 0.00 (0.00–2.16) a |
| IQ (ng/d) | 0.13 (0.00–0.27) | 0.13 (0.00–0.25) a | 0.13 (0.00–0.27) a | 0.00 (0.00–0.17) a |
| Total HAs (sum) (ng/d) | 119.54 (53.34–315.45) | 103.17 (46.23–269.12) a | 125.27 (53.95–381.99) a | 185.87 (83.32–245.86) a |
| Nitrates (mg/d) | 91.15 (55.92–140.60) | 95.09 (65.41–140.60) a | 69.44 (55.74–113.17) a | 97.39 (54.94–186.96) a |
| Nitrites (mg/d) | 2.39 (1.52–4.17) | 2.48 (1.74–4.34) a | 2.37 (1.31–4.17) a | 2.44 (1.73–2.62) a |
| NDMA (µg/d) | 0.16 (0.11–0.30) | 0.16 (0.10–0.28) a | 0.16 (0.11–0.30) a | 0.17 (0.13–0.35) a |
| NPIP (µg/d) | 0.07 (0.04–0.11) | 0.08 (0.05–0.11) a | 0.07 (0.03–0.11) a | 0.06 (0.04–0.08) a |
| NPYR (µg/d) | 0.11 (0.06–0.18) | 0.12 (0.08–0.17) a | 0.10 (0.05–0.18) a | 0.09 (0.07–0.12) a |
| Acrylamide (µg/d) | 14.70 (8.66–24.20) | 15.07 (8.66–25.11) a | 14.70 (8.06–21.42) a | 14.15 (13.29–36.22) a |
| N (%) | Mean ± SD | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| GBD factors | ||||
| BMI | ||||
| 5 kg/m2 | 93 (100) | 27.24 ± 4.06 | 1.705 (0.975–2.980) | 0.061 |
| Alcoholic beverages (g/d) | ||||
| ≤48.00 | 32 (37) | 11.10 ± 14.11 | – | – |
| >48.00 | 54 (63) | 442.81 ± 559.93 | 2.539 (0.997–6.467) | 0.051 |
| Whole grains (g/d) | ||||
| ≤50.00 | 78 (91) | 5.47 ± 11.66 | – | – |
| >50.00 | 8 (9) | 130.69 ± 107.33 | 0.168 (0.029–0.966) | 0.046 * |
| Bioactives | ||||
| Soluble pectin (g/d) | ||||
| ≤0.57 | 32 (37) | 0.43 ± 0.09 | – | – |
| 0.57–0.85 | 29 (34) | 0.71 ± 0.08 | 0.357 (0.117–1.089) | 0.070 |
| ≥0.85 | 25 (29) | 1.33 ± 0.60 | 0.408 (0.125–1.327) | 0.136 |
| Flavonoids (mg/d) | ||||
| ≤82.18 | 28 (33) | 44.96 ± 22.29 | – | – |
| 82.18–251.34 | 30 (35) | 152.20 ± 55.08 | 0.343 (0.112–1.052) | 0.061 |
| ≥251.34 | 28 (33) | 525.49 ± 323.63 | 1.099 (0.347–3.482) | 0.872 |
| Other polyphenols (mg/d) | ||||
| ≤16.45 | 30 (35) | 11.26 ± 4.56 | – | – |
| 16.45–32.15 | 28 (33) | 23.91 ± 5.00 | 0.761 (0.249–2.324) | 0.631 |
| ≥32.15 | 28 (33) | 74.05 ± 52.77 | 0.358 (0.116–1.107) | 0.074 |
| Carinogens | ||||
| Ethanol (g/d) | ||||
| ≤1.70 | 29 (34) | 0.39 ± 0.56 | – | – |
| 1.70–11.62 | 28 (33) | 5.33 ± 3.24 | 1.720 (0.575–5.148) | 0.332 |
| ≥11.62 | 29 (34) | 35.12 ± 25.93 | 3.542 (1.117–11.234) | 0.032 * |
| DiB(a)A (µg/d) | ||||
| ≤0.01 | 29 (34) | 0.00 ± 0.00 | – | – |
| 0.01–0.07 | 28 (33) | 0.04 ± 0.01 | 0.587 (0.191–1.803) | 0.352 |
| ≥0.07 | 29 (34) | 0.34 ± 0.33 | 3.100 (0.950–10.118) | 0.061 |
| Total PAH (µg/d) | ||||
| ≤0.75 | 30 (35) | 0.57 ± 0.14 | – | – |
| 0.75–1.29 | 27 (31) | 1.07 ± 0.14 | 3.753 (1.154–12.204) | 0.028 * |
| ≥1.29 | 29 (34) | 1.77 ± 0.37 | 1.530 (0.510–4.595) | 0.448 |
| Nitrates (mg/d) | ||||
| ≤63.75 | 29 (34) | 44.77 ± 13.95 | – | – |
| 63.75–106.65 | 28 (33) | 85.25 ± 12.54 | 0.561 (0.182–1.729) | 0.314 |
| ≥106.65 | 29 (34) | 206.06 ± 102.67 | 0.371 (0.121–1.133) | 0.082 |
| Nitrites (mg/d) | ||||
| ≤1.69 | 30 (35) | 1.16 ± 0.38 | – | – |
| 1.69–3.34 | 26 (30) | 2.45 ± 0.45 | 0.297 (0.094–0.944) | 0.040 * |
| ≥3.34 | 30 (35) | 8.38 ± 13.07 | 0.515 (0.168–1.584) | 0.247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Saavedra, S.; Zapico, A.; del Rey, C.G.; Gonzalez, C.; Suárez, A.; Díaz, Y.; de los Reyes-Gavilán, C.G.; González, S. Dietary Xenobiotics Derived from Food Processing: Association with Fecal Mutagenicity and Gut Mucosal Damage. Nutrients 2022, 14, 3482. https://doi.org/10.3390/nu14173482
Ruiz-Saavedra S, Zapico A, del Rey CG, Gonzalez C, Suárez A, Díaz Y, de los Reyes-Gavilán CG, González S. Dietary Xenobiotics Derived from Food Processing: Association with Fecal Mutagenicity and Gut Mucosal Damage. Nutrients. 2022; 14(17):3482. https://doi.org/10.3390/nu14173482
Chicago/Turabian StyleRuiz-Saavedra, Sergio, Aida Zapico, Carmen González del Rey, Celestino Gonzalez, Adolfo Suárez, Ylenia Díaz, Clara G. de los Reyes-Gavilán, and Sonia González. 2022. "Dietary Xenobiotics Derived from Food Processing: Association with Fecal Mutagenicity and Gut Mucosal Damage" Nutrients 14, no. 17: 3482. https://doi.org/10.3390/nu14173482






